Abstract
Background
This study assessed sex differences in treatments, all‐cause mortality, relative survival, and excess mortality following acute myocardial infarction.
Methods and Results
A population‐based cohort of all hospitals providing acute myocardial infarction care in Sweden (SWEDEHEART [Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies]) from 2003 to 2013 was included in the analysis. Excess mortality rate ratios (EMRRs), adjusted for clinical characteristics and guideline‐indicated treatments after matching by age, sex, and year to background mortality data, were estimated. Although there were no sex differences in all‐cause mortality adjusted for age, year of hospitalization, and comorbidities for ST‐segment–elevation myocardial infarction (STEMI) and non‐STEMI at 1 year (mortality rate ratio: 1.01 [95% confidence interval (CI), 0.96–1.05] and 0.97 [95% CI, 0.95–0.99], respectively) and 5 years (mortality rate ratio: 1.03 [95% CI, 0.99–1.07] and 0.97 [95% CI, 0.95–0.99], respectively), excess mortality was higher among women compared with men for STEMI and non‐STEMI at 1 year (EMRR: 1.89 [95% CI, 1.66–2.16] and 1.20 [95% CI, 1.16–1.24], respectively) and 5 years (EMRR: 1.60 [95% CI, 1.48–1.72] and 1.26 [95% CI, 1.21–1.32], respectively). After further adjustment for the use of guideline‐indicated treatments, excess mortality among women with non‐STEMI was not significant at 1 year (EMRR: 1.01 [95% CI, 0.97–1.04]) and slightly higher at 5 years (EMRR: 1.07 [95% CI, 1.02–1.12]). For STEMI, adjustment for treatments attenuated the excess mortality for women at 1 year (EMRR: 1.43 [95% CI, 1.26–1.62]) and 5 years (EMRR: 1.31 [95% CI, 1.19–1.43]).
Conclusions
Women with acute myocardial infarction did not have statistically different all‐cause mortality, but had higher excess mortality compared with men that was attenuated after adjustment for the use of guideline‐indicated treatments. This suggests that improved adherence to guideline recommendations for the treatment of acute myocardial infarction may reduce premature cardiovascular death among women.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02952417.
Keywords: excess mortality, mortality, non–ST‐segment–elevation acute coronary syndrome, relative survival, sex, ST‐segment–elevation myocardial infarction, survival
Subject Categories: Epidemiology, Myocardial Infarction, Mortality/Survival
Clinical Perspective
What Is New?
We found a survival disadvantage for women with ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation myocardial infarction who were followed for 10 years after acute myocardial infarction.
These sex differences in excess mortality persisted after adjusting for age and comorbidities.
However, the differences in excess mortality decreased or disappeared after adjusting for the use of evidence‐based treatments.
What Are the Clinical Implications?
Our novel findings suggest that if treatments for acute myocardial infarction were provided equally between sexes, then differences in deaths between men and women would be smaller and premature cardiovascular deaths among women would be reduced.
Coronary artery disease is a major health problem that contributes considerably to the global mortality and disease burden in men and women.1 Estimates of the impact of sex on survival following acute myocardial infarction (AMI) have varied to some extent.2, 3 The reasons for these inconsistent findings are numerous and include the use of different populations with regard to age and type of AMI, as well as differing times to censorship and adjustments for confounders.
Several studies have suggested that sex is no longer a major independent predictor of death, after adjustment for age and comorbidities.4, 5, 6, 7, 8, 9, 10, 11, 12 However, these studies have not adjusted for the fact that women without AMI have a better underlying prognosis than men without AMI. Notably, most of the literature reporting survival has used all‐cause mortality as the clinical outcome.5, 6 Such analyses are not able to disentangle deaths related to the disease of interest (ie, AMI in this study) from deaths due to competing risks. To correct for estimated mortality due to other causes using background population mortality data, the method of choice is relative survival.13, 14, 15, 16
To our knowledge, no large‐scale studies have used this technique to evaluate sex differences in survival after AMI. Consequently, we aimed to estimate the impact of sex on relative survival and excess mortality following AMI using a population‐based cohort within a relative survival framework and to identify factors associated with differences in survival.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Institutional review board approval was obtained, according to the JAHA guidelines. No informed consent was required.
Patients, Setting, and Inclusion Criteria
We included all hospitals in Sweden (n=73) that provided care for patients with AMI. Eligible patients (n=180 368) were those aged >18 years who had been hospitalized for AMI between January 1, 2003, and December 31, 2013 (Table S1). All patients were censored on December 31, 2013. For multiple admissions, we used the earliest record. Patient‐level data concerning demographics, comorbidities, cardiovascular risk factors, and treatments at discharge were extracted from SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies), a population‐based registry of outcomes for patients hospitalized with acute coronary syndrome. SWEDEHEART is a Web‐based system, and all data are registered by the caregiver (physicians and nurses) during acute care. The registry is regularly monitored with 95% to 96% agreement between register and electronic health records; more details about the registry and validity have been published elsewhere.17 Baseline data were also enriched by linking SWEDEHEART to the National Patient Register, including all International Classification of Diseases codes for all admissions to Swedish hospitals since 1987. Cases of AMI were defined as ST‐segment–elevation myocardial infarction (STEMI) and non‐STEMI (NSTEMI), according to the current European Society of Cardiology, American College of Cardiology, and American Heart Association guidelines and determined at the local level by the attending physician.18 The data flow for the derivation of the analytical cohort is shown in Figure 1.
Figure 1.
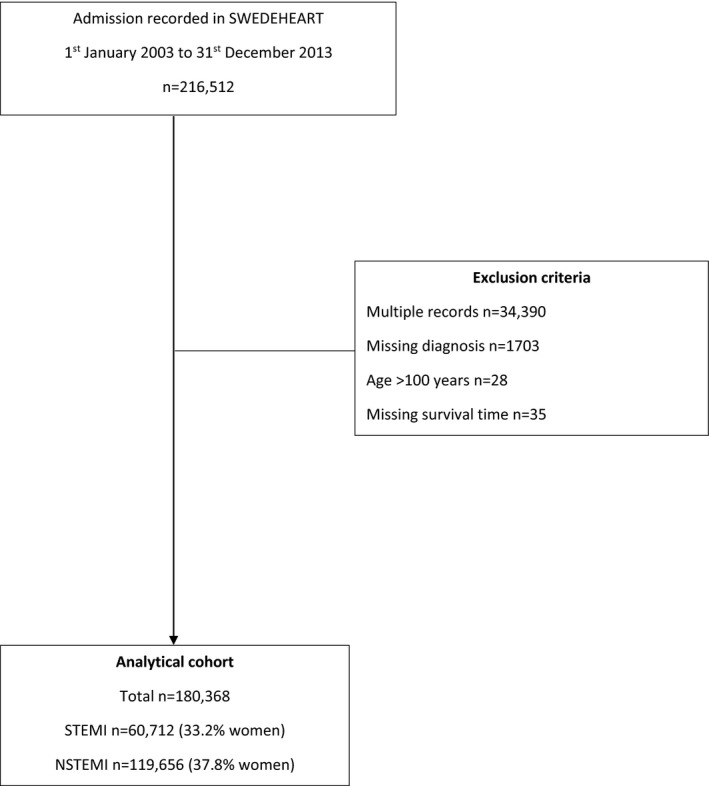
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram of exclusion of cases from the SWEDEHEART data set to derive the analytical cohort. NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Observed Survival
Data for all‐cause mortality for patients recorded in SWEDEHEART were extracted through linkage to the National Population Registry in Sweden using each patient's unique identifier. Patients were followed up for their vital status after hospitalization for AMI, with censoring at the end of follow‐up on December 31, 2013 (Figure 1). Survival time was defined as the duration between date of hospital admission for AMI and date of death, date of last follow‐up for vital status, or end of the study censoring period.
Expected Survival
Expected survival was derived from the general population of Sweden matched by age, sex, and year of hospitalization to observed survival for the SWEDEHEART registry patients. This analysis was undertaken using life tables obtained from the Human Mortality Database of Sweden (http://www.mortality.org) and included 1 093 480 matched deaths.
Primary Outcome
The primary outcome was excess mortality at 6 months, 1 year, and 5 years following hospitalization with AMI.
Statistical Analyses
Differences in baseline characteristics by sex were evaluated using percentages to describe categorical variables and means and standard deviations for continuous normally distributed variables (all continuous variables were normally distributed). Logistic regression, with and without adjustment for age and comorbidities, was used to evaluate sex differences in the odds of receiving treatment at discharge, represented as odds ratios with 95% confidence intervals (CIs).
Relative survival was defined as the observed survival among patients with STEMI and NSTEMI divided by expected survival in the age‐, sex‐, and year‐matched populace of Sweden.
We used flexible parametric survival models to calculate excess mortality following AMI, associated with sex. The flexible parametric approach allowed the incorporation of time‐dependent effects and used restricted cubic spline functions to estimate the baseline cumulative hazard function.19 The base model (model 1) included age (≤55, 56 to ≤65, 66 to ≤75 [reference group], 76 to ≤85, and >85 years), sex, and year of hospitalization (2003–2005 [reference group], 2006–2008, 2009–2011, and 2012–2013). Subsequently, a case mix model (model 2) was fitted that included the base variables (age, sex, year of hospitalization) and comorbidities (history of diabetes mellitus, hypertension, myocardial infarction, stroke, peripheral vascular disease, heart failure, chronic renal failure, and chronic obstructive pulmonary disease) to test whether the effect of sex on excess mortality would change. To this model we then added (model 3) the use of guideline‐indicated treatments for AMI including reperfusion therapy (fibrinolysis or primary percutaneous coronary intervention, only for patients with STEMI), any revascularization (percutaneous coronary intervention or coronary artery bypass grafting surgery), and medications at discharge from the hospital (aspirin, β‐blockers, HMG‐CoA [5‐hydroxy‐3‐methylglutaryl–coenzyme A] reductase inhibitors [statins], angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, P2Y12 inhibitors). Obtained estimates from these incremental models were presented at 6 months, 1 year, and 5 years of follow‐up and calculated separately for STEMI and NSTEMI. Results were presented as adjusted excess mortality rate ratios (EMRRs).20, 21 Evidence of excess mortality is observed when the EMRR is >1 (an EMRR of 1.5 for women compared with men indicates that women experience 50% higher excess mortality than men).
For all models, the hazard was selected as the model's scale. The degrees of freedom for the fitted model were determined using the Akaike information criterion, the Bayesian information criterion, and the likelihood ratio test. The proportional excess hazards assumption was assessed by incorporating time‐dependent effects among age, sex, year, and timescale and assessed using the likelihood ratio test. Given the effects of age, sex, and year of hospitalization varied across time, interaction terms between age, sex, and year of hospitalization and time were added to the models. Adding the age×sex interaction to the models had no significant effect on improving the model fit, as assessed using the Akaike information criterion, the Bayesian information criterion, and the likelihood ratio test. We compared mortality rate ratios (MRRs) calculated using a Cox model (all‐cause mortality) with those from the flexible parametric relative survival model. Finally, we investigated the impact of increasing age on excess mortality from AMI due to sex by fitting the full flexible parametric survival models according to age group and comparing the relative risk of change of EMRR between patients aged ≤55 years with those aged >85 years. All analyses were performed using complete case data because of the limited number of missing values (Table S2). All tests were 2‐tailed, with the level of statistical significance prespecified at 5% (P<0.05). Statistical analyses were performed using Stata version 14.2 (StataCorp).
Results
There were 60 712 (33.7%) patients with STEMI (mean age: 68.9 years [SD: 12.6]; 33.2% women) and 119 656 (66.3%) with NSTEMI (mean age: 72.4 years [SD: 12.0]; 37.8% women). Women with STEMI (mean age: 73.7 years [SD: 12.2] versus 66.5 years [SD: 12.1]) and NSTEMI (mean age: 75.6 years [SD: 11.5] versus 70.4 years [SD: 11.9]) were older than men and also more comorbid, being more likely to have diabetes mellitus, heart failure, hypertension, and chronic obstructive pulmonary disease. However, they were less likely to be smokers and to have had prior myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting surgery (Table 1 and Tables S3 and S4). At presentation to the hospital, women were more likely to have a systolic blood pressure <90 mm Hg, heart rate >110 beats/min, and receive loop diuretics during their hospital stay (Table 1). Women were less likely than men to receive guideline‐indicated pharmacological and invasive coronary treatments (Table 1, Figure 2, and Table S5).
Table 1.
Baseline Characteristics Stratified by Sex for STEMI and NSTEMI
| STEMI (N=60 712) | NSTEMI (N=119 656) | |||||
|---|---|---|---|---|---|---|
| Women (n=20 140) | Men (n=40 572) | Difference in Means/Proportions (95% CI)a | Women (n=45 254) | Men (n=74 402) | Difference in Means/Proportions (95% CI)a | |
| Mean (SD) age, y | 73.7 (12.2) | 66.5 (12.1) | −7.22 (−7.43 to −7.02)b | 75.6 (11.5) | 70.4 (11.9) | −5.19 (−5.33 to −5.05)b |
| Year of hospitalization, n (%) | ||||||
| 2003–2005 | 5903 (29.3) | 11 208 (27.6) | 1.69 (0.26–3.12)b | 13 054 (28.9) | 21 099 (28.4) | 0.49 (−0.50 to 1.48) |
| 2006–2008 | 5549 (27.6) | 10 911 (26.9) | 0.66 (−0.78 to 2.10) | 12 704 (28.1) | 20 722 (27.9) | 0.22 (−0.77 to 1.21) |
| 2009–2011 | 5263 (26.1) | 11 217 (27.7) | −1.52 (−3.00 to −0.07)b | 11 675 (25.8) | 19 448 (26.1) | −0.34 (−1.34 to 0.67) |
| 2012–2013 | 3425 (17.0) | 7236 (17.8) | −0.82 (−2.36 to 0.72) | 7821 (17.3) | 13 133 (17.7) | −0.37 (−1.43 to 0.69) |
| Risk factors, n (%) | ||||||
| Diabetes mellitus | 3524 (17.5) | 5972 (14.7) | 2.78 (1.24–4.32)b | 10 510 (23.2) | 16 384 (22.0) | 1.2 (0.17–2.23)b |
| Hypertension | 9724 (48.3) | 14 701 (36.2) | 12.05 (10.80–13.31)b | 24 456 (54.0) | 33 436 (44.9) | 9.1 (8.28–9.92)b |
| Current or ex‐smoker | 8574 (47.3) | 24 075 (63.8) | −16.47 (−17.70 to −15.25)b | 16 569 (41.2) | 42 269 (62.0) | −20.78 (21.66 to −19.90)b |
| Prior cardiovascular diseases, n (%) | ||||||
| Myocardial infarction | 2346 (11.7) | 5278 (13.0) | −1.36 (−2.94 to 0.22) | 10 126 (22.4) | 19 192 (25.8) | −3.42 (−4.44 to −2.40)b |
| Heart failure | 1269 (6.3) | 1526 (3.8) | 2.54 (0.90–4.18)b | 6170 (13.6) | 8382 (11.3) | 2.36 (1.27–3.45)b |
| PCI | 721 (3.6) | 2440 (6.0) | −2.43 (−4.08 to −0.78)b | 2857 (6.3) | 7763 (10.4) | −4.12 (−5.24 to −3.00)b |
| CABG | 415 (2.1) | 1626 (4.0) | −1.95 (−3.62 to −0.28) | 2493 (5.5) | 8699 (11.7) | −6.18 (−7.30 to −5.06)b |
| Cerebrovascular disease | 1775 (8.8) | 2746 (6.8) | 2.04 (0.42–3.66)b | 5335 (11.8) | 8189 (11.0) | 0.78 (−0.32 to 1.88) |
| PVD | 833 (4.1) | 1287 (3.2) | 0.97 (−0.69 to 2.63) | 3121 (6.9) | 5041 (6.8) | 0.12 (−1.01 to 1.25) |
| Other comorbidities, n (%) | ||||||
| Chronic renal failure | 372 (1.9) | 717 (1.8) | 0.08 (−1.60 to 1.76) | 1522 (3.4) | 2991 (4.0) | −0.66 (−1.81 to −0.49) |
| COPD | 1358 (6.7) | 1656 (4.1) | 2.66 (1.02–4.30)b | 4130 (9.1) | 5208 (7.0) | 2.13 (1.01–3.25)b |
| Presentation characteristics | ||||||
| Systolic BP, mm Hg, mean (SD) | 140.0 (31.4) | 140.9 (29.6) | 0.87 (0.32–1.43)b | 150.4 (30.8) | 148.5 (28.6) | −1.83 (−2.21 to −1.46)b |
| Systolic BP, ≤90 mm Hg, n (%) | 1032 (5.1) | 1555 (3.8) | 1.29 (−0.36 to 2.94) | 924 (2.0) | 1323 (1.8) | 0.26 (−0.90 to 1.42) |
| Heart rate, beats/min, mean (SD) | 79.7 (22.4) | 76.8 (21.0) | −2.87 (−3.26 to −2.47)b | 86.5 (24.5) | 81.3 (23.9) | −5.24 (−5.54 to −4.93)b |
| Heart rate, >110 beats/min, n (%) | 1430 (8.4) | 2068 (6.0) | 2.39 (0.63–4.15)b | 5521 (14.4) | 6380 (10.1) | 4.31 (3.13–5.49)b |
| ST‐segment deviation, n (%) | 18 841 (93.8) | 37 909 (93.8) | 0 (−0.42 to 0.42) | 16 852 (37.9) | 26 483 (36.3) | 1.64 (0.71–2.57)b |
| In‐hospital course, n (%) | ||||||
| Cardiac arrest | 1265 (6.3) | 2491 (6.1) | 0.14 (−1.50 to 1.78) | 961 (2.1) | 1878 (2.5) | −0.41 (−1.56 to 0.74) |
| Use of a loop diuretic | 5703 (28.5) | 8181 (20.3) | 8.23 (6.77–9.70)b | 13 511 (30.0) | 16 177 (21.9) | 8.15 (7.15–9.15)b |
| Reperfusion | 13 768 (68.5) | 32 093 (79.2) | −10.7 (−11.60 to −9.81)b | NA | NA | NA |
| Revascularization | 13 023 (64.7) | 32 446 (80.0) | −15.31 (−16.24 to −14.38)b | 14 528 (32.1) | 37 616 (50.6) | −18.46 (−19.37 to −17.55)b |
| Treatments at discharge | ||||||
| Aspirin | 17 304 (87.1) | 36 873 (91.8) | −4.66 (−5.23 to −4.09)b | 38 451 (85.7) | 65 956 (89.4) | −3.68 (−4.10 to −3.26)b |
| β‐Blocker | 16 530 (83.1) | 35 417 (88.0) | −4.89 (−5.55 to −4.23)b | 37 535 (83.6) | 63 518 (86.0) | −2.37 (−2.83 to −1.91)b |
| Statin | 14 612 (73.5) | 34 431 (85.6) | −12.12 (−12.93 to −11.31)b | 30 419 (67.8) | 58 677 (79.5) | −11.68 (−12.30 to −11.06)b |
| ACEi or ARB | 13 070 (70.3) | 29 372 (77.7) | −7.35 (−8.27 to −6.43)b | 27 271 (65.3) | 47 912 (69.4) | −4.15 (−4.85 to −3.45)b |
| P2Y12 inhibitor | 14 176 (71.2) | 32 519 (80.8) | −9.56 (−10.42 to −8.70)b | 26 329 (58.6) | 49 396 (66.8) | −8.23 (−8.96 to −7.50)b |
Difference in means for continuous variables and proportions for categorical variables. Reperfusion comprises fibrinolysis or primary PCI. Revascularization comprises PCI or CABG surgery. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CABG, coronary artery bypass grafting; CI, confidence interval; COPD, chronic obstructive pulmonary disease; NSTEMI indicates non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST‐segment–elevation myocardial infarction.
significance level of p value <0.05..
Significance level <0.05.
Figure 2.

Odds of receipt of guideline‐indicated care for women compared with men, by (A) ST‐segment–elevation myocardial infarction and (B) non–ST‐segment–elevation myocardial infarction. Odds ratios were calculated using univariate and multivariable logistic regression. *Adjusted odds ratios for age, diabetes mellitus, hypertension, previous myocardial infarction, cerebrovascular disease, peripheral vascular disease, and heart failure. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; OR, odds ratio.
Standard Survival of All‐Cause Mortality (Cox Model)
For women, there were 30 202 (46.2%) deaths over 271 824 person‐years at risk, and for men there were 39 702 (34.5%) deaths over 540 771 person‐years at risk. The median time to death was shorter for women than for men (1.7 years [interquartile range: 0.3–4.3 years] versus 1.9 years (interquartile range: 0.3–4.6 years]; P<0.001). For STEMI, all‐cause mortality analysis showed no sex difference in mortality adjusted for age, year of hospitalization, and comorbidities at 6 months (MRR: 1.02 [95% CI, 0.98–1.06]), 1 year (MRR: 1.01 [95% CI, 0.96–1.05]), and 5 years (MRR: 1.03; 95% CI, 0.99–1.07]). For NSTEMI, women showed a small reduction in mortality compared with men at 6 months (MRR: 0.96 [95% CI, 0.94–0.99]), 1 year (MRR: 0.97 [95% CI, 0.95–0.99]), and 5 years (MRR: 0.97 [95% CI, 0.95–0.99]). However, after further adjusting for treatments, women with STEMI and NSTEMI each had reduced all‐cause mortality at 6 months (MRR: 0.94 [95% CI, 0.91–0.98]) and 0.88 [95% CI, 0.86–0.90]), 1 year (MRR: 0.92 [95% CI, 0.89–0.96] and 0.88 [95% CI, 0.87–0.90]), and 5 years (MRR: 0.93 [95% CI, 0.89–0.96] and 0.89 [95% CI, 0.87–0.91]; Table 2).
Table 2.
Adjusted MRR and EMRR at 6 Months, 1 Year, and 5 Years for Women With 95% CIs for STEMI and NSTEMI
| Model | STEMI, n=60 712 | NSTEMI, n=119 656 | ||
|---|---|---|---|---|
| MRR (95% CI) | EMRR (95% CI) | MRR (95% CI) | EMRR (95% CI) | |
| Model 1: age, year of hospitalization | ||||
| 6 mo | 1.01 (0.97–1.05) | 2.12 (1.85–2.42)a | 0.93 (0.91–0.95)a | 1.14 (1.10–1.18)a |
| 1 y | 1.01 (0.95–1.05) | 3.29 (2.40–4.51)a | 0.93 (0.92–0.95)a | 1.24 (1.19–1.29)a |
| 5 y | 1.03 (1.00–1.07) | 1.91 (1.73–2.10)a | 0.95 (0.92–0.97)a | 1.35 (1.28–1.42)a |
| Model 2: age, year of hospitalization, comorbiditiesb | ||||
| 6 mo | 1.02 (0.98–1.06) | 1.65 (1.50–1.81)a | 0.96 (0.94–0.99) | 1.15 (1.11–1.19)a |
| 1 y | 1.01 (0.96–1.05) | 1.89 (1.66–2.16) | 0.97 (0.95–0.99) | 1.20 (1.16–1.24)a |
| 5 y | 1.03 (0.99–1.07) | 1.60 (1.48–1.72)a | 0.97 (0.95–0.99) | 1.26 (1.21–1.32)a |
| Model 3: age, year of hospitalization, comorbiditiesb, treatments at dischargec | ||||
| 6 mo | 0.94 (0.91–0.98)a | 1.26 (1.16–1.37)a | 0.88 (0.86–0.90)a | 0.97 (0.94–1.00) |
| 1 y | 0.92 (0.89–0.96)a | 1.43 (1.26–1.62)a | 0.88 (0.87–0.90)a | 1.01 (0.97–1.04) |
| 5 y | 0.93 (0.89–0.96)a | 1.31 (1.19–1.43)a | 0.89 (0.87–0.91)a | 1.07 (1.02–1.12)a |
CI indicates confidence interval; EMRR, excess mortality rate ratio; MRR, mortality rate ratio; NSTEMI indicates non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Significance level <0.05.
Diabetes mellitus, hypertension, myocardial infarction, cerebrovascular diseases, peripheral vascular disease, heart failure.
Aspirin, β‐blockers, statin, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, P2Y12 inhibitors, reperfusion (fibrinolysis or primary PCI), revascularization (PCI or coronary artery bypass grafting surgery).
Relative Survival and Excess Mortality
The estimated average relative survival adjusted for age and year was lower for women with STEMI than for men at 6 months (84.8% versus 87.6%), 1 year (83.2% versus 86.8%), and 5 years (75.1% versus 82.4%). For NSTEMI, the difference in relative survival between men and women was not evident at 6 months (90.0% versus 89.6%) and 1 year (86.9% versus 87.0%); however, women with NSTEMI had lower relative survival than men at 5 years (73.1% versus 76.0%).
Women with STEMI had a 2‐fold increase in excess mortality after adjustment for age and year of hospital admission at 6 months (EMRR: 2.12 [95% CI, 1.85–2.42]), a 3‐fold increase at 1 year (EMRR: 3.29 [95% CI, 2.40–4.51]), and an almost 2‐fold increase at 5 years (EMRR: 1.91[ 95% CI, 1.73–2.10]). For NSTEMI, the effects were smaller but statistically significant at 6 months (EMRR: 1.14 [95% CI, 1.10–1.18]), 1 year (EMRR: 1.24 [95% CI, 1.19–1.29]), and 5 years (EMRR: 1.35 [95% CI, 1.28–1.42]; Table 2 and Figure 3).
Figure 3.
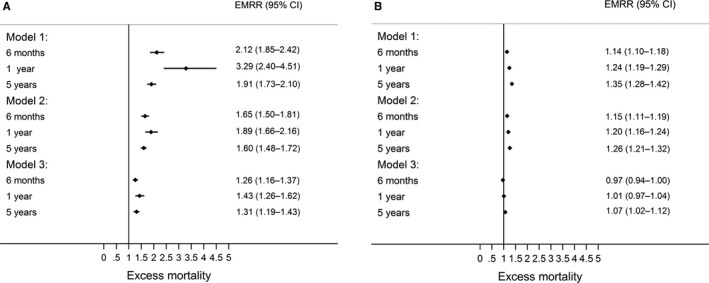
Risk of death at 6 months, 1 year, and 5 years for women compared with men by (A) ST‐segment–elevation myocardial infarction (STEMI) and (B) non–ST‐segment–elevation myocardial infarction (NSTEMI). Age and year of hospitalization (model 1); age, year of hospitalization, and comorbidities (model 2); and age, year of hospitalization, comorbidities, and treatments at discharge (aspirin, β–blockers, statin, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, P2Y12 inhibitors, revascularization [NSTEMI], reperfusion. and revascularization [STEMI]; model 3). CI indicates confidence interval; EMRR, excess mortality rate ratio.
After additive adjustment for comorbidities, women with STEMI and NSTEMI continued to demonstrate significant excess mortality at each of the follow‐up time points (Table 2 and Figure 3). After further adjustment for the use of guideline‐indicated treatments, excess mortality among women with NSTEMI was no longer significant at 6 months (EMRR: 0.97 [95% CI, 0.94–1.00]) and 1 year (EMRR: 1.01 [95% CI, 0.97–1.04]), but remained at 5 years (EMRR: 1.07 [95% CI, 1.02–1.12]). For STEMI, adjustment for treatments reduced excess mortality for women compared with men at 6 months (EMRR: 1.26 [95% CI, 1.16–1.37]), 1 year (EMRR: 1.43[ 95% CI, 1.26–1.62]), and 5 years (EMRR: 1.31 [95% CI, 1.19–1.43]; Table 2 and Figure 3). With increasing age, the risk of excess mortality after adjustment for comorbidities and treatments increased for women with STEMI (age >85 versus ≤55 years, EMRR: 2.85 [95% CI, 2.26–3.60]) and NSTEMI (age >85 versus ≤55 years, EMRR: 4.07 [95% CI, 3.38–4.92]). A similar association of excess mortality and increased age was found for men with STEMI (age >85 versus ≤55 years, EMRR: 2.27 [95% CI, 1.85–2.80]; Figure 4A) and NSTEMI (age >85 versus ≤55 years, EMRR: 2.93 [95% CI, 2.56–3.35; Figure 4B), and there was no significant interaction between sex and age.
Figure 4.
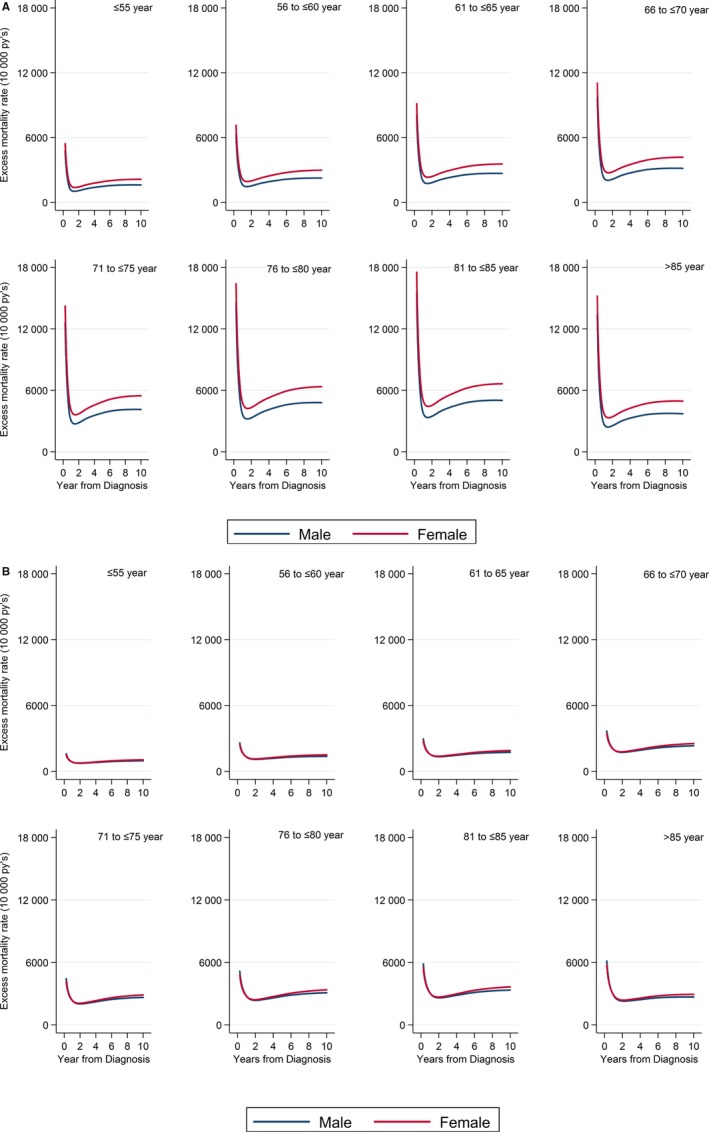
A, Adjusted cumulative excess mortality (model 3) by age group stratified by sex for (A) ST‐segment–elevation myocardial infarction and (B) non–ST‐segment–elevation myocardial infarction.
Discussion
This nationwide study of >180 000 patients with AMI contains several novel and important findings relevant to clinical practice. We found that women after AMI, despite similar adjusted all‐cause mortality, had much worse relative survival and higher excess mortality than men. This was most evident for patients with STEMI. Whilst the magnitude of the association was reduced when comorbidities were considered; however, after additional adjustment for the use of evidence‐based treatments, the differences in excess mortality between women and men were further reduced for STEMI and eliminated at up to 1 year for NSTEMI. Moreover, sex‐dependent differences in excess mortality increased with increasing age. Taken together, these data suggest that the impact of AMI is greater in women and that better adherence to guideline‐indicated care for women may result in improved survival of AMI and more equitable cardiovascular outcomes between the sexes.
In line with results of previous studies, we found that women were older than men and more likely to have comorbidities but less likely to smoke.5, 6, 22 Despite the international recommendations for equal treatment of women and men presenting with AMI,23 we found that women were less likely to receive reperfusion and revascularization therapies and to be prescribed guideline‐indicated pharmacological therapies at the time of discharge from the hospital. We found that the lower rates of use of evidence‐based medications and invasive procedures could not be fully explained by confounding factors such as age and comorbidities. These differences may partly be explained by a higher incidence of myocardial infarction with nonobstructive coronary arteries in women,24, 25 for which most treatments lack evidence from clinical trials. Still, this may indicate inequalities in medical care provision between sexes and may lead to increased mortality among women with AMI compared with men.
When we studied all‐cause mortality in patients with AMI (rather than relative survival), we found that sex differences were no longer significant for STEMI, whereas women with NSTEMI had a better prognosis than men. These findings agree with most earlier studies4, 5, 6, 7, 8, 9, 10, 11, 12, 26 in which standard survival analyses were applied. Using the relative survival approach, we found that women with STEMI and NSTEMI had higher excess mortality rates compared with their male counterparts. Unlike standard survival, relative survival accounts for differences in background mortality across groups, allowing for the distinction between death due to the index AMI and deaths due to other causes, and avoids the risk of classification errors in cause‐of‐death records.27 Our results and the findings of a recent study by Baart et al13 illustrate that measuring relative survival and excess mortality may have important implications for acute coronary care.
Earlier studies suggested that sex differences following AMI could be due to the higher cardiovascular risk factor profile, older age, later presentation, delayed revascularization, underdiagnosis, and less aggressive and evidence‐based treatment in women compared with men.28, 29, 30, 31, 32, 33 Although these remain plausible, we found that women with STEMI and NSTEMI had excess mortality compared with men despite adjustment for their demographics and cardiovascular profile. It was only after we further adjusted for treatments used that the differences in excess mortality between the sexes decreased and, for NSTEMI, disappeared. For NSTEMI, the differences in excess mortality were mitigated after adjustment for the use of treatments at up to, but not beyond 1 year following hospitalization. It is possible that such higher risk cases of AMI require more intensive and persistent or novel therapeutic interventions to achieve ongoing equipoise in outcomes between the sexes.34, 35
We also found that excess mortality increased with increasing age. This was evident both for STEMI and NSTEMI in women >85 years, with >2‐fold higher excess mortality than their younger counterparts. Similarly, men with STEMI and NSTEMI who were aged >85 years had 2‐fold higher excess mortality compared with men <55 years. Age was significantly associated with excess mortality even after adjusting for sex, comorbidities, and treatments. However, care must be taken in interpreting this result because it is possible that other unmeasured comorbidities were present in the older adults that may have biased the estimates.36, 37
Our findings have important implications regarding care and outcomes in women, particularly as patients with AMI are living much longer.38 We clearly showed that the gap in mortality between the sexes decreased for STEMI and almost disappeared for NSTEMI after adjusting for treatments at discharge. Thus, there may be greater potential to realize the benefits of healthcare quality improvement efforts in women than in men.
Although this study has strengths, including the size, the high quality, and the completeness of the data set, there are also limitations. Expected survival probabilities were calculated from general population life tables that are stratified only by age, sex, and calendar year. The concept of relative survival relies on comparing the survival probabilities of a diseased population (eg, patients with AMI) to the survival probabilities of the general population of the same demographic structure. However, we were not able to match for other factors influencing outcome, such as comorbidities, socioeconomical status, and ethnicity. Consequently, the excess mortality may be related not only to AMI but also to other differences between the diseased and the general population. Such differences may be more pronounced in older adults.39 We did not correct for the prevalence of AMI in the general population, and this may have overinflated survival estimates in our AMI population group.20, 40 However, because the prevalence of AMI in the Swedish general population is small, adjustment to address this issue would most likely have a small impact. Although extensive adjustments have been made, our study still lacks data regarding some important patient characteristics, such as coronary anatomy, left ventricular ejection fraction, and signs of hemodynamic instability and subsequent outpatient follow‐up data, which may have shed more light on the reasons for the differences in outcome. Another limitation is that data were available only at discharge. It is important to investigate the level of adherence to evidence‐based treatments after AMI discharge and its impact on long‐term survival.
Conclusion
Women with AMI in Sweden had lower relative survival and higher excess mortality compared with men, which persisted from date of hospitalization and increased with age. Excess mortality among women reduced only after adjustment for the use of guideline‐indicated treatments. This finding suggests that improved adherence to guideline recommendations for the treatment of AMI may reduce premature cardiovascular death among women.
Author Contributions
Alabas performed the data management and all analyses and wrote the article. Jernberg and Gale conceived the study. All authors contributed to critical revision of the article and approved the final version. Ethical approval: 2012/60‐31/2 Data Sharing: No additional data are available. The lead author (Jernberg) affirms that the article is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Sources of Funding
This study was supported by grants from the Swedish Heart and Lung Foundation and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institute. Hall was funded by the British Heart Foundation (Project Grant PG/13/81/30474).
Disclosures
None.
Supporting information
Table S1. Years of Diagnosis and Years of Follow‐up
Table S2. Baseline and Clinical Characteristics for the 2003–2013 Acute Myocardial Infarction Cohort With Missing Levels, Stratified by Clinical Presentation
Table S3. Baseline Characteristics Stratified by Sex and Age Group for ST‐Segment–Elevation Myocardial Infarction
Table S4. Baseline Characteristics Stratified by Sex and Age Group for Non–ST‐Segment–Elevation Myocardial Infarction
Table S5. Unadjusted and Adjusted Odd Ratios With 95% Confidence Intervals for ST‐Segment–Elevation Myocardial Infarction and Non–ST‐Segment–Elevation Myocardial Infarction Women Who Received Treatment at Discharge Compared With Men
(J Am Heart Assoc. 2017;6:e007123 DOI: 10.1161/JAHA.117.007123.)29242184
References
- 1. Mnatzaganian G, Braitberg G, Hiller JE, Kuhn L, Chapman R. Sex differences in in‐hospital mortality following a first acute myocardial infarction: symptomatology, delayed presentation, and hospital setting. BMC Cardiovasc Disord. 2016;16:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. Time trends in STEMI—improved treatment and outcome but still a gender gap: a prospective observational cohort study from the SWEDEHEART register. BMJ Open. 2012;2:e000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bavishi C, Bangalore S, Patel D, Chatterjee S, Trivedi V, Tamis‐Holland JE. Short and long‐term mortality in women and men undergoing primary angioplasty: a comprehensive meta‐analysis. Int J Cardiol. 2015;198:123–130. [DOI] [PubMed] [Google Scholar]
- 4. Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam CS, McEntegart M, Claggett B, Liu J, Skali H, Lewis E, Kober L, Rouleau J, Velazquez E, Califf R, McMurray JJ, Pfeffer M, Solomon S. Sex differences in clinical characteristics and outcomes after myocardial infarction: insights from the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail. 2015;17:301–312. [DOI] [PubMed] [Google Scholar]
- 6. Perl L, Bental T, Assali A, Vaknin‐Assa H, Lev E, Kornowski R, Porter A. Impact of female sex on long‐term acute coronary syndrome outcomes. Coron Artery Dis. 2015;26:11–16. [DOI] [PubMed] [Google Scholar]
- 7. Johnston N, Jonelid B, Christersson C, Kero T, Renlund H, Schenck‐Gustafsson K, Lagerqvist B. Effect of gender on patients with ST‐elevation and non‐ST‐elevation myocardial infarction without obstructive coronary artery disease. Am J Cardiol. 2015;115:1661–1666. [DOI] [PubMed] [Google Scholar]
- 8. Redfors B, Angeras O, Ramunddal T, Petursson P, Haraldsson I, Dworeck C, Odenstedt J, Ioaness D, Ravn‐Fischer A, Wellin P, Sjoland H, Tokgozoglu L, Tygesen H, Frick E, Roupe R, Albertsson P, Omerovic E. Trends in gender differences in cardiac care and outcome after acute myocardial infarction in Western Sweden: a report from the Swedish web system for enhancement of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). J Am Heart Assoc. 2015;4:e001995 DOI: 10.1161/JAHA.115.001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J. 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 10. Brown TM, Deng L, Becker DJ, Bittner V, Levitan EB, Rosenson RS, Safford MM, Muntner P. Trends in mortality and recurrent coronary heart disease events after an acute myocardial infarction among Medicare beneficiaries, 2001–2009. Am Heart J. 2015;170:249–255. [DOI] [PubMed] [Google Scholar]
- 11. Alfredsson J, Stenestrand U, Wallentin L, Swahn E. Gender differences in management and outcome in non‐ST‐elevation acute coronary syndrome. Heart. 2007;93:1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. A gender perspective on short‐ and long term mortality in ST‐elevation myocardial infarction—a report from the SWEDEHEART register. Int J Cardiol. 2013;168:1041–1047. [DOI] [PubMed] [Google Scholar]
- 13. Baart SJ, van Domburg RT, Janssen‐Heijnen MLG, Deckers JW, Akkerhuis KM, Daemen J, van Geuns RJ, Boersma E, Kardys I. Impact of relative conditional survival estimates on patient prognosis after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2017;10:e003344. [DOI] [PubMed] [Google Scholar]
- 14. Alabas OA, Hall M, Dondo TB, Rutherford MJ, Timmis AD, Batin PD, Deanfield JE, Hemingway H, Gale CP. Long‐term excess mortality associated with diabetes following acute myocardial infarction: a population‐based cohort study. J Epidemiol Community Health. 2017;71:25–32. [DOI] [PubMed] [Google Scholar]
- 15. Alabas OA, Brogan RA, Hall M, Almudarra S, Rutherford MJ, Dondo TB, Feltbower R, Curzen N, de Belder M, Ludman P, Gale CP. Determinants of excess mortality following unprotected left main stem percutaneous coronary intervention. Heart. 2016;102:1287–1295. [DOI] [PubMed] [Google Scholar]
- 16. Hall M, Alabas OA, Dondo TB, Jernberg T, Gale CP. Use of relative survival to evaluate non‐ST‐elevation myocardial infarction quality of care and clinical outcomes. Eur Heart J Qual Care Clin Outcomes. 2015;1:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 19. Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–290. [Google Scholar]
- 20. Nelson CP, Lambert PC, Squire IB, Jones DR. Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med. 2007;26:5486–5498. [DOI] [PubMed] [Google Scholar]
- 21. Eloranta S, Lambert PC, Andersson TM, Czene K, Hall P, Bjorkholm M, Dickman PW. Partitioning of excess mortality in population‐based cancer patient survival studies using flexible parametric survival models. BMC Med Res Methodol. 2012;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Worrall‐Carter L, McEvedy S, Wilson A, Rahman MA. Gender differences in presentation, coronary intervention, and outcomes of 28,985 acute coronary syndrome patients in Victoria, Australia. Women's Health Issues. 2016;26:14–20. [DOI] [PubMed] [Google Scholar]
- 23. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the task force for the management of Acute Coronary Syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 24. Baron T, Hambraeus K, Sundstrom J, Erlinge D, Jernberg T, Lindahl B. Impact on long‐term mortality of presence of obstructive coronary artery disease and classification of myocardial infarction. Am J Med. 2016;129:398–406. [DOI] [PubMed] [Google Scholar]
- 25. Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation. 2017;135:1490–1493. [DOI] [PubMed] [Google Scholar]
- 26. Bell DM, Nappi J. Myocardial infarction in women: a critical appraisal of gender differences in outcomes. Pharmacotherapy. 2000;20:1034–1044. [DOI] [PubMed] [Google Scholar]
- 27. Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. [DOI] [PubMed] [Google Scholar]
- 28. Diercks DB, Owen KP, Kontos MC, Blomkalns A, Chen AY, Miller C, Wiviott S, Peterson ED. Gender differences in time to presentation for myocardial infarction before and after a national women's cardiovascular awareness campaign: a temporal analysis from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress ADverse Outcomes with Early Implementation (CRUSADE) and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network‐Get with the Guidelines (NCDR ACTION Registry‐GWTG). Am Heart J. 2010;160:80–87.e3. [DOI] [PubMed] [Google Scholar]
- 29. Kaul P, Armstrong PW, Sookram S, Leung BK, Brass N, Welsh RC. Temporal trends in patient and treatment delay among men and women presenting with ST‐elevation myocardial infarction. Am Heart J. 2011;161:91–97. [DOI] [PubMed] [Google Scholar]
- 30. Kang SH, Suh JW, Yoon CH, Cho MC, Kim YJ, Chae SC, Yoon JH, Gwon HC, Han KR, Kim JH, Ahn YK, Jeong MH, Kim HS, Choi DJ. Sex differences in management and mortality of patients with ST‐elevation myocardial infarction (from the Korean Acute Myocardial Infarction National Registry). Am J Cardiol. 2012;109:787–793. [DOI] [PubMed] [Google Scholar]
- 31. Corrada E, Ferrante G, Mazzali C, Barbieri P, Merlino L, Merlini P, Presbitero P. Eleven‐year trends in gender differences of treatments and mortality in ST‐elevation acute myocardial infarction in northern Italy, 2000 to 2010. Am J Cardiol. 2014;114:336–341. [DOI] [PubMed] [Google Scholar]
- 32. Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long‐term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, Walker S, Collinson PO, Apple FS, Gray AJ, Fox KA, Newby DE, Mills NL. High sensitivity cardiac troponin and the under‐diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wallentin L, Lindhagen L, Arnstrom E, Husted S, Janzon M, Johnsen SP, Kontny F, Kempf T, Levin LA, Lindahl B, Stridsberg M, Stahle E, Venge P, Wollert KC, Swahn E, Lagerqvist B. Early invasive versus non‐invasive treatment in patients with non‐ST‐elevation acute coronary syndrome (FRISC‐II): 15 year follow‐up of a prospective, randomised, multicentre study. Lancet. 2016;388:1903–1911. [DOI] [PubMed] [Google Scholar]
- 35. Donataccio MP, Puymirat E, Parapid B, Steg PG, Eltchaninoff H, Weber S, Ferrari E, Vilarem D, Charpentier S, Manzo‐Silberman S, Ferrieres J, Danchin N, Simon T. In‐hospital outcomes and long‐term mortality according to sex and management strategy in acute myocardial infarction. Insights from the French ST‐elevation and non‐ST‐elevation Myocardial Infarction (FAST‐MI) 2005 Registry. Int J Cardiol. 2015;201:265–270. [DOI] [PubMed] [Google Scholar]
- 36. Rashid M, Kwok CS, Gale CP, Doherty P, Olier I, Sperrin M, Kontopantelis E, Peat G, Mamas MA. Impact of co‐morbid burden on mortality in patients with coronary heart disease, heart failure and cerebrovascular acciden: a systematic review and meta‐analysis. Eur Heart J Qual Care Clin Outcomes. 2017;3:20–36. [DOI] [PubMed] [Google Scholar]
- 37. Simon T, Mary‐Krause M, Cambou JP, Hanania G, Gueret P, Lablanche JM, Blanchard D, Genes N, Danchin N. Impact of age and gender on in‐hospital and late mortality after acute myocardial infarction: increased early risk in younger women: results from the French nation‐wide USIC registries. Eur Heart J. 2006;27:1282–1288. [DOI] [PubMed] [Google Scholar]
- 38. Alabas OA, Allan V, McLenachan JM, Feltbower R, Gale CP. Age‐dependent improvements in survival after hospitalisation with acute myocardial infarction: an analysis of the Myocardial Ischemia National Audit Project (MINAP). Age Ageing. 2014;43:779–785. [DOI] [PubMed] [Google Scholar]
- 39. Talback M, Dickman PW. Estimating expected survival probabilities for relative survival analysis—exploring the impact of including cancer patient mortality in the calculations. Eur J Cancer. 2011;47:2626–2632. [DOI] [PubMed] [Google Scholar]
- 40. Graffeo N, Jooste V, Giorgi R. The impact of additional life‐table variables on excess mortality estimates. Stat Med. 2012;31:4219–4230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Years of Diagnosis and Years of Follow‐up
Table S2. Baseline and Clinical Characteristics for the 2003–2013 Acute Myocardial Infarction Cohort With Missing Levels, Stratified by Clinical Presentation
Table S3. Baseline Characteristics Stratified by Sex and Age Group for ST‐Segment–Elevation Myocardial Infarction
Table S4. Baseline Characteristics Stratified by Sex and Age Group for Non–ST‐Segment–Elevation Myocardial Infarction
Table S5. Unadjusted and Adjusted Odd Ratios With 95% Confidence Intervals for ST‐Segment–Elevation Myocardial Infarction and Non–ST‐Segment–Elevation Myocardial Infarction Women Who Received Treatment at Discharge Compared With Men


