Abstract
Background
The matrine‐type alkaloids are bioactive components extracted from Sophora flavescens, which is used in treatment of diabetes mellitus in traditional Chinese medicine. Advanced glycation end products mediate diabetic vascular complications. This study was aimed to investigate the protective effects and molecular mechanisms of matrine‐type alkaloids on advanced glycation end products–induced reactive oxygen species–mediated endothelial apoptosis.
Methods and Results
Rats aorta and cultured rat aortic endothelial cells were exposed to advanced glycation end products. Matrine‐type alkaloids, p38 mitogen‐activated protein kinase (MAPK) inhibitor, and small interference RNAs against p38 MAPK kinases MAPK kinase kinase (MKK)3 and MKK6 were administrated. Intracellular reactive oxygen species production, cell apoptosis, phosphorylation of MKKs/p38 MAPK, and expression levels of heme oxygenase/NADPH quinone oxidoreductase were assessed. The nuclear factor erythroid 2‐related factor 2 nuclear translocation and the binding activity of nuclear factor erythroid 2‐related factor 2 with antioxidant response element were also evaluated. Matrine‐type alkaloids suppressed intracellular reactive oxygen species production and inhibited endothelial cell apoptosis in vivo and in vitro by recovering phosphorylation of MKK3/6 and p38 MAPK, nuclear factor erythroid 2‐related factor 2 nuclear translocation, and antioxidant response element binding activity, as well as the expression levels of heme oxygenase/NADPH quinone oxidoreductase. p38 MAPK inhibitor treatment impaired the effects of matrine‐type alkaloids in vivo and in vitro. MKK3/6 silencing impaired the effects of matrine‐type alkaloids in vitro.
Conclusions
Matrine‐type alkaloids exert endothelial protective effects against advanced glycation end products induced reactive oxygen species–mediated apoptosis by targeting MKK3/6 and enhancing their phosphorylation.
Keywords: apoptosis, diabetes mellitus, endothelial cell, matrine‐type alkaloids, reactive oxygen species
Subject Categories: Basic Science Research, Cell Signalling/Signal Transduction, Oxidant Stress, Vascular Biology
Clinical Perspective
What Is New?
The intracellular antioxidant system in the arterial endothelium is impaired by advanced glycation end products exposure.
The deactivation of mitogen‐activated protein kinase kinases/p38 mitogen‐activated protein kinase/erythroid 2‐related factor 2 signaling pathway is involved in imbalance of antioxidant/oxidant leverage.
Matrine‐type alkaloids exert endothelial protective effects against advanced glycation end products–induced oxidative stress‐mediated apoptosis by targeting mitogen‐activated protein kinase kinase 3/6 and enhancing their phosphorylation.
What Are the Clinical Implications
Matrine‐type alkaloids and related compounds have the potential clinical application value in treating diabetic vascular complications.
Restoring the phosphorylation of mitogen‐activated protein kinase kinases could be a novel strategy for enhancing intracellular antioxidant capacity, which protects against oxidative stress–associated cell injury.
Introduction
Atherosclerosis is believed to be one of the fundamental pathological manifestations of diabetic vascular complications.1 Evidence from previous clinical trials indicated the role of hyperglycemia in the pathogenesis of diabetic vascular complications.2 Under circumstances of sustained hyperglycemia, glucose participates in nonenzymatic glycation reactions with lipids, nucleic acids, and amino groups of proteins, generating Schiff bases and Amadori products. As a result, high‐molecular‐weight protein and fluorescent entities are produced. These products are referred to as advanced glycation end products (AGEs).3 Studies pointed out that AGEs were closely correlated with the occurrence, progression, and development of diabetic vascular complications.4
Investigations have suggested that AGEs were responsible for induction and maintenance of oxidative stress.5 After interacting with their receptors, AGEs trigger activation of multiple signaling pathways to induce generation of reactive oxygen species (ROS), including hydroxy radical (OH˙), superoxide anion (), and peroxyl radical (RO˙). ROS participates in many intracellular pathological changes.6, 7 In accord with our and other previous studies, ROS triggers programmed cell death through the mitochondrial pathway, death receptor pathway, and endoplasmic reticulum stress‐mediated pathway.8, 9, 10 Previous studies indicated that AGEs induced endothelial cell apoptosis by exacerbating ROS‐mediated apoptosis.11
Activation of antioxidant response element (ARE) is believed to be a modulator of the intracellular antioxidant defense system, which protects against ROS‐mediated cell death. The nuclear factor erythroid 2‐related factor 2 (Nrf2) signaling pathway triggers the activation of ARE. By initiating transcriptions of a set of antioxidant enzymes genes, Nrf2 exerts its crucial role in enhancing cellular antioxidant defense system. Kelch‐like ECH‐associated protein 1 (Keap1) binds to Nrf2 to maintain static status of Nrf2.12 When encountering harmful stimuli, Nrf2 dissociates from Keap1 and further translocates to the nucleus to trigger the transcriptions of antioxidant enzymes genes by binding with ARE, which locates in the promoter region of these genes.13 The activation of Nrf2/ARE signaling is regulated by the upstream kinases. Theses kinases are referred to as mitogen‐activated protein kinases (MAPKs).14 As a member of the MAPKs family, p38 MAPK is a key signaling transducer in response to harmful stimuli, including ROS.15 The activation of p38MAPK is regulated by its upstream kinases, MAPK kinase kinase (MKK)3 and MKK6.16
Matrine, oxymatrine, and sophocarpine are typical matrine‐type alkaloids, which are believed to be the main bioactive components extracted from Chinese medical herb Sophora flavescens 17 which was used in treatment of diabetes mellitus from ancient times. These alkaloids have been found to show a variety of pharmacological activities, including anti‐inflammatory, ‐viral, ‐fibrotic, and ‐cancer properties. According to our and others’ previous studies, the antioxidative and cardioprotective activities of matrine were featured.18, 19, 20 Several previous studies indicated that matrine regulated some protein kinases, such as AMP‐activated protein kinase and Janus‐activated kinase.21, 22 The pharmacological activities of matrine‐type alkaloids may be similar because they share several of the same key molecular structures.23 Thus, it is reasonable for us to speculate that the matrine‐type alkaloids may suppress AGEs‐induced oxidative stress by affecting MKKs, which further regulates p38 MAPK/Nrf2 signaling. In the present study, both in vivo and in vitro investigations were implemented. The involvement of MKKs/p38 MAPK/Nrf2 signaling in matrine‐type alkaloids’ effects of attenuating AGEs induced oxidative stress–mediated endothelial cell apoptosis was investigated.
Materials and Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Preparation of AGEs‐BSA
The preparation procedure was carried out in accord with previous studies.24 Briefly, under sterile conditions, BSA (Hyclone Laboratories Inc, South Logan, UT) was incubated with glyceraldehyde (0.1 mmol/L; Sigma‐Aldrich, St. Louis, MO) in NaPO4 buffer (0.2 mmol/L; pH=7.4) at 37°C for 7 days. Then, by using chromatography with PD‐10 desalting columns (GE Healthcare, Little Chalfont, UK) and dialysis against PBS, the unicorporated sugars were removed. Meanwhile, control nonglycated BSA was prepared by the same procedure without glyceraldehyde.
Animals, Grouping, and Treatment
Seven‐week‐old male Sprague–Dawley rats (weighting 180–220 g) were provided by the Experimental Center of Xi'an Jiaotong University (Xi'an, China). The experimental procedures concerning animal studies were carried out according to the Recommended Guideline for the Care and Use of Laboratory Animals issued by Chinese Council on Animal Research. The experimental protocol in this study was approved by the Ethics Committee of Xi'an Jiaotong University.
For AGEs exposure, rats received an intraperitoneal injection of 1 mg of prepared AGEs‐BSA daily for 10 consecutive days. Meanwhile, rats were treated with intraperitoneal injections of matrine‐type alkaloids (matrine, oxymatrine, and sophocarpine), respectively. Dosage for matrine (Sigma‐Aldrich) was 200 mg/kg/day,18 for oxymatrine (Sigma‐Aldrich) 120 mg/kg/day,25 and for sophocarpine (Invitrogen, Carlsbad, CA) 40 mg/kg/day for 10 consecutive days.26 Several rats also received treatment of specific inhibitor of p38 MAPK SB203580 (1 mg/kg/day; Selleck Chemicals, Houston, TX) simultaneously.27 For control animals, they received treatment of physiological saline with equal volume to agents. According to different treatment, an equal amount of rats (8 rats for each group) were assigned to control (control animals), AGEs (animals exposed to AGEs), AGEs+Mat (AGEs‐exposed animals treated with matrine), AGEs+Oxy (AGEs‐exposed animals treated with oxymatrine), AGEs+Soh (AGEs‐exposed animals treated with sophocarpine), AGEs+Mat+SB (AGEs‐exposed animals cotreated with matrine and SB203580), AGEs+Oxy+SB (AGEs‐exposed animals cotreated with oxymatrine and SB203580), and AGEs+Soh+SB (AGEs‐exposed animals cotreated with sophocarpine and SB203580). Animals were euthanitized by carbon dioxide asphyxia before samples were harvested.
Cell Culture, Grouping, and Treatment
Rat aortic endothelial cells (RAECs) were isolated and cultured in accord with the method described previously.28 RAECs were extracted from aorta segments. Isolated RAECs were cultured in endothelial‐basal medium (EBM‐2; Lonza, Basel, Switzerland) supplemented with FBS (5%; Hyclone) and an antibiotics mix (Invitrogen) in an incubator providing a humidified environment, a constant temperature at 37°C, and an atmosphere with 5%CO2/95% fresh air. AGEs, SB203580 (final concentration, 10 μmol/L), and matrine‐type alkaloids were used to incubate the cells, respectively. Several cells were also transfected with small interfering RNA (siRNAs). Specifically, the concentrations of matrine were 0.25, 0.5, 1.0, 2.0, and 2.5 mmol/L18; the concentrations of oxymatrine were 0.0625, 0.125, 0.25, 0.5, 1.0, and 2.0 mmol/L29; the concentrations of sophocarpine were 0.0625, 0.125, 0.25, 0.5, 1.0, and 2.0 mmol/L.30
siRNA Transfection
In this study, MKK3 and MKK6 were silenced in RAECs by using an siRNA transfection technique. SignalSilence MKK3 siRNA (Cat.6294S; Cell Signaling Technology, Danvers, MA) was used to knock down mkk3. The specific sequence of siRNA against mkk6 was: 3′‐CUACAGUAGUGAAGAGAUUTT‐3′, which was designed and synthesized by GenePharma Co, Ltd (Shanghai, China). SignalSilence Control siRNA (Cat.6568S; Cell Signaling Technology) was used as control. Cultured RAECs (at 70% confluence) were transfected with the above siRNAs with Mirus TransIT‐TKO reagent (Mirus Bio LLC, Madison, WI) for 48 hours per the manufacturer's instructions.
Cell Viability Assessment
The noncytotoxic concentrations of matrine‐type alkaloids were evaluated by MTT assay. Cultured RAECs were seeded in a 96‐well cell‐culture plate and then cultured with matrine, oxymatrine, and sophocarpine at serial diluted concentrations for 48 hours. MTT (5 mg/mL; Sigma‐Aldrich) was added into the wells to incubate RAECs for 4 hours. Then, DMSO was added to dissolve the formazan crystals. A plate reader was used to determine the absorbance at 490 nm (A490). Cell viability was calculated as (A490 of alkaloids‐treated cell/A490 of DMSO‐treated control cells)×100%.
ARE‐Luciferase Reporter Construct and Transfection
The ARE‐luciferase reporter was constructed according to the description of previous studies13 and accomplished by GenePharma. The pTI‐ARE‐luciferase contains a single copy of ARE of 41‐base‐pair length (5′‐TAGCTTGGAAATGACATTGCTAATGGTGACAAAGCAACTTT‐3′) and a minimal TATA‐Inr promoter. Plasmid was transfected into RAECs with HiPerFect Transfection Reagent (Qiagen, Hilden, Germany) per the manufacturer's instructions. After treatment of transfection reagent, cells were cultured in replaced fresh medium before further experiments.
ARE‐Luciferase Activity Assay
The ARE‐luciferase activity assay was performed in accord with descriptions in previous studies.13 Briefly, after being washed by PBS, RAECs were lysed by reporter lysis buffer (Promega, Fitchburg, WI). The resulting supernatant was used for ludiferase activity assay with a GloMax luminometer (Promega) after centrifugation at 3000g for 10 minutes. Luciferase activity was expressed as folds over control cells.
In Situ ROS Detection
In situ ROS generation detection was performed by using DHE (Beyotime Biotech, Nantong, China) staining. Fresh thoracic aorta was harvested and immersed in optimal cutting temperature compound (Tissue‐Tek) and frozen on dry ice. Tissue was cut into 10‐μm‐thick sections and placed on polylysine‐coated slides. Slides were then incubated with DHE (10 μmol/L) in a humidified dark chamber at 37°C for 45 minutes according to the recommendations provided by the manufacturer. For cultured RAECs, intracellular ROS production was detected by using DCFH‐DA (Molecular Probes, Eugene, OR) staining. After PBS washing, RAECs were incubated with DCFH‐DA at 37°C for 30 minutes in a humidified dark chamber according to the instruction provided by the manufacturer. Fluorescent images were captured by an inverted fluorescence microscope. Fluorescence intensities were analyzed by the software Image‐Pro Plus (ver.5.0).
Total Antioxidant Capacity Assessments
Aorta samples and cultured RAECs were collected and the homogenates were prepared. After centrifugation at 4°C at 12 000g for 5 minutes, the supernatant was used for total antioxidant capacity (TAC) detection. The TAC was measured by using a T‐AOC Assay Kit (Beyotime) according to the manual provided by the manufacturer. A plate reader was used to determine the absorbance of ABTS+ at 734 nm.
Apoptosis Determination
In situ apoptosis was determined by terminal transferase UTP nick end labeling assay. Paraffin‐embedded thoracic aorta tissue was cut into 5‐μm‐thick sections. After deparaffinization, sections were treated with proteinase K (20 μmol/L; Sigma‐Aldrich). Then, terminal transferase UTP nick end labeling assay was carried out using a terminal transferase UTP nick end labeling assay kit (Roche, Indianapolis, IN) per the manufacturer's instructions. Fluorescent images were captured by an inverted fluorescence microscope. Fluorescence intensities were analyzed by the software Image‐Pro Plus (ver.5.0). For cultured RAECs, apoptosis was evaluated by Annexin V/FITC and propidium iodide double staining with flow cytometry. Briefly, cells were resuspended in PBS. A 195‐μL cell suspension (5000/mL) was mixed with 5 μL of Annexin V/FITC (BD Biosciences, San Jose, CA) at room temperature for 10 minutes. Then, cells were washed by PBS and resuspended in 190 μL of deliquated binding buffer. Five microliters of propidium iodide (BD Biosciences, San Jose, CA) was used to incubate RAECs in binding buffer for 15 minutes in a dark chamber. Then, a flow cytometer (FACS Calibur; BD Biosciences, San Jose, CA) was used to analyze apoptosis using CellQuest software.
Nrf2 Nuclear Translocation Evaluation
Nuclear translocation of Nrf2 was assessed by western blotting in vivo and by both western blotting/immunofluorescent staining in vitro. By using a cell lysis buffer system (Santa Cruz Biotechnology), the whole‐cell extract from cultured RAECs and harvested thoracic aorta tissue was prepared. Cytoplasmic protein and nuclear protein were extracted with Cytoplasmic Extraction Reagents (Pierce Biotechnology Inc, Rockford, IL) and Nuclear Extraction Reagents (Pierce), respectively. Protein concentrations were determined by a BCA protein assay kit (Pierce). The protocol of western blotting is described in the below paragraph. Cultured RAECs were fixed and incubated with primary antibody against Nrf2 (1:250; Cell Signaling Technology) at 4°C for 8 hours. Then, the secondary antibody conjugated with Alexa 488 (Invitrogen) and DAPI was used to incubate the cells. Quenching of fluorescence was alleviated with SlowFade Lignt Antifade kit (Molecular Probes). Samples were exited at 519 nm and observed at 442 and 495 nm with an inverse fluorescence microscope (Axio Imager 2; Carl Zeiss, Jena, Germany). Fluorescent intensity was determined by using Zeiss Physiology software (Ver.3.2; Carl Zeiss).
Western Blotting
A 20‐μg protein sample was loaded and separated by vertical SDS‐PAGE. Then, proteins were transferred to PVDF membranes electrically. Specific antibodies against Nrf2 (1:500Cell Signaling Technology), MKK3 (1:500; Sigma‐Aldrich), phospho‐MKK3 (1:500; Sigma‐Aldrich), MKK6 (1:500; Sigma‐Aldrich), phospho‐MKK6 (1:500; Sigma‐Aldrich), p38 (1:250; Cell Signaling Technology), phospho‐p38 (1:250; Cell Signaling Technology), heme oxygenase (HO1; 1:1000; Cell Signaling Technology), NADPH quinone oxidoreductase (NQO1; 1:500; Cell Signaling Technology), histone H3 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), and GAPDH (1:1000; Invitrogen) were used to incubate membranes at 4°C for 10 hours. Then, membranes were washed by Tris‐buffered saline‐Tween 20 (0.02%). Corresponding second antibodies conjugated to HRP (Santa Cruz Biotechnology) were used to incubate membranes at room temperature for 2 hours. Membranes were developed with Super Signal West Pico chemiluminescence reagent and visualized. Intensities of immunoblots were quantified and analyzed by the software Image‐Pro Plus (ver.5.0).
Statistical Analysis
Data acquired in this study are presented in a mean±SD manner. One‐way ANOVA and the Student t test were used for analyze the differences between values. The Bonferroni adjustments were performed as post hoc tests. The data analysis was performed by using SPSS software (ver. 16.0; SPSS, Inc, Chicago, IL). P<0.05 was considered statistically significant.
Results
Matrine, oxymatrine, and sophocarpine administration alleviated AGEs‐induced ROS generation in rat aorta.
The ROS produced in rat aorta was tagged by DHE fluorescent staining. The captured images are demonstrated in Figure 1A. ROS production was significantly upregulated in the AGEs group compared with the control group. However, after being treated with matrine (200 mg/kg/day), oxymatrine (120 mg/kg/ day), and sophocarpine (40 mg/kg/day), ROS production in rat aorta was dramatically reduced. Moreover, the p38 MAPK inhibitor administration significantly impaired matrine, oxymatrine, and sophocarpine's inhibition of ROS production in AGEs‐exposed rat aorta. As shown in Figure 1B, the aortic TAC decreased significantly in rats exposed to AGEs, which was dramatically attenuated by matrine, oxymatrine, and sophocarpine administrations. However, the attenuation was significantly impaired by coadministration of p38 MAPK inhibitor SB203580.
Figure 1.
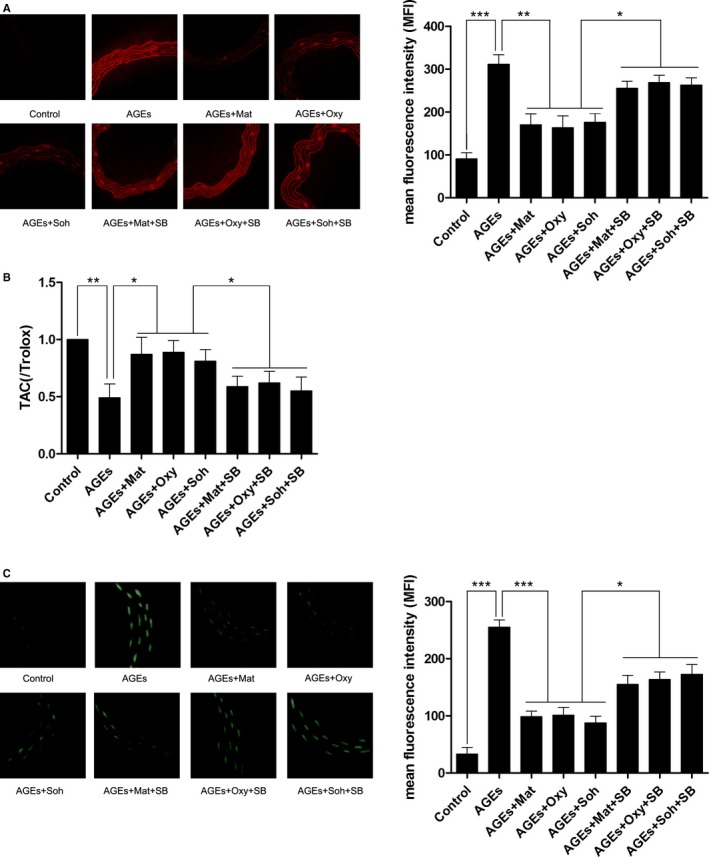
A, The left panel demonstrates the captured fluorescent images of aorta stained with DHE. ROS (reactive oxygen species) was positively stained. Columns on the right panel indicated the mean fluorescence intensity (MFI) of DHE stain in Control, AGEs (advanced glycation end products), AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, and AGEs+Soh+SB, respectively. B, Columns indicate the detected total antioxidant capacity (TAC) of aorta harvested from experimental animals. C, The left panel demonstrates the captured fluorescent images of TUNEL (terminal transferase UTP nick end labeling) assay of aorta. Apoptotic cells were positively stained. Columns on the right panel indicate the MFI of TUNEL staining in Control, AGEs, AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, and AGEs+Soh+SB, respectively (3 independent experiments were performed; *P<0.05; **P<0.01; ***P<0.001).
Matrine, oxymatrine, and sophocarpine administration alleviated AGEs‐induced cell apoptosis in rat aorta, which was reversed by SB203580 treatment.
In vivo cell apoptosis was detected by terminal transferase UTP nick end labeling assay. The captured images are shown in Figure 1C. Compared with control, apoptosis in aorta increased significantly in AGEs‐exposed rats. However, the matrine (200 mg/kg/day), oxymatrine (120 mg/kg/day), and sophocarpine (40 mg/kg/day) administration significantly inhibited aortic cell apoptosis. The inhibitory effect of matrine‐type alkaloids on apoptosis was dramatically impaired by coadministration with p38 MAPK inhibitor SB203580.
Matrine, oxymatrine, and sophocarpine administration recovered phosphorylation of MKK3/6, which facilitated p38 MAPK/Nrf2 antioxidative signaling transduction in AGEs‐treated rat aorta, which was reversed by SB203580 treatment.
Immunoblots and their quantifications are demonstrated in Figure 2. The phosphorylation of MKK3, MKK6, and p38 MAPK was found to be significantly inhibited in AGEs‐exposed aortas compared with control. The administration of matrine (200 mg/kg/day), oxymatrine (120 mg/kg/day), and sophocarpine (40 mg/kg/day) dramatically recovered the phosphorylation of MKK3, MKK6, and p38. The p38 MAPK inhibitor, SB203580, did not affect the phosphorylation levels of MKK3 and MKK6, but suppressed the phosphorylation of p38 MAPK. The nuclear expression of Nrf2 decreased significantly in AGEs‐exposed aorta, indicating that the Nrf2 nuclear translocation was inhibited by AGEs. After matrine‐type alkaloids treatment, however, the nuclear expression of Nrf2 was dramatically increased, indicating that the nuclear translocation of Nrf2 was recovered by matrine‐type alkaloids administration. The expression levels of antioxidant proteins, namely HO1 and NQO1, were increased by administration of matrine‐type alkaloids in aorta exposed to AGEs. The coadministration of SB203508 dramatically impaired the increasing expression of HO1 and NQO1 in AGEs‐exposed aorta treated with matrine‐type alkaloids.
Figure 2.
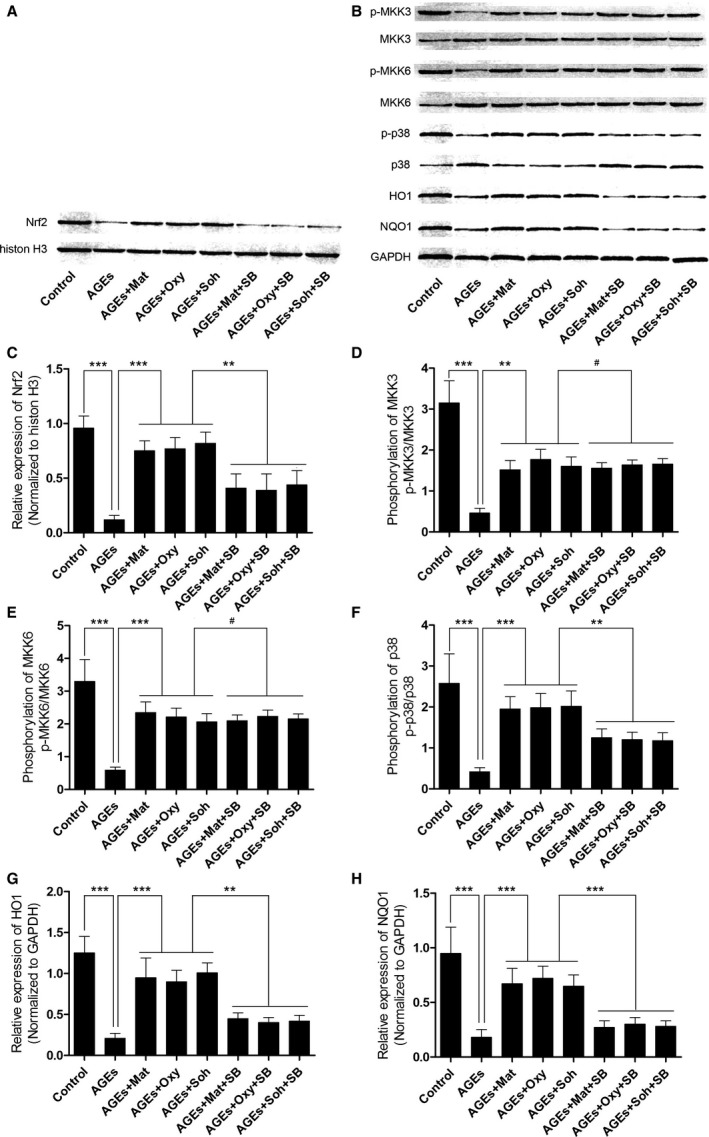
A, The immunoblots of Nrf2 in nuclear protein when histone H3 was used as the internal reference. B, The immunoblots of phosphorylated MKK3 (p‐MKK3), MKK3, p‐MKK6, MKK6, p‐p38, p38, HO1 (heme oxygenase 1), and NQO1 (NADPH quinone oxidoreductase 1) in total protein when GAPDH was introduced as the internal reference. C through H, Columns in these panels indicate the relative expression level of Nrf2 (Nrf2/histon H3), phosphorylation level of MKK3 (p‐MKK3/MKK3), phosporylation level of MKK6 (p‐MKK6/MKK6), phosphorylation level of p38 MAPK, the relative expression levels of HO1 (HO1/GAPDH), and the relative expression level of NQO1 (NQO1/GAPDH), respectively (3 independent experiments were performed; *P<0.05; **P<0.01; ***P<0.001; # P>0.05).
Selection of the Noncytotoxic Concentrations of Matrine, Oxymatrine, and Sophocarpine
The noncytotoxic concentrations of matrine, oxymatrine, and sophocarpin were selected by MTT assay. Matrine began to inhibit RAECs at a concentration of 2.0 mmol/L; both oxymatrine and sophocarpine began to show cytotoxic effects at a concentration of 0.25 mmol/L. Thus, matrine at a concentration of 1.5 mmol/L and oxymatrine and sophocarpin at concentrations of 0.125 mmol/L were selected for subsequent in vitro experiments. These results are demonstrated in Figure 3A.
Figure 3.
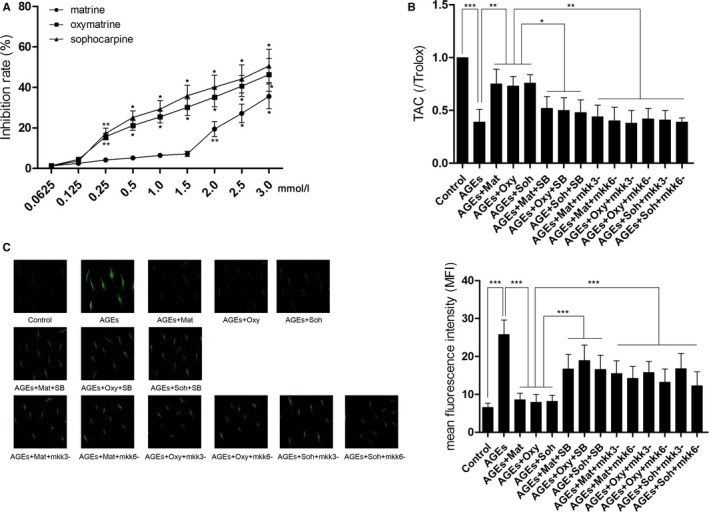
A, Results of MTT assay are indicated by the line chart in this figure. Cultured RAECs (rat aortic endothelial cells) were incubated with matrine, oxymatrine, and sophocarpine at serial diluted concentrations. B, Columns indicate the TAC (total antioxidant capacity) levels in cultured RAECs in Control, AGEs, AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, AGEs+Soh+SB, AGEs+Mat+mkk3−, AGEs+Mat+mkk6−, AGEs+Oxy+mkk3−, AGEs+Oxy+mkk6−, AGEs+Soh+mkk3−, and AGEs+Soh+mkk6−, respectively. C, Left panel demonstrates the captured fluorescent images of cultured RAECs stained with DCFH‐DA. ROS (reactive oxygen species) was positively stained. Columns on the right panel indicate the MFI of DCFH‐DA stain in Control, AGEs, AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, AGEs+Soh+SB, AGEs+Mat+mkk3−, AGEs+Mat+mkk6−, AGEs+Oxy+mkk3−, AGEs+Oxy+mkk6−, AGEs+Soh+mkk3−, and AGEs+Soh+mkk6−, respectively (3 independent experiments were performed; *P<0.05; **P<0.01; ***P<0.001).
Matrine, oxymatrine, and sophocarpine incubation suppressed AGEs‐induced ROS production while strengthening TAC in cultured RAECs, which were impaired by SB203580 treatment and mkk3/6 silencing.
ROS generation was determined by DCFH‐DA staining in cultured RAECs. The captured fluorescent images are demonstrated in Figure 3B. AGEs exposure boosted the generation of ROS in cultured RAECs. The treatment of matrine (1.5 mmol/L), oxymatrine (0.125 mmol/L), and sophocarpine (0.125 mmol/L) significantly suppressed the production of ROS in AGEs‐exposed RAECs. The administration of p38 MAPK inhibitor impaired the antioxidant effects of the matrine‐type alkaloids on AGEs‐exposed RAECs. MKK3 and MKK6 were silenced by specific siRNAs in RAECs, respectively. The results showed that both mkk3 and mkk6 silencing impaired the antioxidant effects of the matrine‐type alkaloids on AGEs‐exposed RAECs. Correspondingly, as shown in Figure 3C, the TAC of AGEs‐exposed RAECs was reduced significantly, which was restored by matrine, oxymatrine, and sophocarpine incubations. However, coadministration with p38 MAPK inhibitor or mkk3/6 silencing decreased TAC in AGEs‐exposed RAECs incubated with matrine, oxymatrine, and sophocarpine.
Matrine, oxymatrine, and sophocarpine incubation suppressed AGEs‐induced cell apoptosis of cultured RAECs, which were impaired by SB203580 treatment and mkk3/6 silencing.
The apoptosis of RAECs was detected by flow cytometry in this study. The charts of flow cytometry are demonstrated in Figure 4. Compared with control, AGEs exposure significantly increased apoptosis of cultured RAECs. The treatment of matrine (1.5 mmol/L), oxymatrine (0.125 mmol/L), and sophocarpine (0.125 mmol/L) dramatically decreased apoptotic percentage of AGEs‐exposed RAECs. Both coadministration of p38 MAPK inhibitor SB203508 and mkk3/6 silencing impaired the matrine‐type alkaloids’ antiapoptotic effects on AGEs‐exposed RAECs.
Figure 4.
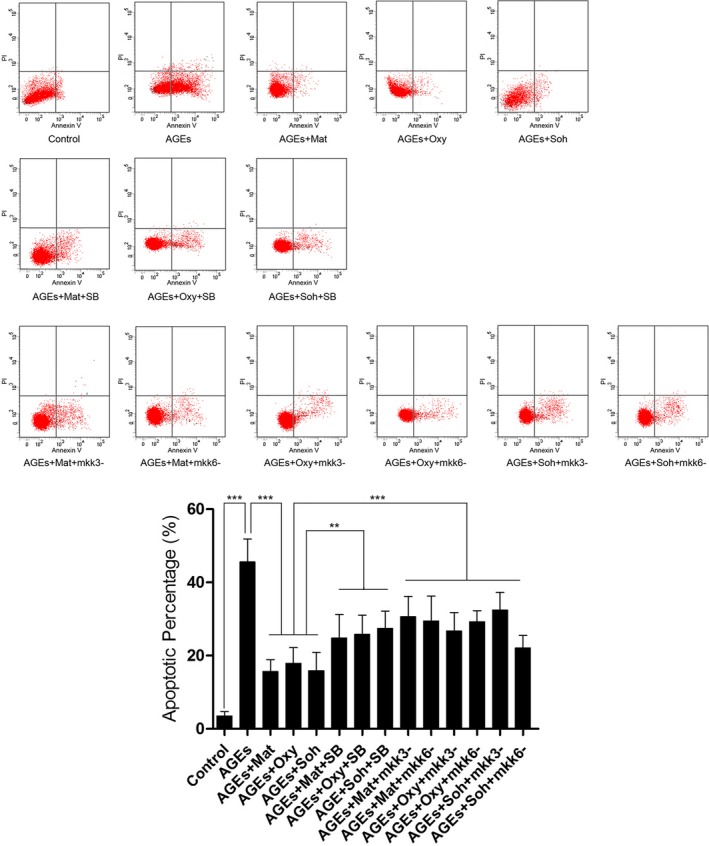
Charts on the upper panel of this figure demonstrate PI/Annexin V double staining detected by a flow cytometer. Columns on the lower panel indicate the apoptotic percentage in Control, AGEs, AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, AGEs+Soh+SB, AGEs+Mat+mkk3−, AGEs+Mat+mkk6−, AGEs+Oxy+mkk3−, AGEs+Oxy+mkk6−, AGEs+Soh+mkk3−, and AGEs+Soh+mkk6−, respectively (3 independent experiments were performed; **P<0.01; ***P<0.001).
Matrine, oxymatrine, and sophocarpine incubation facilitated Nrf2 nuclear translocation and ARE‐binding, which were impaired by SB203580 treatment and mkk3/6 silencing.
Results of Nrf2 nuclear translocation and ARE binding activity are demonstrated in Figure 5. After AGEs exposure, both the Nrf2 nuclear translocation and ARE binding activity were inhibited. Matrine (1.5 mmol/L), oxymatrine (0.125 mmol/L), and sophocarpine (0.125 mmol/L) incubation dramatically restored Nrf2 nuclear translocation and ARE binding activity. However, both coadministration of p38 MAPK inhibitor SB203508 and mkk3/6 silencing impaired the above restoring effects in AGEs‐exposed RAECs.
Figure 5.
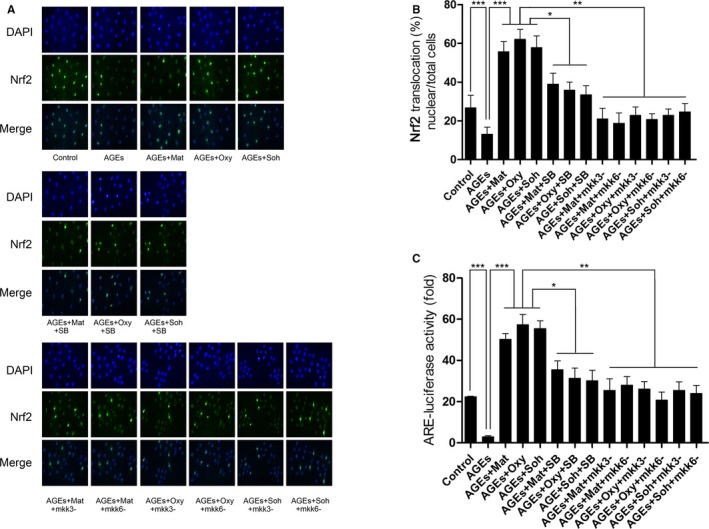
A, This panel demonstrates the captured images of Nrf2 and DAPI fluorescent immunochemistry staining and their merged images in cultured RAECs (rat aortic endothelial cells). B, Columns indicate the ratio of Nrf2 nuclear translocated cell count against total cell count in RAECs in Control, AGEs, AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, AGEs+Soh+SB, AGEs+Mat+mkk3−, AGEs+Mat+mkk6−, AGEs+Oxy+mkk3−, AGEs+Oxy+mkk6−, AGEs+Soh+mkk3−, and AGEs+Soh+mkk6−, respectively. C, Columns indicate the ARE‐luciferase activities in RAECs in Control, AGEs, AGEs+Mat, AGEs+Oxy, AGEs+Soh, AGEs+Mat+SB, AGEs+Oxy+SB, AGEs+Soh+SB, AGEs+Mat+mkk3−, AGEs+Mat+mkk6−, AGEs+Oxy+mkk3−, AGEs+Oxy+mkk6−, AGEs+Soh+mkk3−, and AGEs+Soh+mkk6−, respectively (3 independent experiments were performed; *P<0.05; **P<0.01; ***P<0.001).
Matrine, oxymatrine, and sophocarpine incubation recovered activation of MKK3/6‐p38MAPK/Nrf2 antioxidative signaling in AGEs‐treated RAECs, which were impaired by SB203580 treatment and mkk3/6 silencing.
As shown in Figure 6, the phosphorylation of MKK3, MKK6, and p38 MAPK was found to be significantly inhibited in AGEs‐treated RAECs. Moreover, the expression of Nrf2 in nuclei and expression levels of HO1 and NQO1 were suppressed in AGEs‐treated RAECs. After incubation with matrine (1.5 mmol/L), oxymatrine (0.125 mmol/L), and sophocarpine (0.125 mmol/L), the phosphorylation of MKK3, MKK6, and p38 MAPK were restored. The expression levels of HO1 and NQO1 and nuclear expression of Nrf2 were also recovered. However, the administration of p38 MAPK inhibitor inhibited the restoring effects of matrine‐type alkaloids on p38 MAPK phosphorylation without affecting MKK3/6 phosphorylation. As a result, coadministration with SB203508 decreased nuclear expression of Nrf2 and HO1/NQO1 expressions compared with matrine‐type alkaloids‐incubated AGEs‐exposed RAECs. Treatment of mkk3/6 silencing significantly reduced phosphorylation of MKK3/6, respectively. The mkk3/6 silencing also decreased nuclear expression of Nrf2 and HO1/NQO1 expressions compared with matrine‐type alkaloids‐incubated AGEs‐exposed RAECs.
Figure 6.
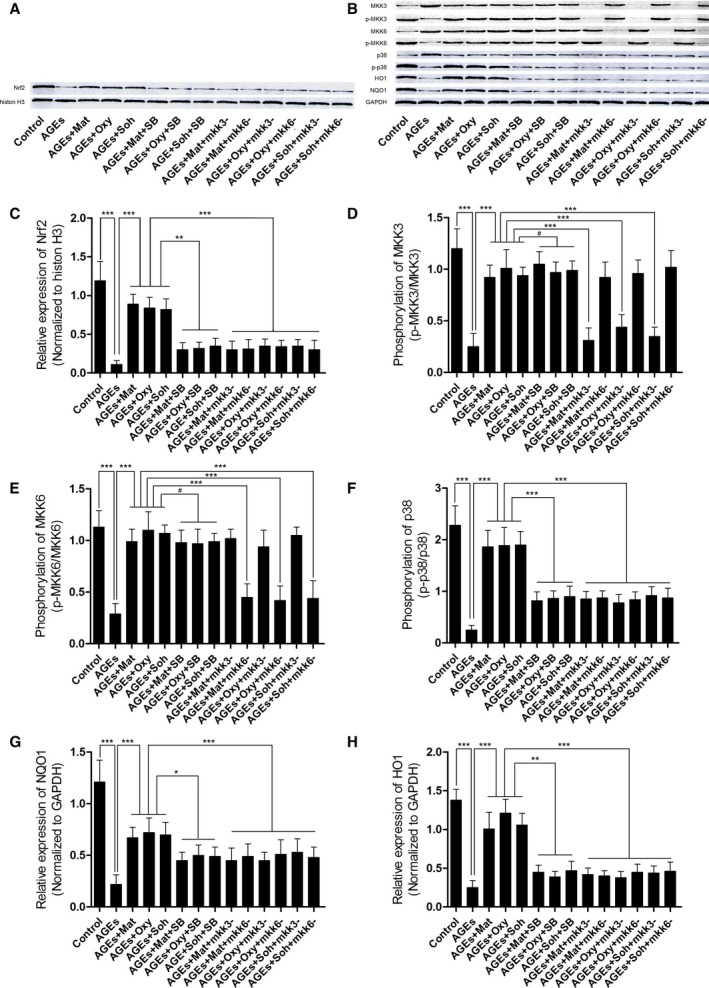
A, The immunoblots of Nrf2 in nuclear protein when histone H3 was used as the internal reference. B, The immunoblots of phosphorylated MKK3 (p‐MKK3), MKK3, p‐MKK6, MKK6, p‐p38, p38, HO1 (heme oxygenase 1), and NQO1 (NADPH quinone oxidoreductase 1) in total protein when GAPDH was introduced as the internal reference. C through H, Columns in these panels indicate the relative expression level of Nrf2 (Nrf2/histone H3), phosphorylation level of MKK3 (p‐MKK3/MKK3), phosporylation level of MKK6 (p‐MKK6/MKK6), phosphorylation level of p38 MAPK, the relative expression levels of HO1 (HO1/GAPDH), and the relative expression level of NQO1 (NQO1/GAPDH), respectively (3 independent experiments were performed; *P<0.05; **P<0.01; ***P<0.001; # P>0.05).
Discussion
AGEs are fostered and further raise oxidative stress to cause cell damages in type 2 diabetes mellitus.31 There is a strong association between apoptosis of endothelial cells and diabetic cardiovascular complications.32 AGEs‐induced oxidative stress plays important roles in the pathogenesis of diabetic cardiovascular complications. It was reported that by coupling with its downstream receptor, receptor for advanced glycation end products, AGEs stimulated ROS production by activating oxidases.11 The AGEs‐induced ROS‐mediated cell apoptosis was linked with AGEs/receptor for advanced glycation end products interaction.33 In this study, rats and cultured RAECs were exposed to AGEs. As a result, the ROS‐mediated apoptosis of RAECs was found to be increased significantly both in vivo and in vitro. Therefore, inhibition of ROS‐mediated RAECs apoptosis would ameliorate vascular injuries caused by AGEs.
A Chinese medical herb named Shan‐Dou‐Gen (S. flavescens) has been used for treatment of diabetes mellitus from ancient times. The effective components were mainly quinolizidine alkaloids, principally matrine‐type alkaloids. Matrine, oxymatrine, and sophocarpine are typical matrine‐type alkaloids extracted from S. flavescens exhibiting similar bioactive effects attributed to their comparable molecular structures. Matrine, oxymatrine, and sophocarpine were known as cardiovascular protective agents and showed potent antioxidant effects.20, 26, 34
The antioxidant system exists and maintains intracellular antioxidant‐oxidant balance. Enhancing the antioxidant system counterweights the cell injuries brought about by excessively produced ROS under certain pathological conditions. As a member of the “cap ‘n’ collar” family of basic leucine zipper transcription factors, Nrf2 induces the transcriptions of antioxidant enzymes by binding with ARE.35 Several genes of antioxidant enzymes were the downstream targets of Nrf2/ARE signaling. HO1, which has the ability of conversing heme into biliverdin, is one of the key phase II enzymes maintaining intracellular redox homeostasis by neutralizing oxidative stress.36 As a detoxifiying enzyme, NQO1 catalyzes the 2‐electron reductive metabolism and detoxification of quinies, protecting cells from oxidative stress–induced injury.37
Indeed, in the current study, the Nrf2 nuclear translocation was found to be inhibited in AGEs‐exposed aorta. After treatment of matrine‐type alkaloids, the nuclear translocation of Nrf2 increased dramatically. Moreover, Nrf2/ARE binding activities were found to be increased by matrine‐type alkaloids treatment. As a result, the expression levels of HO1 and NQO1, as well as TAC, were elevated dramatically in thoracic aorta. These results indicated that matrine‐type alkaloids increased antioxidant capability of aortic endothelium by activating Nrf2/ARE signaling.
The Nrf2/ARE signaling activation was regulated by their upstream kinase, p38 MAPK. In physiological conditions, p38 MAPK is at a static status by binding with its inhibitor, Keap1.14 Various pathological stimuli and intracellular responses activate p38 MAPK by phosphorylation, which facilitates disassociation of Nrf2/Keap1 complex and nuclear translocation of Nrf2.38 In both in vivo and in vitro studies of this investigation, p38 MAPK phosphorylation was found to be impaired in RAECs exposed to AGEs. In the in vitro study, specifically, in RAECs exposed to AGEs, evidenced by ARE‐binding activity assay, the suppression of Nrf2/ARE signaling transduction was found. The matrine‐type alkaloids administration, however, significantly increased the p38 MAPK phosphorylation and Nrf2 nuclear translocation. As a result, the expression levels of antioxidant enzymes HO1 and NQO1, as well as TAC, were upregulated. ROS production and its mediated apoptosis were suppressed in RAECs exposed to AGEs. We also found that the p38 MAPK inhibitor, SB203580, dramatically impaired the protective effects of matrine‐type alkaloids. The treatment of SB203580 blocked p38 MAPK phosphorylation, which further inhibited Nrf2 nuclear translocation and its binding with ARE, as well as the HO1 and NQO1 expressions in RAECs exposed to AGEs, leading to excessive ROS accumulation and ROS‐mediated apoptosis. Results from both in vivo and in vitro studies suggested that matrine‐type alkaloids could increase antioxidant ability of RAECs to inhibit ROS‐mediated apoptosis by regulating the p38 MAPK/Nrf2/ARE pathway.
MKKs are kinase enzymes phosphorylating and activating MAPKs, including extracellular signal‐regulated kinases, c‐Jun N‐terminal kinases, and p38.39, 40 Among them, MKK3 and MKK6 were reported to be specifically involved in the activation of p38 MAPK.16 In this study, the small RNA interfering technique was used to silence MKK3 and MKK6 in AGEs‐exposed RAECs. The results showed that both MKK3 and MKK6 silencing impaired matrine‐type alkaloids’ antioxidant ability in RAECs exposed to AGEs. The results suggested that matrine‐type alkaloids exerted protective effects against oxidative stress–induced cell death by affecting p38 MAPK antioxidant signaling. Their targets were supposed to be the upstream kinases, MKK3 and MKK6, particularly.
In summary, results from the current study indicated that AGEs exposure would trigger ROS overproduction, inducing cell apoptosis in aortic endothelium in vivo and in vitro. The antioxidant signaling transduction by p38 MAPK/Nrf2/ARE was inhibited. Administrations of matrine‐type alkaloids, namely matrine, oxymatrine, and sophocarpine, were found to recover the activation of p38 MAPK/Nrf2/ARE both in vivo and in vitro. p38 MAPK kinases MKK3/6 were believed to be the molecular targets of these matrine‐type alkaloids. The schematic diagram of the molecular mechanisms is shown in Figure 7. We believe results from this study would not only enrich our knowledge of molecular pharmacological mechanisms of matrine‐type alkaloids, but also provide the clues for potential clinical application of matrine‐type alkaloids and related compounds in treatment of diabetic vascular complications.
Figure 7.
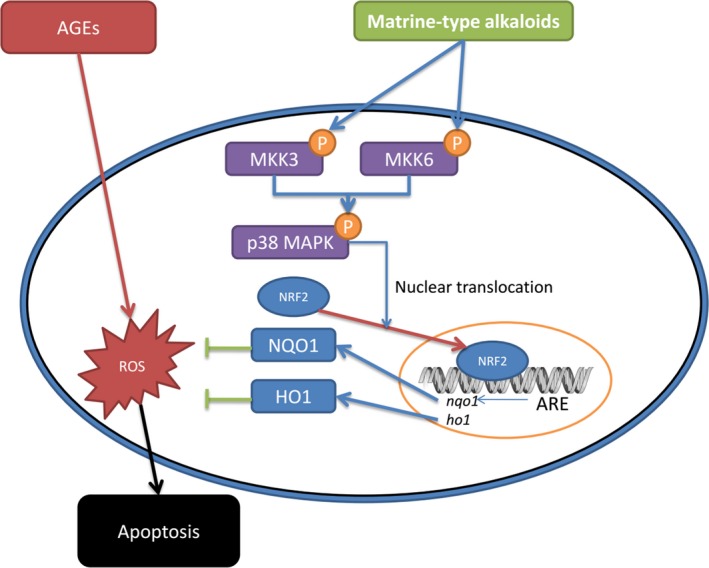
The schematic diagram of the antioxidant molecular mechanisms of matrine‐type alkaloids. AGEs (advanced glycation end products) trigger intracellular ROS (reactive oxygen species) production, which induces apoptosis of aortic endothelial cells. The matrine‐type alkaloids administration promotes the phosphorylation of MKK3 and MKK6. As the kinases of p38 MAPK, phosphorylated MKK3 and MKK6 further phosphorylate p38 MAPK, which facilitate the nuclear translocation of Nrf2. Binding with ARE (antioxidant response element), the nuclear transcription factor Nrf2 initiates the transcriptions of antioxidant proteins such as NQO1 (NADPH quinone oxidoreductase 1) and HO1 (heme oxygenase 1). Thus, the intracellular ROS accumulation is alleviated and apoptosis is suppressed.
Limitations
There were several limitations of this study. (1) Although male experiment animals were more physically stabilized, female animals should also be included because sex is an important biological variant. (2) The concentration gradients of matrine‐type alkaloids were not included in the experiment design of the in vivo study because it would bring a heavy workload. Our pre‐experiments confirmed their effective dosages, which were used in the subsequent experiments. It would be better if in vivo toxicity assays were implemented to prove the biosafety of these alkaloids. Interestingly, we also noticed that the effective dosages of these matrine‐type alkaloids were not consistent in the in vivo and in vitro studies. We think that maybe the effective concentrations of the alkaloids were various, though they share several similar molecular structures; the in vivo metabolisms of these agents may be different. Perhaps studies on the pharmacokinetics of these agents in the future would be helpful to elucidate it. (3) Administration of p38 MAPK inhibitor impaired the effects of matrine‐type alkaloids partially. This result indicated that there might be other signaling pathways modulating matrine‐type alkaloids’ protective effects on RAECs. There are other MKKs regulating MAPKs. For instance, MKK4/7 could regulate the phosphorylation of another MAPK subgroup, the c‐Jun N‐terminal kinase. Moreover, it was reported that c‐Jun N‐terminal kinase was also involved in ROS‐mediated apoptosis of several types of cells.41 Thus, further investigations of other possibly involved pathways would also be meaningful. (4) Results from this study just answered whether the MKK3/6 were phosphorylated by matrine‐type alkaloids, but did not answer how. Maybe some bioinformatic software could be helpful in anticipating the phosphorylation sites of MKK3/6 by these agents, which could be further confirmed by corresponding antibodies. (5) Keap1 binds to Nrf2 to maintain the static status of Nrf2 under normal conditions. When we studied the initiation of nuclear translocation of Nrf2, it would have been better for us to investigate the expression of Keap1 at the same time. This would make the evidence more solid.
Author Contributions
Zhongwei Liu wrote the manuscript and implemented the experiments; Lv implemented the experiments; Zhang researched data; Fuqiang Liu accomplished the figures; Zhu analyzed the data; Pan revised the manuscript; Qiu analyzed the data; Guo participated in the experiments; Yang participated in the experiments; Wang designed the study and revised the manuscript. Prof Wang is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data.
Sources of Funding
This study was supported by the National Scientific Foundation of China (No. 81600646), the China Postdoctoral Science Foundation (No. 2016M590956), and the Sailing Foundation (No. LHJJ20159029).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e007441 DOI: 10.1161/JAHA.117.007441.)29197828
References
- 1. Kiss LZ, Bagyura Z, Vadas R, Polgar L, Lux A, Edes E, Szenczi O, Soos P, Szelid Z, Becker D, Jermendy G, Merkely B. Signs of subclinical atherosclerosis in asymptomatic patients at increased risk of type 2 diabetes mellitus. J Diabetes Complications. 2017;31:1293–1298. [DOI] [PubMed] [Google Scholar]
- 2. Lazzeri C, Valente S, Chiostri M, Attana P, Mattesini A, Nesti M, Gensini GF. Hyperglycemia, acute insulin resistance, and renal dysfunction in the early phase of ST‐elevation myocardial infarction without previously known diabetes: impact on long‐term prognosis. Heart Vessels. 2014;29:769–775. [DOI] [PubMed] [Google Scholar]
- 3. Nass N, Bartling B, Navarrete Santos A, Scheubel RJ, Borgermann J, Silber RE, Simm A. Advanced glycation end products, diabetes and ageing. Z Gerontol Geriatr. 2007;40:349–356. [DOI] [PubMed] [Google Scholar]
- 4. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14:013–0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang QT, Zhang M, Zhong M, Yu YH, Liang WZ, Hang LL, Gao YF, Huang LP, Wang ZJ. Advanced glycation end products as an upstream molecule triggers ROS‐induced sFlt‐1 production in extravillous trophoblasts: a novel bridge between oxidative stress and preeclampsia. Placenta. 2013;34:1177–1182. [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Zhang Y, Ji H, Ji Y, Yang J, Huang J, Sun D. Involvement of hypoxia‐inducible factor‐1alpha in the oxidative stress induced by advanced glycation end products in murine Leydig cells. Toxicol In Vitro. 2016;32:146–153. [DOI] [PubMed] [Google Scholar]
- 7. Ishibashi Y, Matsui T, Isami F, Abe Y, Sakaguchi T, Higashimoto Y, Yamagishi SI. N‐butanol extracts of Morinda citrifolia suppress advanced glycation end products (AGE)‐induced inflammatory reactions in endothelial cells through its anti‐oxidative properties. BMC Complement Altern Med. 2017;17:017–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Chen Y, Cai G, Li X, Wang D. Carnosic acid induces apoptosis of hepatocellular carcinoma cells via ROS‐mediated mitochondrial pathway. Chem Biol Interact. 2017;277:91–100. [DOI] [PubMed] [Google Scholar]
- 9. Liu ZW, Zhu HT, Chen KL, Dong X, Wei J, Qiu C, Xue JH. Protein kinase RNA‐like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)‐mediated endoplasmic reticulum stress‐induced apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol. 2013;12:1475–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tse AK, Cao HH, Cheng CY, Kwan HY, Yu H, Fong WF, Yu ZL. Indomethacin sensitizes TRAIL‐resistant melanoma cells to TRAIL‐induced apoptosis through ROS‐mediated upregulation of death receptor 5 and downregulation of survivin. J Invest Dermatol. 2014;134:1397–1407. [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Kim KM, Kim CS, Sohn E, Lee YM, Jo K, Kim JS. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase‐related oxidative stress. Free Radic Biol Med. 2012;53:357–365. [DOI] [PubMed] [Google Scholar]
- 12. Li C, Zhang WJ, Frei B. Quercetin inhibits LPS‐induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38‐mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016;9:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bak MJ, Truong VL, Ko SY, Nguyen XN, Jun M, Hong SG, Lee JW, Jeong WS. Induction of Nrf2/ARE‐mediated cytoprotective genes by red ginseng oil through ASK1‐MKK4/7‐JNK and p38 MAPK signaling pathways in HepG2 cells. J Ginseng Res. 2016;40:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang G, Hu Y, Liu L, Cai J, Peng C, Li Q. Gastrodin protects against MPP(+)‐induced oxidative stress by up regulates heme oxygenase‐1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem Int. 2014;75:79–88. [DOI] [PubMed] [Google Scholar]
- 15. Jin CH, So YK, Han SN, Kim JB. Isoegomaketone upregulates heme oxygenase‐1 in RAW264.7 cells via ROS/p38 MAPK/Nrf2 pathway. Biomol Ther (Seoul). 2016;24:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshino Y, Yamamoto S, Kohsaka S, Oshiro S, Nakajima K. Superoxide anion contributes to the induction of tumor necrosis factor alpha (TNFalpha) through activation of the MKK3/6‐p38 MAPK cascade in rat microglia. Brain Res. 2011;8:1–12. [DOI] [PubMed] [Google Scholar]
- 17. Cai XH, Guo H, Xie B. Structural modifications of matrine‐type alkaloids. Mini Rev Med Chem. 2016;4:4. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Zhang Y, Tang Z, Xu J, Ma M, Pan S, Qiu C, Guan G, Wang J. Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur J Pharmacol. 2017;804:21–30. [DOI] [PubMed] [Google Scholar]
- 19. Liu ZW, Wang JK, Qiu C, Guan GC, Liu XH, Li SJ, Deng ZR. Matrine pretreatment improves cardiac function in rats with diabetic cardiomyopathy via suppressing ROS/TLR‐4 signaling pathway. Acta Pharmacol Sin. 2015;36:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Zhou R, Zheng P, Yan L, Wu Y, Xiao X, Dai G. Cardioprotective effect of matrine on isoproterenol‐induced cardiotoxicity in rats. J Pharm Pharmacol. 2010;62:514–520. [DOI] [PubMed] [Google Scholar]
- 21. Xie SB, He XX, Yao SK. Matrine‐induced autophagy regulated by p53 through AMP‐activated protein kinase in human hepatoma cells. Int J Oncol. 2015;47:517–526. [DOI] [PubMed] [Google Scholar]
- 22. Ma L, Zhu Z, Jiang L, Sun X, Lu X, Zhou M, Qian S, Jianyong L. Matrine suppresses cell growth of human chronic myeloid leukemia cells via its inhibition of the interleukin‐6/Janus activated kinase/signal transducer and activator of transcription 3 signaling cohort. Leuk Lymphoma. 2015;56:2923–2930. [DOI] [PubMed] [Google Scholar]
- 23. Pan QM, Li YH, Hua J, Huang FP, Wang HS, Liang D. Antiviral matrine‐type alkaloids from the rhizomes of Sophora tonkinensis . J Nat Prod. 2015;78:1683–1688. [DOI] [PubMed] [Google Scholar]
- 24. Matsui T, Nakamura N, Ojima A, Nishino Y, Yamagishi SI. Sulforaphane reduces advanced glycation end products (AGEs)‐induced inflammation in endothelial cells and rat aorta. Nutr Metab Cardiovasc Dis. 2016;26:797–807. [DOI] [PubMed] [Google Scholar]
- 25. Fan H, Li L, Zhang X, Liu Y, Yang C, Yang Y, Yin J. Oxymatrine downregulates TLR4, TLR2, MyD88, and NF‐kappaB and protects rat brains against focal ischemia. Mediators Inflamm. 2009;704706:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Li L, Chu H, Sun X, Ge Z. Oral sophocarpine protects rat heart against pressure overload‐induced cardiac fibrosis. Pharm Biol. 2014;52:1045–1051. [DOI] [PubMed] [Google Scholar]
- 27. Yan W, Xiaoli L, Guoliang A, Zhonghui Z, Di L, Ximeng L, Piye N, Li C, Lin T. SB203580 inhibits epithelial‐mesenchymal transition and pulmonary fibrosis in a rat silicosis model. Toxicol Lett. 2016;259:28–34. [DOI] [PubMed] [Google Scholar]
- 28. Tian D, Qiu Y, Zhan Y, Li X, Zhi X, Wang X, Yin L, Ning Y. Overexpression of steroidogenic acute regulatory protein in rat aortic endothelial cells attenuates palmitic acid‐induced inflammation and reduction in nitric oxide bioavailability. Cardiovasc Diabetol. 2012;11:1475–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fei ZW, Qiu MK, Qi XQ, Dai YX, Wang SQ, Quan ZW, Liu YB, Ou JM. Oxymatrine suppresses proliferation and induces apoptosis of hemangioma cells through inhibition of HIF‐1a signaling. Int J Immunopathol Pharmacol. 2015;28:201–208. [DOI] [PubMed] [Google Scholar]
- 30. Song CY, Zeng X, Wang Y, Shi J, Qian H, Zhang Y, Fang JQ, Sheng X, Zheng JM, Chen YX. Sophocarpine attenuates toll‐like receptor 4 in steatotic hepatocytes to suppress pro‐inflammatory cytokines synthesis. J Gastroenterol Hepatol. 2015;30:405–412. [DOI] [PubMed] [Google Scholar]
- 31. Piperi C, Goumenos A, Adamopoulos C, Papavassiliou AG. AGE/RAGE signalling regulation by miRNAs: associations with diabetic complications and therapeutic potential. Int J Biochem Cell Biol. 2015;60:197–201. [DOI] [PubMed] [Google Scholar]
- 32. van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;792393:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J, Sun Z, Jin M, Tu Y, Wang S, Yang X, Chen Q, Zhang X, Han Y, Pi R. Inhibition of AGEs/RAGE/Rho/ROCK pathway suppresses non‐specific neuroinflammation by regulating BV2 microglial M1/M2 polarization through the NF‐kappaB pathway. J Neuroimmunol. 2017;305:108–114. [DOI] [PubMed] [Google Scholar]
- 34. Zhang W, Zhang J, Liu YK, Liu J, Wang X, Xu Q, Wang Y, Xu X, Dai G. Cardioprotective effects of oxymatrine on isoproterenol‐induced heart failure via regulation of DDAH/ADMA metabolism pathway in rats. Eur J Pharmacol. 2014;745:29–35. [DOI] [PubMed] [Google Scholar]
- 35. Ni YH, Huo LJ, Li TT. Antioxidant axis Nrf2‐keap1‐ARE in inhibition of alcoholic liver fibrosis by IL‐22. World J Gastroenterol. 2017;23:2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lei X, Lei L, Zhang Z, Cheng Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemiareperfusion in rats: the role of the Nrf2/HO1 signaling pathway. Mol Med Rep. 2016;13:412–418. [DOI] [PubMed] [Google Scholar]
- 37. Han MH, Park C, Lee DS, Hong SH, Choi IW, Kim GY, Choi SH, Shim JH, Chae JI, Yoo YH, Choi YH. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2‐mediated NQO1 expression via the activation of the ERK pathway. Int J Mol Med. 2017;39:380–386. [DOI] [PubMed] [Google Scholar]
- 38. Liu H, Wu HY, Wang WY, Zhao ZL, Liu XY, Wang LY. Regulation of miR‐92a on vascular endothelial aging via mediating Nrf2‐KEAP1‐ARE signal pathway. Eur Rev Med Pharmacol Sci. 2017;21:2734–2742. [PubMed] [Google Scholar]
- 39. Ren X, Du H, Li Y, Yao X, Huang J, Li Z, Wang W, Li J, Han S, Wang C, Huang K. Age‐related activation of MKK/p38/NF‐kappaB signaling pathway in lung: from mouse to human. Exp Gerontol. 2014;57:29–40. [DOI] [PubMed] [Google Scholar]
- 40. Leite FGG, Torres AA, De Oliveira LC, Da Cruz AFP, Soares‐Martins JA, Pereira A, Trindade GS, Abrahao JS, Kroon EG, Ferreira PC, Bonjardim CA. C‐Jun integrates signals from both MEK/ERK and MKK/JNK pathways upon vaccinia virus infection. Arch Virol. 2017;15:017–3446. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Guo SH, Shang XJ, Yu LS, Zhu JW, Zhao A, Zhou YF, An GH, Zhang Q, Ma B. Triptolide induces Sertoli cell apoptosis in mice via ROS/JNK‐dependent activation of the mitochondrial pathway and inhibition of Nrf2‐mediated antioxidant response. Acta Pharmacol Sin. 2017;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]


