Abstract
Background
NO bioavailability has not been systematically examined in congenital heart disease (CHD). To assess NO in patients with CHD, we measured nasal NO (nNO) generated by the nasal epithelia, given blood NO is difficult to measure (half‐life, <2 ms). Given NO's role in hemodynamic regulation and the association of NO bioavailability with heart failure risk, we hypothesized NO levels may differ with varying severity of CHD physiologic characteristics.
Methods and Results
Six‐hundred eighteen subjects, 483 with CHD and 135 controls, had nNO measured noninvasively via the nares using American Thoracic Society/European Respiratory Society guidelines. Subjects were dichotomized as having low or normal nNO based on age‐specific cutoff values. Prevalence of low nNO was examined by various CHD physiologic feature types. Low nNO was more prevalent with CHD than controls (odds ratio, 2.28; P=0.001). A logistic regression model showed overall significance (P=0.035) for single ventricle, systemic right ventricle, ventricular dysfunction, oxygen desaturation, and heterotaxy predicting low nNO, with systemic right ventricle independently having twice the odds of low nNO (odds ratio, 2.04; P=0.014). Patients with low nNO had a higher risk of experiencing heart transplant or death (hazard ratio, 2.75; P=0.048), and heart transplant recipients (N=16) exhibited 5 times the odds of low nNO (69% versus 30%; odds ratio, 5.1; P=0.001).
Conclusions
Patients with CHD have increased prevalence of low nNO, with highest odds seen with systemic right ventricle and heart transplant. Further studies are needed to investigate heart failure risks in patients with CHD with left versus right systemic ventricle physiologic characteristics and utility of low nNO for predicting heart failure risk.
Keywords: congenital heart disease, nasal NO, NO, right ventricle, single ventricle, transplant
Subject Categories: Biomarkers, Heart Failure, Endothelium/Vascular Type/Nitric Oxide, Transplantation
Clinical Perspective
What Is New?
Patients with congenital heart disease with a systemic right ventricle have twice the odds of having low nasal NO (nNO).
Patients with congenital heart disease with low nNO may be at higher risk of requiring heart transplant or death.
The highest prevalence of low nNO was in patients with congenital heart disease who had received a heart transplant.
What Are the Clinical Implications?
nNO measurements can easily be trended over time, because they are simple and noninvasive.
Deviation from one's nNO curve may help identify patients with congenital heart disease at risk for poor outcomes.
Congenital heart disease (CHD) is the most common birth defect, with an estimated 2.4 million individuals living with CHD in the United States.1, 2 The leading CHD‐related cause of death after having congenital cardiac surgery is heart failure.3 CHD is the cause of heart failure in 55% of infants, 41% of 1‐ to 5‐year‐old children, 35% of 6‐ to 10‐year‐old children, and 23% of adolescents who received heart transplants (HTx) (from 2009 to 2015).4 Norozi et al observed an overall heart failure rate of 26% in patients with a heterogeneous mix of CHD, but 47% among those with single‐ventricle (SV) CHD.5 It is perhaps not surprising that SV CHD has the highest incidence of heart failure among all CHD types,6 because SV physiologic features necessitate that a single functioning ventricle assume the workload typically shared by 2 ventricles. In some instances, the right ventricle (RV) is the systemic ventricle (sysRV), which has been associated with worse outcomes,6 indicating the RV may not be able to meet the demands of a systemic ventricle. Consistent with this, patients with CHD most commonly listed for HTx are those with SV CHD (36%), followed by sysRV (20%), suggesting these unfavorable CHD physiologic features place patients at high risk for heart failure.7
Recent studies revealed a novel paradigm for heart failure, one in which vascular endothelial health and coronary microvascular function are of pivotal importance.8 Because NO is an important mediator of coronary microcirculatory function,9 and its bioavailability may be crucial to the development of heart failure,10, 11 this suggests NO measurements may have clinical utility as a biomarker for assessing heart failure risks. However, NO measurement in blood is difficult because of NO's extremely short half‐life (<2 ms).12 Given NO is also continuously produced by the airway epithelium,13, 14 this suggests the possibility that nasal NO (nNO) measurements, easily obtained noninvasively via the nares,15 might be suitable as a proxy for blood NO. Measurement of nNO is commonly used in assessments of various respiratory diseases,16 and nNO measurements have also been obtained in prior studies of patients with CHD. One such study demonstrated a 42% (18/43) prevalence of low nNO in a small cohort of patients with heterotaxy CHD.17 A subsequent study of a larger cohort of patients with CHD (with and without heterotaxy) found 36% (64/180) had low nNO.18
Whether varying physiologic characteristics among patients with CHD differentially affect nNO levels remains unknown. In the current study, we investigated nNO levels in the context of different CHD physiologic features to assess the hypothesis that patients with unfavorable CHD physiologic features may have lower nNO and that this may portend heart failure risk.
Methods
Study Design
This was a prospective observational study of patients with CHD, with exposure being favorable versus unfavorable CHD physiologic features and the outcome being normal or low nNO status. Secondarily, the relationship between low nNO and the composite end point of HTx or death was investigated. A third and final analysis was performed to compare nNO measurements after HTx with those of patients with CHD who had not undergone HTx.
Patient Recruitment
Patients with CHD of all ages and all structural CHD phenotypes presenting to the Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center (Pittsburgh, PA) were recruited into the study from January 25, 2010 through January 17, 2017. Written parental informed consent was obtained for all minors, and adults gave their own written informed consent. This study was performed in compliance with our full institutional review board–approved protocol (PRO 09090021).
Subjects were recruited from the cardiology clinic for patients being seen for routine appointments and from the surgical preoperative suite for those being seen for cardiac catheterization or surgical intervention. Neonates who were born and admitted directly to the cardiac intensive care unit were recruited and had testing only if clinically stable. Acutely ill or clinically decompensating inpatients were excluded. Patients with isolated dilated or restrictive cardiomyopathies were excluded.
Controls consisted of healthy volunteers, healthy parents and siblings of subjects with CHD, and infants and children without CHD who were being seen for simple outpatient surgical procedures (eg, hernia repair, blepharoplasty, or small lesion/cyst removals). Subjects with nasal/airway pathological features, any trauma, or acutely ill, urgent, or emergent patient status were excluded.
nNO Assessment
nNO was measured as per the American Thoracic Society/European Respiratory Society guidelines (Data S1).19 nNO values (nl/min) were acquired using a CLD88sp chemiluminescence NO analyzer (EcoPhysics, Ann Arbor, MI). Patients were categorized as having normal or low nNO based on established cutoff values from the literature.17, 18, 20
Demographic and Clinical Variables
Each subject's sex, race, and age at nNO measurement were recorded. In addition, for the primary analysis, we chose 5 physiologic variables to examine for correlation with nNO levels. These variables were chosen on the basis of their predilection for heart failure and potential influence on oxygen content/delivery (SV status, systemic ventricle status, ventricular function, and blood oxygenation) or on previous published findings showing correlation with nNO level (heterotaxy status).17, 18
Each subject's surgical stage of correction at nNO measurement and whether the subject had a systemic right or left ventricle were documented. Heterotaxy status was abstracted from the medical record, clinic notes, or imaging studies stating atrial isomerism and/or thoracoabdominal situs abnormalities. Oxygen saturation and systemic ventricular systolic function, as determined by transthoracic echocardiography, were abstracted from the medical record from the same day that subjects had their nNO measured (Data S1). These tests were done as part of their routine care and not specifically as part of the study design. All clinicians were blinded to each patient's nNO status.
Statistical Analysis
All patients sampled were included in the analysis. Summary statistics are presented as the count with percentage and as the mean with SD. Comparisons of the previously mentioned categorical variables with nNO status were analyzed using χ2 testing and the Fisher exact test when appropriate. All subjects were used in the categorical analysis. Logistic regression with Pearson goodness‐of‐fit postestimation testing was used to examine the effect of CHD physiologic features on nNO status. Time‐to‐event distributions for the composite end point of transplant or death, on the basis of nNO status, were analyzed using the log‐rank test and Cox proportional‐hazards modeling (Data S1). P=0.05 was considered statistically significant, with Bonferroni adjustment for correction of the α level for multiple comparisons. Statistical analysis was completed using STATA 14.1 (StataCorp, College Station, TX) and GraphPad Prism 7.0a (GraphPad Software, La Jolla, CA).
Results
Patient Characteristics
For the primary analysis examining physiologic variables and nNO, measurements were available for 606 subjects, composed of 471 patients with CHD (78%), of whom 365 (77%) had biventricular CHD and 106 (23%) had SV CHD; also, 135 (22%) were control subjects (Figure 1A). Along with heterotaxy status, the physiologic variables examined included status for SV and systemic ventricle, ventricular systolic function (458 patients [97%]), and oxygen saturation (415 patients [88%]). From this cohort, 6 patients with CHD (1%) progressed to requiring HTx and 16 (3%) died within the study period, providing 22 patients for composite end point analysis (Figure 1B). Measurement of nNO was repeated on 4 of the 6 patients requiring HTx after HTx, and an additional 12 patients were recruited after their HTx. Together, this yielded 16 patients for the post‐HTx nNO analysis (Figure 1C).
Figure 1.
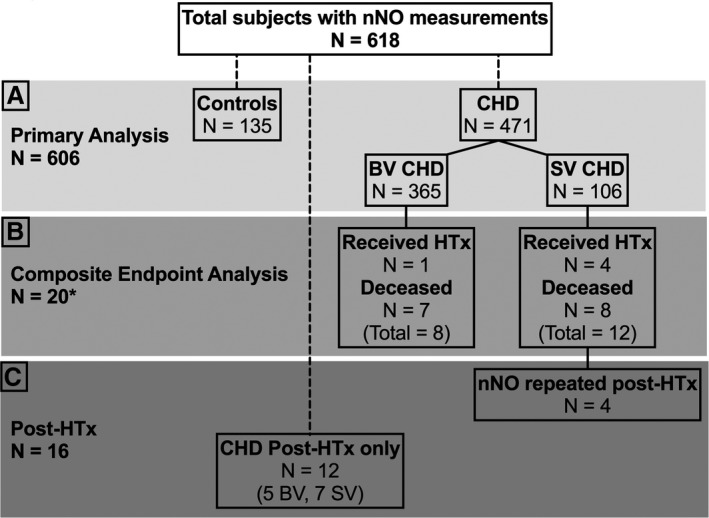
Flow chart showing subject numbers used for analyses. A, A total of 606 subjects with nasal NO (nNO) measurements were available for the primary analysis. B, There were 6 subjects who underwent heart transplant (HTx) and 16 who died. Of the 22 patients who experienced these end points, only 20 (asterisk) were appropriate for the outcome analysis (see Results). C, Of the 6 patients with HTx, 4 had repeated nNO values after HTx in addition to 12 patients who only had post‐HTx nNO measurements, thus providing a total 16 patients after HTx. CHD indicates congenital heart disease; and SV, single ventricle.
General nNO Assessment
We observed an age‐related increase in nNO measurements, which appeared to plateau at ≈10 years old, consistent with prior literature (Figure 2).21 Although nNO increased with age (as expected), the prevalence of low nNO in patients <10 years old versus those ≥10 years old was not different (Table S1). We subdivided the 606 subjects into controls, biventricular CHD, and SV CHD. Both a 1‐phase association and quadratic nonlinear curves were generated, with 1‐phase association being the preferred model in all 3 instances (Figure 2A through 2C, respectively). The nNO regression curves with 95% prediction intervals appear to progressively decrease from controls, to biventricular CHD, to SV CHD.
Figure 2.
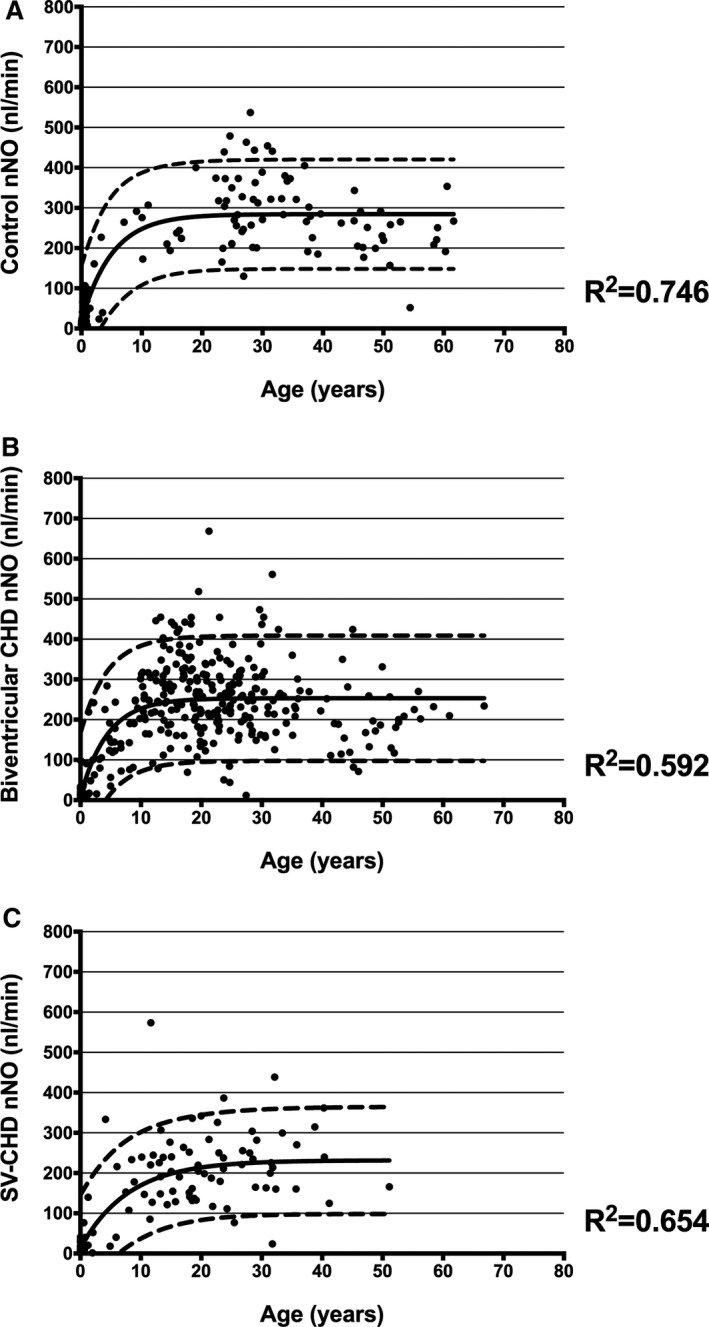
Age‐related changes in nasal NO (nNO) measurements (nl/min). Our study sample demonstrated an age‐related increase in nNO measurements until the age of ≈10 years. Fitted nNO regression curves and 95% prediction intervals appear to decrease from controls (A), to biventricular congenital heart disease (CHD) (B), to single‐ventricle (SV) CHD (C).
Patients with CHD in the primary analysis (Figure 1A) had a significantly higher prevalence of low nNO than control subjects (31% versus 16%; P=0.001) (Table 1). Group comparison showed a significant difference for the prevalence of low nNO between controls (16%), SV CHD (42%), and biventricular CHD (27%) (P<0.001), with additional subgroup analysis showing patients with SV CHD having nearly 4 times the odds of low nNO measurements compared with control subjects (odds ratio, 3.79; P<0.001) (Table 1). There was no significant difference in the prevalence of low nNO by sex, race, or sampling method within either the CHD or control groups (Table S1). The CHD type with the highest prevalence of low nNO was hypoplastic left heart syndrome (17/30 [57%]), followed by congenitally corrected transposition of the great arteries (10/24 [42%]), d‐transposition of the great arteries (18/49 [37%]), and double‐outlet RV (16/45 [36%]) (Figure S1, Table S2). Interestingly, these 4 CHD types were also observed to have the highest percentage of a sysRV (Figure S1). Analysis of the stage of surgical palliation showed patients with both Fontan SV CHD and pre‐Fontan SV CHD had a significantly higher prevalence of low nNO than controls (Figure 3). In addition, Fontan SV CHD also had a higher prevalence of low nNO when compared with biventricular CHD (Figure 3).
Table 1.
Low nNO Prevalence Between Control, Biventricular CHD, and SV CHD Groups
| Group | Total | Low nNO | % Low nNO | P Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Controla | 135 | 22 | 16 | … | … | … |
| CHD (biventricular+SV) | 471 | 145 | 31 | 0.001 | 2.28 | 1.37–3.94 |
| SV CHD | 106 | 45 | 42 | <0.001 | 3.79 | 2.01–7.24 |
| Biventricular CHD | 365 | 100 | 27 | 0.010 | 1.94 | 1.14–3.40 |
N=606. CHD indicates congenital heart disease; CI, confidence interval; nNO, nasal NO; OR, odds ratio; and SV, single ventricle.
P values and ORs reflect comparison to control.
Figure 3.
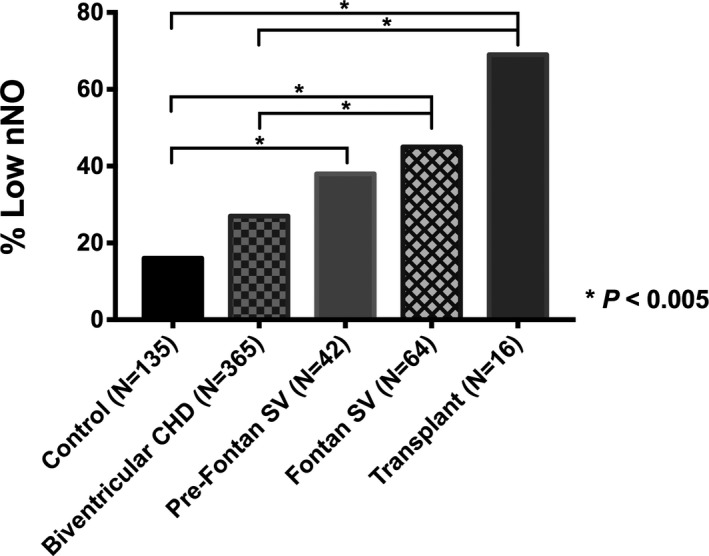
Bar graph illustrating the prevalence of low nasal NO (nNO) by stage of congenital heart disease (CHD) repair. Both patients with Fontan single‐ventricle (SV) and transplanted CHD had a significantly higher prevalence of low nNO than both subjects with biventricular CHD and control subjects. Patients with pre‐Fontan SV CHD had a higher prevalence of low nNO than controls. The asterisk indicates Bonferroni adjustment for 10 comparisons (0.05/10=0.005).
nNO by Various CHD Physiologic Features
The prevalence of low nNO was significantly higher in SV CHD versus biventricular CHD (42% versus 27%; P=0.003) and sysRV versus systemic left ventricle (46% versus 26%; P<0.001) (Table 2). Patients with more systemic ventricular dysfunction had a higher prevalence of low nNO than those with better ventricular function; however, differences between the groups were not significant (Table 2). There were no significant differences in the prevalence of low nNO when comparing patients by oxygenation or heterotaxy status (Table 2). Logistic regression of these 5 variables for predicting low nNO produced an overall significant model (P=0.035) with a postestimation goodness‐of‐fit test that supports the model (P=0.592) (Table 3). On the basis of this model, having a sysRV was an independent predictor of low nNO, with odds twice as high for low nNO in sysRV patients (odds ratio, 2.04; P=0.014) (Table 3). Because nNO increases dynamically with age until it plateaus at ≈10 years old,21 patients were separated into older (≥10 years old) and younger (<10 years old) groups, and continuous nNO data were analyzed only for the older group. nNO measurements were significantly lower in those with SV CHD, sysRV, and oxygen saturation <90% (Table S3).
Table 2.
Prevalence of Low nNO by Various CHD Physiologic Variables
| Variables | Total | Low nNO | % Low nNO | P Value | OR | 95% CI |
|---|---|---|---|---|---|---|
| N=471a | ||||||
| Single‐ventricle CHD | 106 | 45 | 42 | 0.003 | 1.95 | 1.21–3.13 |
| Biventricular CHD | 365 | 100 | 27 | … | … | … |
| Systemic RV | 112 | 52 | 46 | <0.001 | 2.48 | 1.56–3.94 |
| Systemic LV | 359 | 93 | 26 | … | … | … |
| Ventricular dysfunction | 0.135 | |||||
| None | 320 | 93 | 29 | … | … | |
| Low‐normal | 61 | 19 | 31 | … | … | |
| Mild | 52 | 19 | 37 | … | … | |
| Moderate | 22 | 11 | 50 | … | … | |
| Severe | 3 | 2 | 67 | … | … | |
| Spo 2 <90% | 77 | 32 | 42 | 0.069 | … | … |
| Spo 2 ≥90% | 338 | 104 | 31 | … | … | |
| Heterotaxy | 42 | 14 | 33 | 0.708 | … | … |
| Nonheterotaxy | 429 | 131 | 31 | … | … | |
| N=483 | ||||||
| Transplanted | 16 | 11 | 69 | 0.001 | 5.09 | 1.59–18.96 |
| Not transplanted | 467 | 141 | 30 | … | … | |
CHD indicates congenital heart disease; CI, confidence interval; LV, left ventricle; nNO, nasal NO; OR, odds ratio; RV, right ventricle; and Spo 2, oxygen saturation.
Bonferroni adjustment for 5 CHD characteristics (0.05/5=0.01).
Table 3.
Logistic Regression Model for Low nNO in the Population With CHD
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Single‐ventricle CHD | 1.57 | 0.88–2.79 | 0.124 |
| Systemic RV | 2.04 | 1.16–3.59 | 0.014 |
| Ventricular dysfunction | |||
| Low‐normal | 0.67 | 0.33–1.33 | 0.25 |
| Mild | 0.97 | 0.49–1.94 | 0.934 |
| Moderate | 1.68 | 0.65–4.33 | 0.286 |
| Severe | 2.25 | 0.19–26.72 | 0.52 |
| Spo 2 <90% | 1.02 | 0.55–1.88 | 0.961 |
| Heterotaxy | 0.69 | 0.33–1.44 | 0.319 |
N=413. Overall model: P=0.035. Pearson goodness of fit: P=0.592. CHD indicates congenital heart disease; CI, confidence interval; nNO, nasal NO; OR, odds ratio; RV, right ventricle; and Spo 2, oxygen saturation.
Composite End Point Analysis
There were 16 deaths and 6 patients with pre‐HTx nNO values who progressed to requiring HTx, thus providing 22 possible subjects for end point analysis comprising either HTx or death. One deceased patient (a 2‐month‐old patient who was the only patient with isolated total anomalous pulmonary venous return) had no suitable CHD match and was excluded from the analysis. One patient experienced both HTx and death, but because the pre‐HTx nNO was measured the day of HTx, only the death event and post‐HTx/predeath nNO measurement were used. This resulted in 20 patients for the final composite end point analysis (Figure 1B). For comparison, 80 patients with CHD were matched to these 20 on the basis of CHD type and age at nNO measurement (Table S4).
When these 100 subjects were divided into low nNO (N=44) versus normal nNO (N=56) groups, there were no significant differences in SV status, systemic ventricle, heterotaxy status, ventricular dysfunction, or oxygenation level, although there was a trend for more SV CHD in the low nNO group (66% versus 44%; P=0.052) (Table S5). The composite end point comprising death or transplant was observed in a higher percentage of the low nNO group (13/44 [30%]) than those with normal nNO (7/56 [12%]) (P=0.034) (Table S5). Survival analysis for end point comprising either HTx or death showed a lower percentage of event‐free subjects through the 7‐year study period for those with low nNO compared with those with normal nNO (Figure 4), but this difference did not reach statistical significance (log‐rank P=0.074). We performed a Cox proportional‐hazards regression analysis for the composite end point, with low nNO as a predictor and ventricular function and oxygenation status as covariates (anatomic covariates were controlled by matching). Low nNO was found to be associated with increased risk for HTx/death (hazard ratio, 2.75; P=0.048) (Table 4).
Figure 4.
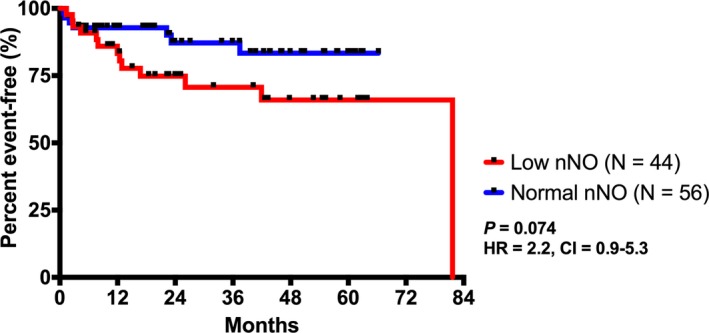
Kaplan‐Meier plot showing time from nasal NO (nNO) measurement to either heart transplant or death. There was a lower proportion of event‐free patients in the low nNO group within the 7‐year study period, although this did not reach statistical significance. CI indicates confidence interval; and HR, hazard ratio.
Table 4.
Proportional‐Hazards Model for the Composite End Point Analysis
| Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Low nNO | 2.75 | 1.01–7.47 | 0.048 |
| Ventricular dysfunction | |||
| Low‐normal | 0.67 | 0.2–2.27 | 0.522 |
| Mild | 0.33 | 0.07–1.6 | 0.169 |
| Moderate | 2.13 | 0.55–8.19 | 0.273 |
| Severe | 2.01 | 0.25–16.29 | 0.514 |
| Spo 2 <90% | 1.63 | 0.63–4.22 | 0.317 |
N=95. Overall model: P=0.098. Harrell C: 0.677. CI indicates confidence interval; nNO, nasal NO; and Spo 2, oxygen saturation.
Post‐HTx Analysis
Comprising the 11 of 16 post‐HTx patients with low nNO measurements were 10 patients with SV CHD (91%), 9 (83%) of whom had sysRV (Table S6). Of the 5 patients with normal post‐HTx nNO, 3 (60%) were prior patients with SV CHD, 2 (40%) of whom had a sysRV. When compared against patients with nontransplanted CHD, significantly more post‐HTx patients had a low nNO (69% versus 30%; P=0.001) (Table 2). Overall, the post‐HTx group showed a significantly higher prevalence of low nNO compared with the biventricular CHD group and control subjects (Figure 3).
Discussion
To our knowledge, this is the largest study of nNO measurements in patients with CHD. Our study included a wide spectrum of CHD phenotypes and varying physiologic features. Each group, control, biventricular CHD, and SV CHD, appreciated the same age‐related increase in nNO levels as described in the literature21; however, there was a progressive decline in the best‐fit line and 95% prediction intervals from controls, to patients with biventricular CHD, to patients with SV CHD. Overall, we observed patients with CHD had twice the odds of having low nNO compared with non‐CHD control subjects. We further showed having a sysRV was an independent predictor for having low nNO. An association was also observed between low nNO and having SV CHD, ventricular dysfunction, and low oxygen saturation. Analysis of outcomes showed the highest prevalence of low nNO at 69% (11/16) in patients with CHD who had undergone HTx. More important, we observed fewer event‐free patients in the low nNO group using the composite end point of HTx or death (log‐rank P=0.074), and our proportional‐hazards model was significant for low nNO having a hazard ratio of 2.75 (P=0.048).
In contrast to the study of Garrod et al,18 we did not see a significant difference in the prevalence of low nNO in patients with heterotaxy. Because the population with heterotaxy‐CHD in the study by Nakhleh et al included a large proportion of subjects with double‐outlet RV and congenitally corrected transposition of the great arteries, the high prevalence of low nNO (42%) in this cohort of patients with heterotaxy may, in fact, reflect the high proportion of patients with sysRV physiologic features.17 Similarly, in the study of Garrod et al, the proportion of patients with heterotaxy CHD was 18% compared with only 9% in our current CHD cohort and, thus, those results may have been skewed by a similar trend.18
Physiologic Variables Associated With Low nNO
Our finding of a high prevalence of low nNO in patients with CHD with SV and sysRV amalgamates prior publications showing a central role for NO in the development of heart failure8 and the known increased risk for heart failure among patients with SV/sysRV‐CHD.6 We showed an association of low nNO with ventricular dysfunction and with oxygen desaturation, begging the question as to whether low nNO is the cause or effect of these undesirable physiologic states. Lundberg and Weitzberg suggested low nNO may result from impaired substrate delivery.21 A lack of substrate (hypoxemia) or inadequate substrate delivery (ventricular dysfunction) to the nasal epithelia may manifest outwardly as low nNO, and this could be accompanied by a parallel reduction in substrate availability for coronary and myocardial NO production. Because NO is an essential effector of myocardial microvascular function,22 insufficient NO can cause cardiac dysfunction and increased tissue ischemia/acidosis. This can lead to oxidative stress, resulting in NO synthase (NOS) uncoupling,23 further impairing NO production and leading to even more NO/nitrite consumption and further reduction in substrate availability. This spiraling effect may lead to heart failure and could contribute to the increased heart failure risks in patients with CHD with low nNO. In addition, patients with complex CHD are at higher risk of NO scavenging, because some of the palliative shunts and valve replacements used in CHD surgery can result in high shear stress that may injure blood cells, causing decompartmentalized hemoglobin, which can scavenge endothelial‐derived NO 600‐fold faster than hemoglobin within intact erythrocytes.24 This may be exacerbated by the fact that at the low oxygen concentrations seen in patients with cyanotic CHD, the NO binding affinity for various globin species is even higher.25 Thus, an NO scavenging mechanism may play an important role in NO bioavailability in patients with complex CHD.
Interestingly, the patients with pre‐Fontan SV CHD had a higher prevalence of low nNO compared with controls, but those who had undergone the Fontan operation showed an even higher prevalence of low nNO. This was unexpected because the Fontan procedure should resolve the hypoxemia experienced by the patients with pre‐Fontan SV CHD. However, it is possible that these older patients may have been in more advanced states of heart failure at their nNO measurement, so although adequate substrate (oxygen) may have been available in the patients with Fontan SV CHD physiologic features, the delivery may have been impaired. This may suggest that NO‐producing capacity is an intrinsic individual trait, under the theory that the functionality of the vascular endothelium (and other NO‐producing tissues) may play a larger role than appreciated in this population. Furthermore, because mutations in endothelial NOS can result in CHD,26, 27 it is possible that genetic causes of impaired NO production capacity may both drive abnormal cardiac development, causing CHD, and result in low NO bioavailability in postnatal and adult life. Whether it is vascular endothelium, myocardial cells, or nasal epithelium, NO is produced by either the oxygen‐dependent NOS pathway or nitrite reduction, the latter predominating with ischemia and/or acidosis. nNO can be informative of NO bioavailability given all 3 NOS isoforms have been identified in both the nasal epithelia13, 14 and the myocardium.28
Low nNO and Outcomes
Our analysis of the 20 patients with preevent nNO measurements who progressed to the end point of either HTx or death and 80 matched, censored subjects showed no significant difference in the prevalence of SV or sysRV, ventricular dysfunction, or oxygen desaturation between those with low or normal nNO. Without any significant difference in their underlying CHD physiologic features, those who experienced the composite end point had a higher prevalence of low nNO than their CHD‐ and age‐matched controls. Although our univariate survival analysis did not reach statistical significance, our proportional‐hazards model showed low nNO to be a significant factor. These analyses demonstrate the potential utility of using nNO as a biomarker for monitoring patients longitudinally to identify those at risk for heart failure. Thus, by trending a patient's nNO over time, it might be possible to predict impending heart failure in patients showing a decrease on their nNO curve, similar to that of a failure‐to‐thrive patient deviating from his or her curve on a height and weight growth chart. This would allow earlier intervention for at‐risk patients to improve outcome.29
HTx and Low nNO
The single parameter associated with the highest prevalence of low nNO was being post‐HTx (11/16 [69%] with low nNO). Interestingly, of the 6 patients with pre‐HTx nNO, all 6 (100%) had low nNO. Of these 6 patients, 4 had both pre‐ and post‐HTx nNO measurements, with all 4 (100%) showing the same low nNO after HTx. There are several potential causes for NO bioavailability remaining low in patients after HTx. First, all common immunosuppressant agents can either directly or indirectly inhibit NOS.30, 31 An additional untoward effect of immunosuppression is the vulnerability for opportunistic infections and the use of prophylactic antibiotics. Prophylactic antibiotic use can negatively affect the enterosalivary circulation of nitrate through the unintended alteration of commensal bacteria in the oral cavity, which are responsible for reduction of nitrate to nitrite and, thus, contributing to a reduced NO bioavailability.32
However, if immunosuppression and antibiotics were the sole causes for low nNO after HTx, all patients with HTx would be predicted to have low nNO, not the 69% observed. On the contrary, one could expect the prevalence of low nNO to be much lower after HTx, because HTx should remedy low nNO that may arise from the abnormal physiologic features associated with the SV CHD or sysRV, systemic ventricular dysfunction, and arterial hypoxemia. Instead, our observations suggest other underlying mechanisms causing low nNO in these patients. We hypothesize this may involve intrinsic endothelial dysfunction with resultant poor NO production and this may contribute to impaired cardiovascular development and continuing pathologic features because of the intrinsic limited bioavailability of NO that cannot be corrected by the palliative surgical procedures.
Indeed, if low nNO is reflective of systemic NO bioavailability and, therefore, myocardial NO bioavailability, this would contribute prominently to the progression towards end‐stage heart failure and the subsequent need for HTx. Cardiac allograft vasculopathy continues to be a leading cause of death after HTx for CHD, and it may be that NO bioavailability plays an important role in this critical outcome.
Airway NO and Heart Failure
There is mounting interest in NO research with regard to cardiovascular function and heart failure. We should point out that, to our knowledge, all heart failure studies use exhaled NO (eNO) measurements, whereas our study used nNO measurements. Exhaled NO largely measures NO generated in the lower airway, whereas nNO measures NO produced in the nasal sinuses. The dilution, fluctuations, and confounders that can affect lower airway eNO measurements are important reasons why we believe nNO may be a superior measurement compared with eNO.
In contrast to our nNO results, a study by Hare et al concluded that adult patients with heart failure have increased eNO.33 Conversely, a few years after the publication by Hare et al,33 Katz et al showed results similar to ours, where adults with low eNO were more likely to experience the same end point we examined, that of HTx or death.34 Indeed, Hare et al33 point out, in their discussion, that there are several conflicting studies on eNO and heart failure. Again, we believe nNO is likely more sensitive as a marker for systemic NO bioavailability compared with lower airway NO (eNO), because the eNO would be more diluted by the larger lung volume compared with nNO measurements. Also of great importance in studying patients with CHD is the fact that nNO measurement does not require patient cooperation, which allows for data to be more easily obtained from the pediatric population, including infants. Overall, we agree with both Hare et al33 and Katz et al34 that airway NO, at least to some degree, does, in fact, reflect endothelial NO production capacity, and this is supported by the fact that all NOS isoforms, including endothelial NOS, have been identified in nasal/airway epithelium.13
Although some heart failure studies have yielded evidence of pathophysiologic features arising from increased peroxynitrite production attributable to elevated NO production, we note at the same time that there is also extensive evidence of decreased NO bioavailability playing a role in heart failure. Relevant to this is a point emphasized in the Pall review, where it is described that a basic mechanism of the NO cycle in disease must involve local effects, given the limited half‐lives of NO metabolites in biological tissues.10 This would suggest, in the context of CHD, given the likely presence of oxidative stress, there may be NOS uncoupling and alterations of numerous bioenergetic mechanisms (mitochondrial dysfunction, intracellular calcium abnormalities, and tetrahydrobiopterin depletion) that could affect NO levels. Thus, the role of NO in the pathophysiologic features of heart failure in patients with CHD will require further analysis of the complex interplay between different NOS isoforms in regulating myocardial versus vascular NO homeostasis and its effects on systemic NO.
Limitations of the Study
Despite having >600 subjects in this study, we recognize the sample sizes for the composite end point and post‐HTx analyses were relatively small; hence, these were included only as secondary analyses. We acknowledge that 7 years may not be sufficient time to experience HTx/death attributable to CHD, especially for those enrolled in the later years of the study.
Another limitation is the fact that we do not have oxygen saturation values for 12% and transthoracic echocardiograms for 2% of the CHD cohort. The standard practice of our pediatric cardiologists does not include routine measurement of oxygen saturation on predominantly healthy patients with biventricular CHD being seen at the outpatient clinic for routine assessment.
In addition, we chose to apply the Bonferroni adjustment to our P values for analyses with multiple comparisons/hypotheses. This highly conservative adjustment, while accurately identifying statistical insignificance, may also result in false negatives by rejecting clinically significant associations of low nNO with various physiologic parameters. We chose to err on the conservative side to ensure our study does not overstate the evidence supporting the potential use of nNO as a clinical biomarker for assessing heart failure risk.
Conclusion
Low nNO observed in some patients with CHD is especially prevalent in those with sysRV, suggesting a subpopulation of patients with CHD may have a defect in NO generation and/or overuse. Low nNO was associated with increased risk of HTx and/or mortality, with low nNO continuing to be highly prevalent in the patients after HTx. This would suggest the low nNO results from intrinsic patient factors and is not solely attributable to the physiologic characteristics of the congenitally malformed heart.
Sources of Funding
Lo is supported by grants from the National Institutes of Health (NIH; R01GM104412 and HL132024) and Department of Defense (DOD) (W81XWH‐15‐1‐0649). Adams is supported, in part, by a training grant from the NIH (T32GM075770). We thank the Heart Institute at the Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center for their support.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods.
Table S1. Prevalence of Low nNO Between Various Patient Characteristics for Both CHD and Control Subjects
Table S2. Spectrum of CHD Subtypes and Proportions With Low nNO (nNO), SV‐CHD, and sysRV
Table S3. Comparison of nNO Values for Physiologic Variables in CHD Subjects ≥10 Years‐Old
Table S4. Congenital Heart Disease (CHD) Patients Used for the Survival Analysis. Censored Patients Were Matched Based on CHD Type and Age at nNO Measurements
Table S5. Variable Comparison Between the Low nNO Versus Normal nNO Groups Used in the Composite Endpoint Analysis
Table S6. List of Patients Who Underwent HTx With Pre‐ and/or Post‐HTx nNO Measurements
Figure S1. Bar graph illustrating the prevalence of low nNO by specific CHD phenotypes (magenta bars).
(J Am Heart Assoc. 2017;6:e007447 DOI: 10.1161/JAHA.117.007447.)29212650
References
- 1. Gilboa SM, Devine OJ, Kucik JE, Oster ME, Riehle‐Colarusso T, Nembhard WN, Xu P, Correa A, Jenkins K, Marelli AJ. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 3. Raissadati A, Nieminen H, Haukka J, Sairanen H, Jokinen E. Late causes of death after pediatric cardiac surgery: a 60‐year population‐based study. J Am Coll Cardiol. 2016;68:487–498. [DOI] [PubMed] [Google Scholar]
- 4. Rossano JW, Dipchand AI, Edwards LB, Goldfarb S, Kucheryavaya AY, Levvey BJ, Lund LH, Meiser B, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: nineteenth pediatric heart transplantation report‐2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35:1185–1195. [DOI] [PubMed] [Google Scholar]
- 5. Norozi K, Wessel A, Alpers V, Arnhold JO, Geyer S, Zoege M, Buchhorn R. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol. 2006;97:1238–1243. [DOI] [PubMed] [Google Scholar]
- 6. Hinton RB, Ware SM. Heart failure in pediatric patients with congenital heart disease. Circ Res. 2017;120:978–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamour JM, Kanter KR, Naftel DC, Chrisant MR, Morrow WR, Clemson BS, Kirklin JK. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol. 2009;54:160–165. [DOI] [PubMed] [Google Scholar]
- 8. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 9. Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite‐Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. [DOI] [PubMed] [Google Scholar]
- 10. Pall ML. The NO/ONOO‐cycle as the central cause of heart failure. Int J Mol Sci. 2013;14:22274–22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paulus WJ. The role of nitric oxide in the failing heart. Heart Fail Rev. 2001;6:105–118. [DOI] [PubMed] [Google Scholar]
- 12. Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim‐Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–H2035. [DOI] [PubMed] [Google Scholar]
- 13. Furukawa K, Harrison DG, Saleh D, Shennib H, Chagnon FP, Giaid A. Expression of nitric oxide synthase in the human nasal mucosa. Am J Respir Crit Care Med. 1996;153:847–850. [DOI] [PubMed] [Google Scholar]
- 14. Jackson CL, Lucas JS, Walker WT, Owen H, Premadeva I, Lackie PM. Neuronal NOS localises to human airway cilia. Nitric Oxide. 2015;44:3–7. [DOI] [PubMed] [Google Scholar]
- 15. Collins SA, Gove K, Walker W, Lucas JS. Nasal nitric oxide screening for primary ciliary dyskinesia: systematic review and meta‐analysis. Eur Respir J. 2014;44:1589–1599. [DOI] [PubMed] [Google Scholar]
- 16. Manna A, Montella S, Maniscalco M, Maglione M, Santamaria F. Clinical application of nasal nitric oxide measurement in pediatric airway diseases. Pediatr Pulmonol. 2015;50:85–99. [DOI] [PubMed] [Google Scholar]
- 17. Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, Yagi H, Khalifa O, Kureshi S, Chatterjee B, Sabol SL, Swisher M, Connelly PS, Daniels MP, Srinivasan A, Kuehl K, Kravitz N, Burns K, Sami I, Omran H, Barmada M, Olivier K, Chawla KK, Leigh M, Jonas R, Knowles M, Leatherbury L, Lo CW. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125:2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garrod AS, Zahid M, Tian X, Francis RJ, Khalifa O, Devine W, Gabriel GC, Leatherbury L, Lo CW. Airway ciliary dysfunction and sinopulmonary symptoms in patients with congenital heart disease. Ann Am Thorac Soc. 2014;11:1426–1432. [DOI] [PubMed] [Google Scholar]
- 19. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. [DOI] [PubMed] [Google Scholar]
- 20. Adams PS, Tian X, Zahid M, Khalifa O, Leatherbury L, Lo CW. Establishing normative nasal nitric oxide values in infants. Respir Med. 2015;109:1126–1130. [DOI] [PubMed] [Google Scholar]
- 21. Lundberg JO, Weitzberg E. Nasal nitric oxide in man. Thorax. 1999;54:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pellens M, Gillijns H, Van de Werf F, Bloch KD, Janssens S. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007;50:808–817. [DOI] [PubMed] [Google Scholar]
- 23. Gielis JF, Lin JY, Wingler K, Van Schil PE, Schmidt HH, Moens AL. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic Biol Med. 2011;50:765–776. [DOI] [PubMed] [Google Scholar]
- 24. Gladwin MT. Role of the red blood cell in nitric oxide homeostasis and hypoxic vasodilation. Adv Exp Med Biol. 2006;588:189–205. [DOI] [PubMed] [Google Scholar]
- 25. Tejero J, Gladwin MT. The globin superfamily: functions in nitric oxide formation and decay. Biol Chem. 2014;395:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Feng Q. NOing the heart: role of nitric oxide synthase‐3 in heart development. Differentiation. 2012;84:54–61. [DOI] [PubMed] [Google Scholar]
- 27. Zhou K, Wang Y, Peng W, Sun J, Qing YM, Mo XM. Genetic variants of the endothelial NO synthase gene (eNOS) may confer increased risk of sporadic congenital heart disease. Genet Mol Res. 2014;13:3805–3811. [DOI] [PubMed] [Google Scholar]
- 28. Tang L, Wang H, Ziolo MT. Targeting NOS as a therapeutic approach for heart failure. Pharmacol Ther. 2014;142:306–315. [DOI] [PubMed] [Google Scholar]
- 29. Dodd DA. Pediatric heart failure and transplantation: where are we in 2013? Curr Opin Pediatr. 2013;25:553–560. [DOI] [PubMed] [Google Scholar]
- 30. Bogossian H, Frommeyer G, Ninios I, Bandorski D, Seyfarth M, Matzaroglou C, Lemke B, Eckardt L, Zarse M, Kafchitsas K. Expression of NO synthase under medication with cyclosporine A, mycophenolate mofetil, and tacrolimus during development of transplant vasculopathy on rat cardiac allograft. Cardiovasc Ther. 2016;34:183–190. [DOI] [PubMed] [Google Scholar]
- 31. Fruhwurth S, Krieger S, Winter K, Rosner M, Mikula M, Weichhart T, Bittman R, Hengstschlager M, Stangl H. Inhibition of mTOR down‐regulates scavenger receptor, class B, type I (SR‐BI) expression, reduces endothelial cell migration and impairs nitric oxide production. Biochim Biophys Acta. 2014;1841:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017;105:48–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hare JM, Nguyen GC, Massaro AF, Drazen JM, Stevenson LW, Colucci WS, Fang JC, Johnson W, Givertz MM, Lucas C. Exhaled nitric oxide: a marker of pulmonary hemodynamics in heart failure. J Am Coll Cardiol. 2002;40:1114–1119. [DOI] [PubMed] [Google Scholar]
- 34. Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Prevalence of Low nNO Between Various Patient Characteristics for Both CHD and Control Subjects
Table S2. Spectrum of CHD Subtypes and Proportions With Low nNO (nNO), SV‐CHD, and sysRV
Table S3. Comparison of nNO Values for Physiologic Variables in CHD Subjects ≥10 Years‐Old
Table S4. Congenital Heart Disease (CHD) Patients Used for the Survival Analysis. Censored Patients Were Matched Based on CHD Type and Age at nNO Measurements
Table S5. Variable Comparison Between the Low nNO Versus Normal nNO Groups Used in the Composite Endpoint Analysis
Table S6. List of Patients Who Underwent HTx With Pre‐ and/or Post‐HTx nNO Measurements
Figure S1. Bar graph illustrating the prevalence of low nNO by specific CHD phenotypes (magenta bars).


