Abstract
Background
Subclinical atrial fibrillation is one possible cause of embolic stroke of undetermined source (ESUS). It remains to be elucidated if a specific infarction site has a predictive value for detecting subclinical atrial fibrillation. We aimed to investigate the predictive value of infarction site in patients with ESUS for the detection of atrial tachyarrhythmia (AT) using an insertable cardiac monitor.
Methods and Results
Consecutive 146 patients (84 men; aged 62±12 years) underwent insertable cardiac monitor implantation after diagnosis of ESUS. The detection of AT >30 seconds was evaluated. The ESUS infarction sites were categorized into internal carotid artery and vertebral artery (VA) territories, with ophthalmic artery, anterior cerebral artery, and middle cerebral artery as internal carotid artery subterritories, and posterior cerebral artery and other vertebrobasilar arteries as VA subterritories. During a median follow‐up of 387 days, AT was detected in 33 patients (23%). Subclinical AT detection was significantly more frequent after VA territorial infarction opposed to internal carotid artery infarction (20/57 [35%] versus 13/89 [15%]; P=0.0039). Kaplan‐Meier analysis demonstrated a significantly higher AT detection rate after VA infarction (log‐rank, P=0.0076). Regression analysis revealed that VA territorial infarction, and particularly posterior cerebral artery area infarction, was an independent predictor of AT detection.
Conclusions
Patients with ESUS in the posterior cerebral artery territory had a higher rate of subclinical AT detection than those with other infarct localizations. Our data suggest that the possible usefulness of ESUS site to identify candidates for direct oral anticoagulation should be confirmed in future research.
Keywords: atrial fibrillation, embolic stroke, ischemic, stroke
Subject Categories: Ischemic Stroke, Atrial Fibrillation
Clinical Perspective
What Is New?
Patients with embolic stroke of undetermined source in the territory of the vertebral artery (especially the posterior cerebral artery) had a higher incidence of subclinical atrial tachyarrhythmias compared with other embolic stroke of undetermined source locations during follow‐up using an insertable cardiac monitor.
What Are the Clinical Implications?
The site of embolic stroke of undetermined source may be a possible indicator to help identify patients who would benefit from oral anticoagulation, instead of antiplatelet therapy.
The validity of including the infarction site in therapeutic decision making in patients with embolic stroke of undetermined source should be confirmed in further research.
After the diagnosis of stroke, if the source of embolism remains unidentified after thorough diagnostic workup, the diagnosis of embolic stroke of undetermined source (ESUS) should be given.1
The current guidelines recommend the use of antiplatelet agents rather than oral anticoagulation (OAC) in patients with ESUS, unless atrial fibrillation (AF) has been demonstrated.2 However, even under antiplatelet therapy, patients with ESUS have a higher risk of stroke recurrence compared with patients with stroke of other causes.3
Up to 30% of patients with diagnosis of cryptogenic stroke showed “covert” AF and subsequently had an indication for OAC.4 Other possible causes of cryptogenic stroke are myxomatous valvulopathy, aortic valve stenosis, atrial septal aneurysm, patent foramen ovale, and tumor emboli from occult cancer.1
Although these previously confirmed data suggest that a considerable number of patients diagnosed as having ESUS may be candidates for OAC to prevent stroke recurrence, clinical predictors of covert AF in ESUS have not been determined yet.
In the present study, we intended to evaluate the relationship between the localization of ESUS and the detection of atrial tachyarrhythmias (ATs) after implantation of an insertable cardiac monitor (ICM).
Methods
The study population consisted of consecutive patients who underwent ICM implantation after diagnosis of ESUS and without history of AF or atrial flutter in our hospital between August 1, 2011 and May, 31 2016.
ESUS was defined according to the criteria proposed by the Cryptogenic Stroke/ESUS International Working Group, as follows: (1) stroke detected by computed tomography or magnetic resonance imaging that is not lacunar, (2) absence of extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in arteries supplying the area of ischemia, (3) no major‐risk cardioembolic source, and (4) no other specific cause of stroke identified (eg, arteritis, dissection, migraine/vasospasm, or drug misuse).1
The infarction sites were categorized into internal carotid artery and vertebral artery (VA) territories, with ophthalmic artery, anterior cerebral artery, middle cerebral artery as internal carotid artery subterritories, and posterior cerebral artery (PCA) and other vertebrobasilar arteries (ie, cerebellum and brainstem) as VA subterritories.
Twelve‐lead electrocardiography, transthoracic or transesophageal echocardiography, and cardiac monitoring at least for 24 hours were performed before determining the indication for ICM implantation.
After written informed consent, the patients underwent ICM implantation under local anesthesia. The patients were routinely followed up in the outpatient clinic, and the detection of AT was evaluated. AT was defined as AF and atrial flutter documented over 30 seconds. Time 0 was the day of ICM implantation, and observation was censored at first AT detection, end of observation, or explantation of ICM.
Continuous data were shown as mean±SD. In case of nonnormal distributed data, they were shown as median value (lower‐upper quartile). The χ2 test, Kruskal‐Wallis test, Student t test, Fisher exact test, or 1‐way analysis of variance was performed when appropriate. For global test statistics, we used a significance level of 5%. To evaluate the relationship between AT detection and ESUS sites, Cox regression analysis was performed, incorporating other clinical characteristics (sexuality, age, and comorbidities). For the multivariable regression analysis, we have included the variables that showed statistical significance in the single‐variable regression analysis. The analyses of our data were performed using JMP (SAS, Version 10).
This study was approved by local institutional review board. All authors had full access to the data, and have read and agreed to the article as written.
Results
In total, 146 patients consecutively underwent ICM implantation without any complication. Patient characteristics are shown in Table 1.
Table 1.
Baseline Characteristics
| Characteristics | Value (N=146) |
|---|---|
| Male sex, n (%) | 84 (58) |
| Age, mean±SD, y | 62.0±12.1 |
| Hypertension, n (%) | 107 (73) |
| Diabetes mellitus, n (%) | 23 (16) |
| Coronary artery disease, n (%) | 17 (12) |
| CHA2DS2‐VASc score, mean±SD | 4.1±1.3 |
| Follow‐up after implantation of ICM, median (lower‐upper quartile), d | 387 (283–552) |
ICM indicates insertable cardiac monitor.
Figure 1 shows infarction sites of ESUS diagnosed by magnetic resonance imaging or computed tomography. More than 50% of infarction was localized in the territory of the middle cerebral artery. The infarction in the territory of the PCA, and in the other vertebrobasilar artery, accounted for 22%, and 15%, respectively.
Figure 1.
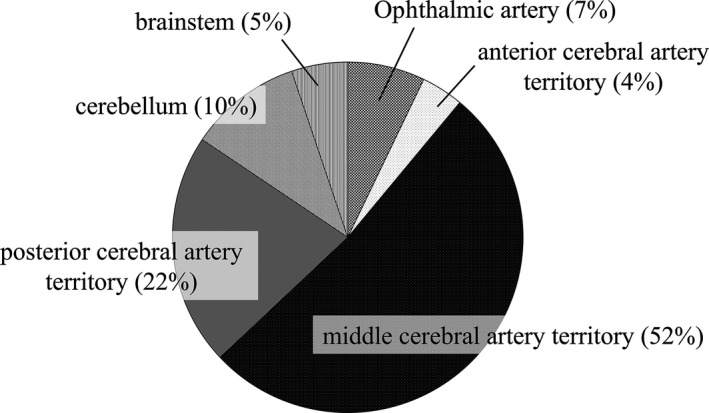
Localization of infarction sites. Approximately 50% of patients in our cohort had strokes in the middle cerebral artery territory. Infarction in the territory of the vertebrobasilar system was noted in ≈37% of patients.
During a median follow‐up of 387 (lower‐upper quartile, 283–552) days, AT >30 seconds was detected in 33 patients (23%). The detected AT in 3 patients was atrial flutter, and AF was detected in the remaining 30 patients.
The patients’ characteristics according to AT detection are shown in Table 2. There were no significant differences in clinical characteristics and comorbidities between patients with and without AT detection, except for age, which tended to be higher in patients with AT detection.
Table 2.
AT Detection and Baseline Characteristics
| Characteristics | AT Detection (Absent) (N=113) | AT Detection (Present) (N=33) | P Value |
|---|---|---|---|
| Male sex, n (%) | 67 (59) | 17 (52) | 0.43 |
| Age, mean±SD, y | 61.0±12.3 | 65.2±11.3 | 0.084 |
| Hypertension, n (%) | 84 (74) | 23 (70) | 0.60 |
| Diabetes mellitus, n (%) | 20 (18) | 3 (9) | 0.23 |
| Coronary heart disease, n (%) | 13 (12) | 4 (12) | 0.92 |
| PFO, n (%) | 19 (17) | 4 (12) | 0.52 |
| CHA2DS2‐VASc score, mean±SD | 4.0±1.3 | 4.3±1.5 | 0.35 |
| Follow‐up, median (lower‐upper quartile), d | 398 (283–552) | 369 (282–550) | 0.99 |
AT indicates atrial tachyarrhythmia; and PFO, patent foramen ovale.
AT detection according to the infarction sites is shown in Table 3. There were no patients with ophthalmic artery symptoms at the diagnosis of ESUS in whom AT was detected. The patients with AT detection showed higher prevalence of infarction in the territory of PCA compared with patients without AT detection (13/33 [39%] versus 20/113 [18%]; P=0.0088). There was no significant difference in incidence of AT detection in the other territories.
Table 3.
AT Detection According to Infarct Site
| Infarct Site | AT Detection (Absent) (N=113) (117 Sites) | AT Detection (Present) (N=33) (37 Sites) | P Value |
|---|---|---|---|
| Ophthalmic artery | 11 (9.7) | 0 (0) | 0.070 |
| Anterior cerebral artery | 5 (4.4) | 1 (3.0) | 1.0 |
| Middle cerebral artery | 64 (57) | 16 (48) | 0.41 |
| Posterior cerebral artery | 20 (18) | 13 (39) | 0.0088 |
| OVAs | 17 (15) | 7 (21) | 0.43 |
Data are given as number (percentage). AT indicates atrial tachyarrhythmia; and OVA, other vertebrobasilar artery.
Kaplan‐Meier analysis demonstrated that the patients with ESUS in the territory of VA showed a higher AT detection rate compared with the patients with ESUS in the internal carotid artery territory (log‐rank, P=0.0076; Figure 2A). Particularly, Kaplan‐Meier analysis demonstrated the higher incidence of AT detection in patients with PCA territory infarction opposed to the patients with other territory infarction (log‐rank, P=0.0091; Figure 2B).
Figure 2.
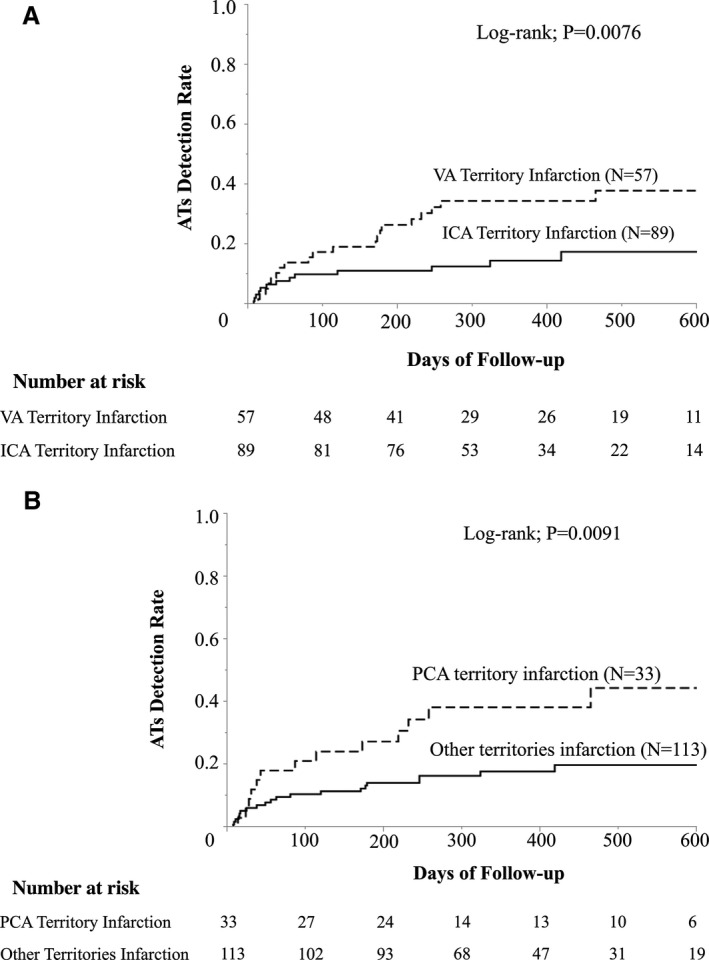
Kaplan‐Meier analysis on atrial tachyarrhythmia (AT) detection during follow‐up. A, Vertebrobasilar system infarction and anterior system infarction. Atrial tachycardia detection incidence was significantly higher in patients with vertebral artery (VA) territorial infarction compared with internal carotid artery (ICA) territorial infarction. B, Posterior cerebral artery infarction and all other infarctions. Atrial tachycardia detection incidence was significantly higher in patients with infarction in the posterior cerebral artery territory opposed to the patients with infarction in other subterritories.
Table 4 shows the results of regression analysis. Single‐variable regression analyses showed that age and the location of ESUS in the PCA territory were statistically significant indicators of AT detection. Multivariable regression analysis incorporating these 2 indicators demonstrated that ESUS in the territory of PCA was an independent predictor of AT detection. Other clinical characteristics and other ESUS sites could not predict the incidence of AT. In addition, the calculated CHA2DS2‐VASc score was not a significant predictor of AT detection by single‐variable analysis (hazard ratio, 1.15; 95% confidence interval, 0.88–1.46; P=0.30).
Table 4.
Result of Single‐Variable and Multivariable Analysis for Detection of Atrial Tachycardia
| Variable | Single‐Variable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Anterior cerebral artery | 0.77 (0.04–3.60) | 0.79 | … | … |
| Middle cerebral artery | 0.82 (0.41–1.64) | 0.58 | … | … |
| Posterior cerebral artery | 2.46 (1.19–4.89) | 0.016 | 2.27 (1.09–4.57) | 0.029 |
| OVAs | 1.30 (0.52–2.84) | 0.55 | … | … |
| Male sex | 0.75 (0.38–1.50) | 0.41 | … | … |
| Age (1 y) | 1.03 (1.00–1.06) | 0.050 | 1.02 (1.00–1.06) | 0.093 |
| Hypertension | 0.80 (0.39–1.75) | 0.55 | … | … |
| Diabetes mellitus | 0.51 (0.12–1.44) | 0.23 | … | … |
| Coronary heart disease | 1.04 (0.31–2.66) | 0.94 | … | … |
| PFO | 0.75 (0.22–1.91) | 0.58 | … | … |
CI indicates confidence interval; HR, hazard ratio; OVA, other vertebrobasilar artery; and PFO, patent foramen ovale.
Discussion
The main finding of this study is that patients with ESUS in the territory of VA, in particular in the territory of PCA, independently showed a higher incidence of subclinical AT detection. To the best of our knowledge, this is a first detailed analysis on the relationship between infarction site in ESUS and the subsequent detection of AT after ICM implantation.
The current guidelines and the data recommend antiplatelet therapy after a diagnosis of ESUS.2, 5 However, a recent study that demonstrated a higher recurrence rate suggested that this therapeutic decision without any “determined source of embolism” may not be sufficient.6
Previously, a subgroup analysis demonstrated that warfarin therapy was superior to aspirin in patients with cryptogenic stroke.7 Several randomized studies are underway to evaluate the efficacy of direct anticoagulation after ESUS.8, 9
Our data in the present study may support the hypothesis that patients with ESUS in the PCA territory can be candidates for OAC, instead of antiplatelet therapy, compared with patients with ESUS in the other territories. This hypothesis should be confirmed in a larger randomized trial. The confirmation of these data may identify patients with ESUS in the PCA territory as candidates for direct OAC.
The mechanism of higher AT detection in patients with ESUS in the territory of VA, in particular of PCA, could not be clarified in the present study. Several studies demonstrated that the cardiac embolism attributable to patent foramen ovale favors the posterior circulation, particularly the PCA region.10, 11 Kim et al reported that a patent foramen ovale stroke was more frequently observed in the vertebrobasilar artery territory compared with an AF stroke. However, their data were obtained from the patients who had been already diagnosed as having AF. Therefore, the patient population of the present study (ESUS) is different, and in the patients with ESUS, we report herein, for the first time, the relevance of the ESUS location for subsequent subclinical AF documentation using ICM. Sacco et al reported that warfarin showed an advantage in preventing recurrent stroke compared with aspirin for patients after stroke in the posterior circulation‐sparing brainstem.7 Our data in the present study correspond well to their report, in that PCA stroke may be more frequently related to AF than other stroke localizations in ESUS.
In this study, no AT was detected in the patients with infarctions in the ophthalmic artery. This corresponds to a recent study by Golsari et al, which revealed that in the pathogenesis of acute retinal ischemia, large‐artery atherosclerosis plays a major role, although the number of patients with ophthalmic artery territorial infarction was small in our cohort.12
This study has some limitations. This is a single‐center cohort study with relatively few patients. All these patients were referred to us after diagnostic workup did not reveal a clear cause for stroke. Therefore, there could be a referral bias because of the feature of our institute as a university hospital, and the distribution of infarct location could be different from that in the general patient population with stroke. However, we used established criteria for the diagnosis of ESUS1 and have consecutively enrolled the patients; the mean follow‐up of patients was ≈1 year, with a minimum follow‐up of 3 months. Accordingly, the detection rate of subclinical AT/AF corresponded well to those of the previous publications, which suggests that the patient population in the present study should not be strongly biased.13, 14 Therefore, we believe the validity of our data in the present study.
Conclusions
Patients with ESUS in the territory of the PCA had a higher detection rate of subclinical ATs than other infarct localizations. Our data suggest the possible usefulness of ESUS site to identify candidates for direct OAC. Further studies should be conducted to investigate the validity of including the infarction site in therapeutic decision making in patients with ESUS.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e007448 DOI: 10.1161/JAHA.117.007448.)29187386
References
- 1. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke/ESUS International Working Group . Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 2. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 3. Putaala J, Nieminen T, Haapaniemi E, Meretoja A, Rantanen K, Heikkinen N, Kinnunen J, Strbian D, Mustanoja S, Curtze S, Pakarinen S, Lehto M, Tatlisumak T. Undetermined stroke with an embolic pattern: a common phenotype with high early recurrence risk. Ann Med. 2015;47:406–413. [DOI] [PubMed] [Google Scholar]
- 4. Glotzer TV, Ziegler PD. Cryptogenic stroke: is silent atrial fibrillation the culprit? Heart Rhythm. 2015;12:234–241. [DOI] [PubMed] [Google Scholar]
- 5. Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen‐Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, Alonso‐Coello P, Guyatt GH, Akl EA. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e601S–e636S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ntaios G, Papavasileiou V, Milionis H, Makaritsis K, Vemmou A, Koroboki E, Manios E, Spengos K, Michel P, Vemmos K. Embolic strokes of undetermined source in the Athens Stroke Registry: an outcome analysis. Stroke. 2015;46:2087–2093. [DOI] [PubMed] [Google Scholar]
- 7. Sacco RL, Prabhakaran S, Thompson JL, Murphy A, Sciacca RR, Levin B, Mohr JP; WARSS Investigators . Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin‐Aspirin Recurrent Stroke Study. Cerebrovasc Dis. 2006;22:4–12. [DOI] [PubMed] [Google Scholar]
- 8. Diener HC, Easton JD, Granger CB, Cronin L, Duffy C, Cotton D, Brueckmann M, Sacco RL; RE‐SPECT ESUS Investigators . Design of Randomized, double‐blind, Evaluation in secondary Stroke Prevention comparing the EfficaCy and safety of the oral Thrombin inhibitor dabigatran etexilate vs. acetylsalicylic acid in patients with Embolic Stroke of Undetermined Source (RE‐SPECT ESUS). Int J Stroke. 2015;10:1309–1312. [DOI] [PubMed] [Google Scholar]
- 9. Geisler T, Poli S, Meisner C, Schreieck J, Zuern CS, Nägele T, Brachmann J, Jung W, Gahn G, Schmid E, Bäezner H, Keller T, Petzold GC, Schrickel JW, Liman J, Wachter R, Schön F, Schabet M, Lindner A, Ludolph AC, Kimmig H, Jander S, Schlegel U, Gawaz M, Ziemann U. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): rationale and study design. Int J Stroke. 2016. pii: 1747493016681019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10. Kim BJ, Kim NY, Kang DW, Kim JS, Kwon SU. Provoked right‐to‐left shunt in patent foramen ovale associates with ischemic stroke in posterior circulation. Stroke. 2014;45:3707–3710. [DOI] [PubMed] [Google Scholar]
- 11. Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, Kwon SU. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke. 2013;44:3350–3356. [DOI] [PubMed] [Google Scholar]
- 12. Golsari A, Bittersohl D, Cheng B, Griem P, Beck C, Hassenstein A, Nedelmann M, Magnus T, Fiehler J, Gerloff C, Thomalla G. Silent brain infarctions and leukoaraiosis in patients with retinal ischemia: a prospective single‐center observational study. Stroke. 2017;48:1392–1396. [DOI] [PubMed] [Google Scholar]
- 13. Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology. 2013;80:1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J; CRYSTAL AF Investigators . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]


