Abstract
Background
Percutaneous edge‐to‐edge mitral valve repair (PMVR) has become an established treatment option for mitral regurgitation in patients not eligible for surgical repair. Currently, most procedures are performed under general anesthesia (GA). An increasing number of centers, however, are performing the procedure under deep sedation (DS). Here, we compared patients undergoing PMVR with GA or DS.
Methods and Results
A total of 271 consecutive patients underwent PMVR at our institution between May 2014 and December 2016. Seventy‐two procedures were performed under GA and 199 procedures under DS. We observed that in the DS group, doses of propofol (743±228 mg for GA versus 369±230 mg for DS, P<0.001) and norepinephrine (1.1±1.6 mg for GA versus 0.2±0.3 mg for DS, P<0.001) were significantly lower. Procedure time, fluoroscopy time, and dose area product were significantly higher in the GA group. There was no significant difference between GA and DS with respect to overall bleeding complications, postinterventional pneumonia (4% for GA versus 5% for DS), or C‐reactive protein levels (361±351 nmol/L for GA versus 278±239 nmol/L for DS). Significantly fewer patients with DS needed a postinterventional stay in the intensive care unit (96% for GA versus 19% for DS, P<0.001). Importantly, there was no significant difference between DS and GA regarding intrahospital or 6‐month mortality.
Conclusions
DS for PMVR is safe and feasible. No disadvantages with respect to procedural outcome or complications in comparison to GA were observed. Applying DS may simplify the PMVR procedure.
Keywords: anesthesia, mitral regurgitation, percutaneous mitral valve repair
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Treatment
Clinical Perspective
What Is New?
Deep sedation in patients undergoing percutaneous mitral valve repair (PMVR) is not associated with an increased rate of pneumonia compared with procedures performed under general anesthesia.
PMVR performed under deep sedation results in reduction of mitral regurgitation similar to PMVR performed under general anesthesia.
Performing PMVR under deep sedation reduces the need for catecholamines as well as postinterventional intensive care unit and overall hospital length of stay.
What Are the Clinical Implications?
Patients undergoing PMVR are often frail, present with multiple comorbidities, and experience severe heart failure.
PMVR performed under deep sedation is safe and comparable to general anesthesia with regard to mitral regurgitation reduction.
Thus, deep sedation may be considered an alternative to general anesthesia in patients undergoing PMVR.
Introduction
Mitral valve regurgitation (MR) is one of the most common valvular diseases worldwide and leads to heart failure and other potentially severe complications.1 Since there is still a high rate of patients with an indication for mitral valve repair, who cannot receive surgical repair because of an increased perioperative risk,2 percutaneous edge‐to‐edge mitral valve reconstruction (PMVR) using the MitraClip system (Abbott Vascular) has become a safe and viable alternative to treat severe mitral regurgitation in these patients.3 The 5‐year results of the EVEREST II (Endovascular Valve Edge‐to‐Edge Repair Study II) randomized trial suggest that patients treated with PMVR more commonly required surgery for residual MR during the first year after treatment, while, afterwards, comparably low rates of surgery for mitral valve dysfunction with either PMVR or surgical therapy endorse the durability of MR reduction with both repair techniques.3 Most PMVR procedures are currently performed under general anesthesia (GA),4, 5 whereas other percutaneous interventional approaches to treat valve diseases such as transcatheter aortic valve replacement are increasingly performed under conscious sedation or deep sedation (DS).6 While GA has advantages such as the absence of a relevant risk for aspiration, there may also be potential disadvantages. For instance, perioperative episodes of hypotension can be observed regularly in patients undergoing GA.7 Among other factors, this hypotension is caused by the vasodilatory effect of anesthetic drugs.8 Therefore, GA may result in an increased use of catecholamines and consecutive catecholamine‐induced cardiotoxicity.8 It has been demonstrated that perioperative hypotension and duration of anesthesia (deep hypnotic time) are predictors for complications and prolonged hospital stay.9 No randomized data are available on the incidence of pulmonary infections or pneumonia after PMVR in GA versus DS. So far, only a few case studies with patients undergoing PMVR in DS or nonrandomized cohort studies addressing this topic were performed10, 11 and additional studies are warranted to clarify the clinical value of this approach.
Here, we addressed whether patients with GA or with DS during PMVR show significant differences in clinical parameters and outcome. For instance, time of hospitalization, need for catecholamines, incidence of pneumonia, and improvement of 6‐minute walk test at follow‐up were analyzed for both approaches.
Methods
Study Population and Preprocedural Evaluation
This study included 271 consecutive patients who underwent PMVR using the MitraClip system between May 2014 and December 2016 at the Department of Cardiology and Cardiovascular Medicine, University of Tuebingen. We retrospectively evaluated clinical and procedural outcomes using medical records of these patients. Before the PMVR procedure, every patient was assessed and judged unsuitable for a surgical approach by an interdisciplinary heart team of cardiologists, cardiothoracic surgeons, and cardio‐anesthesiologists. During these weekly heart team meetings, electronic documents such as videos of transthoracic echocardiography, transesophageal echocardiography (TEE), and coronary angiographies were evaluated and decision was based on these and on the documented and discussed clinical evaluation of each patient. Furthermore, decisions were based on risk scores (EuroSCORE and Society of Thoracic Surgeons score) and comorbidities not covered in these scores, as well as the specific anatomy of the mitral valve. The decisions of the heart team were documented in electronic forms and stored using an electronic system accessible to all departments participating in the heart team decisions.
Written informed consent was given by each patient. The study was approved by our local ethics committee (260/2015R). Preprocedural evaluation standards consisted of transthoracic echocardiography and TEE to determine the grade and etiology of the mitral regurgitation, the left ventricular (LV) ejection fraction, and the anatomy of the mitral valve. In addition to clinical assessment, a full laboratory workup and ECG were obtained. To assess the patient`s functional capacity, we performed a 6‐minute walk test (6MWT) at baseline and follow‐up and analyzed the results, if both parameters were available. Patients who were not able to walk at baseline because of cardiac decompensation or other comorbidities were given a 6MWT of 0 m.
PMVR Procedure
The PMVR procedure was performed in a hybrid operating room. In addition to fluoroscopy, 2‐ and 3‐dimensional TEE were used to guide the procedure. Technical details of the MitraClip procedure have been previously described.12, 13 In summary, a guiding catheter is positioned in the left atrium after transfemoral access and transseptal puncture. Then, the clip is advanced into the left atrium and into the left ventricle below the mitral valve plane. The clip is then retracted and consecutively closed to grasp and coapt the mitral valve leaflets. The clip can be reopened and repositioned in case of a suboptimal result as evaluated by echocardiography and hemodynamic changes.14
Mode of Anesthesia
The PMVR procedure was performed either under GA or under DS. Based on the decision of the heart team, 72 procedures were performed under GA and 199 procedures under DS. While DS has advantages such as a more simplified peri‐interventional setup, PMVR under GA can offer advantages such as the benefit from specific ventilation maneuvers in cases of challenging anatomical conditions.12 GA was performed via endotracheal intubation and mechanical ventilation using total intravenous anesthesia. Extubation was performed as soon as possible after the procedure.
For sedation in the DS group, we used propofol (average dose 369±230 mg) and midazolam (average dose 1.5±1.6 mg) and for pain control we used piritramid (average dose 6.4±2.6 mg). In addition, 10 mL of 2% lidocaine were administered subcutaneously for local anesthesia in the inguinal region in the DS group. Norepinephrine (average dose 1.1±1.6 mg in the GA group and 0.2±0.3 mg in the DS group) was applied intravenously via a central venous line if necessary to keep arterial blood pressure (BP) within normal ranges. For GA, propofol (average dose 743±228 mg) and remifentanil (average dose 2.6±1.8 mg) were used for the procedure. In the DS and GA groups, intraprocedural monitoring included continuous invasive measurement of arterial BP via a radial artery catheter as well as measurement of pulmonary artery pressure with a Swan‐Ganz catheter. Continuous ECG monitoring and pulse oxymetry for transcutaneous arterial oxygen saturation were performed. In addition, patients under GA had continuous expiratory CO2 monitoring. BP goals were a mean arterial pressure of >60 mm Hg and a systolic BP of <140 mm Hg in both groups. BP was measured invasively via an intraarterial line. GA was performed by an anesthesiologist and DS was performed by an anaesthesiologist and/or experienced cardiologist as required by official recommendations and federal law. Decision for transferal of a patient to the intensive care unit (ICU) post intervention was based on the individual situation of each patient, eg, the persistent need for pharmacological circulatory support or the patient's respiratory situation. Time point of extubation in the GA group was decided by the present anesthesiologist. Patients with no need for ICU stay were transferred to the intermediate care ward.
Follow‐Up
Medical records were used to identify the necessity and duration of postprocedural ICU stay, in‐hospital stay, and complications, as well as the overall length of stay. Follow‐up was evaluated routinely after 8±5 months post PMVR. The evaluation contained need for rehospitalization, survival rate, New York Heart Association functional class, clip insertion, necessity and doses of heart failure medication, 6MWT, TEE, transthoracic echocardiography, ECG, and clinical examination. Pneumonia was diagnosed according to the current guidelines.15 Stroke was diagnosed according to neurologic symptoms and imaging studies as recommended in the current guidelines.16
Statistical Analysis
Statistical analysis was performed using SPSS version 24 (IBM). Categorical variables are displayed in percentages and absolute numbers. The level of significance in these variables was tested using chi‐square test. Ordinally scaled and continuous data are shown as mean±standard deviation. Kolmogorov–Smirnov and Shapiro–Wilk tests were used to check for normal distribution. In case of not normally distributed data, Mann–Whitney U test and Wilcoxon log‐rank test were used for intergroup comparisons. Two‐tailed P values were calculated with a P<0.05 considered statistically significant. Patients who had to be converted from DS to GA were analyzed in the DS group on an intention‐to‐treat basis, as previously reported.17
Results
We compared the DS and GA groups with regard to clinical parameters such as baseline characteristics, length of postprocedural ICU and hospital stay, procedural outcome, and postprocedural complications including pneumonia, stroke, and bleeding. In total, 271 patients were treated with the MitraClip system. The mean age was 77±9 years, and 42% were women. The etiology of MR was functional in 56%. A total of 96% of the patients had a preprocedural MR grade III or worse. A total of 49% of patients had an LV ejection fraction <35%, and the mean EuroSCORE II was 11±11 (Table 1). There were significant differences regarding the baseline characteristics between the DS and GA groups. Patients in the GA group were significantly younger (74±10 years in the GA group and 78±8 years in the DS group, P=0.005), but had higher EuroSCORE II levels (14±12 for the GA group and 11±10 for the DS group, P=0.036) and more often New York Heart Association class ≥3 (86% in the GA group versus 73% in the DS group, P=0.023), chronic renal failure (65% in the GA versus 40% in the DS group, P<0.001), and previous cardiac surgery (47% in the GA group versus 30% in the DS group, P=0.009). Pulmonary hypertension was present more commonly in the DS group (51% in the GA group and 71% in the DS group, P=0.002). In addition, the GA and DS groups were balanced and comparable regarding baseline characteristics. Detailed data are depicted in Table 1.
Table 1.
Baseline Characteristics
| All Patients (N=271) | GA (n=72) | DS (n=199) | P Value | |
|---|---|---|---|---|
| Age, y | 77±9 | 74±10 | 78±8 | 0.005 |
| Women | 42 (114) | 38 (27) | 44 (87) | 0.36 |
| NYHA classification (grade) | 3±1 | 3±1 | 3±1 | 0.14 |
| NYHA class ≥3 | 77 (207) | 86 (62) | 73 (145) | 0.023 |
| EuroSCORE II | 11±11 | 14±12 | 11±10 | 0.036 |
| Chronic renal failure | 47 (127) | 65 (47) | 40 (80) | <0.001 |
| Coronary artery disease | 76 (205) | 78 (56) | 75 (149) | 0.62 |
| Atrial fibrillation | 66 (179) | 64 (46) | 67 (133) | 0.65 |
| Pulmonary hypertension | 66 (179) | 51 (37) | 71 (142) | 0.002 |
| Hypertension | 69 (188) | 74 (53) | 68 (135) | 0.36 |
| Diabetes mellitus | 29 (79) | 38 (27) | 26 (52) | 0.69 |
| Insulin dependent | 11 (29) | 10 (7) | 11 (22) | 0.75 |
| Previous cardiac surgery | 35 (94) | 47 (34) | 30 (60) | 0.009 |
| Chronic lung disease | 10 (28) | 11 (8) | 10 (20) | 0.8 |
| Recent myocardial infarction | 13 (36) | 18 (13) | 12 (23) | 0.16 |
| Extracardiac arteriopathy | 26 (70) | 29 (21) | 25 (49) | 0.45 |
| Hyperlipidemia | 46 (124) | 54 (39) | 43 (85) | 0.95 |
| MR severity preprocedural ≥3 | 96 (261) | 99 (71) | 96 (190) | 0.28 |
| MR genesis functional | 56 (152) | 58 (42) | 55 (110) | 0.35 |
| LVEF ≤35% | 49 (134) | 54 (39) | 48 (95) | 0.35 |
Values are expressed as mean±SD or percentage (number). DS indicates deep sedation: GA, general anesthesia; LVEF, left ventricular ejection fraction; MR, mitral valve regurgitation; NYHA, New York Heart Association.
Successful clip implantation was achieved in 98% of the cases, and 95% of the patients had a postprocedural MR grade ≤2+ with no significant differences between the groups (Table 2, Figure S1). In 5 patients (2%), no clip could be implanted. Procedure time was defined as the time from puncture of the femoral vein until closure of the access site and was evaluated using standardized procedure protocols. Significantly shorter procedure time (139±52 minutes in the GA group and 128±63 minutes in the DS group, P=0.031) and fluoroscopy time (23±11 minutes in the GA group versus 16±10 minutes in the DS group, P<0.001) and a smaller dose area product (36±30 Gy/cm2 in the GA group and 25±27 Gy/cm2 in the DS group, P<0.001) were observed in patients under DS (Table 2 and Figure 1). Likewise, the used doses of propofol (743±228 mg in the GA group and 369±230 mg in the DS group, P<0.001) and norepinephrine (1.1±1.6 mg in the GA group versus 0.2±0.3 mg in the DS group, P<0.001) were significantly decreased in the DS group (Table 2 and Figure 2).
Table 2.
Procedural Data, Outcome, and Complications
| All Patients (N=271) | GA (n=72) | DS (n=199) | P Value | |
|---|---|---|---|---|
| Clip implantation successful | 98 (266) | 99 (71) | 98 (195) | 0.74 |
| MR severity postprocedural ≤2 | 95 (258) | 94 (68) | 96 (190) | 0.76 |
| Mean MR reduction (grades) | 2.3±0.6 | 2.3±0.5 | 2.3±0.6 | 0.58 |
| Implantation ≥2 clips | 51 (137) | 60 (44) | 47 (94) | 0.69 |
| Procedure time, min | 131±61 | 139±52 | 128±63 | 0.031 |
| Fluoroscopy time, min | 17±10 | 23±11 | 16±10 | <0.001 |
| Dose area product, Gy/cm2 | 28±29 | 36±30 | 25±27 | <0.001 |
| Postprocedural need for ICU stay | 39 (106) | 96 (69) | 19 (37) | <0.001 |
| Mean postprocedural length of ICU stay, d | 2±4 | 3±7 | 1±3 | <0.001 |
| Stroke/TIA | 1 (3) | 1 (1) | 1 (2) | 0.79 |
| Bleeding according to VARC‐2 criteria | ||||
| All | 14 (38) | 13 (9) | 15 (29) | 0.66 |
| Life‐threatening | 1 (3) | 0 (0) | 2 (3) | 0.3 |
| Major | 9 (23) | 10 (7) | 8 (16) | 0.66 |
| Minor | 4 (12) | 3 (2) | 5 (10) | 0.43 |
| Intrahospital mortality | 4 (12) | 3 (2) | 5 (10) | 0.43 |
| Conversion from DS to GA | 1 (3) | ··· | 2 (3) | ··· |
| Conversion to surgery | 0 | 0 | 0 | ··· |
| Pneumonia | 5 (13) | 4 (3) | 5 (10) | 0.77 |
Values are expressed as mean±SD or percentage (number). DS indicates deep sedation; GA, general anesthesia; ICU, intensive care unit; MR, mitral valve regurgitation; TIA, transient ischemic attack; VARC, Valve Academic Research Consortium.
Figure 1.
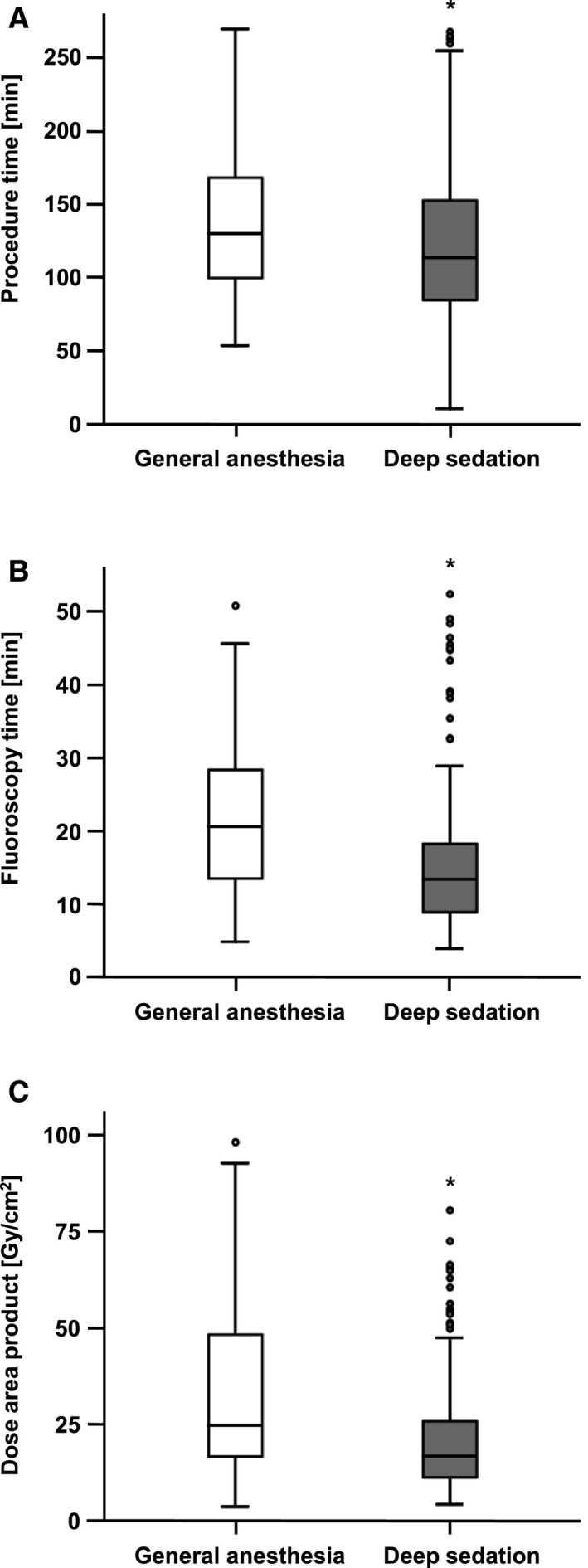
Intraprocedural data comparing deep sedation (DS) vs general anesthesia (GA) groups during percutaneous mitral valve repair. Boxplot diagrams showing values in the DS and GA groups. The boxes consist of the 25% to the 75% quartiles of all measurements. The crossline marks the median of measurements (50% quartile). The whiskers mark the smallest and largest measurements. The circles are outliers. A, The procedure time was significantly shorter in patients undergoing mitral valve repair in DS, *P=0.031. B, Similarly, fluoroscopy time was significantly shorter in the DS group, *P<0.001. C, The dose area product was significantly smaller in the DS group, *P<0.001.
Figure 2.
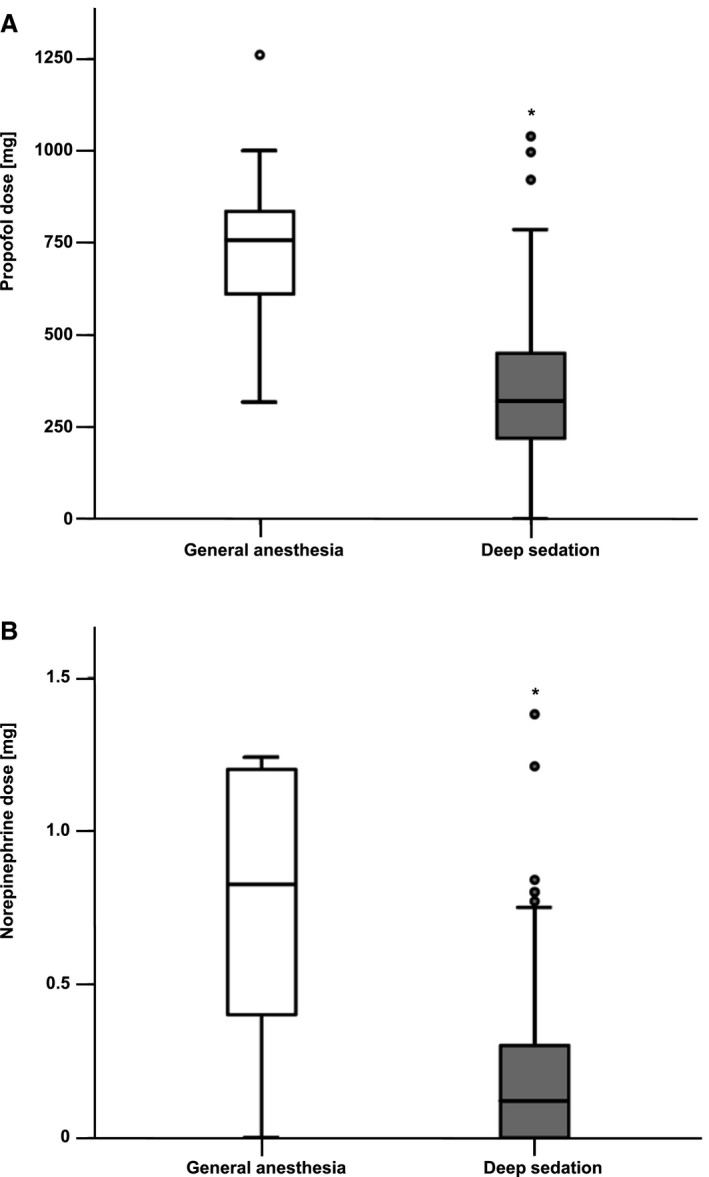
Intraprocedural drug administration during percutaneous mitral valve repair in general anesthesia (GA) vs deep sedation (DS). Boxplot diagrams showing doses in the DS and GA groups. The boxes consist of the 25% to the 75% quartiles of all measurements. The crossline marks the median of measurements (50% quartile). The whiskers mark the smallest and largest measurements. The circles are outliers. A, Compared with GA, the DS group received significantly lower doses of propofol, *P<0.001. B, Norepinephrine doses in the DS group were significantly smaller than in the GA group, *P<0.001.
The occurrence of in‐hospital complications was similar in both groups. There were no significant differences regarding postprocedural bleeding complications (13% in the GA group versus 15% in the DS group, P=0.66) (Figure 3A). Reasons for bleeding complications were groin bleeding, urogenital and gastrointestinal bleeding, endobronchial bleeding, or bleeding from the injection site of the central venous catheter, which led to either minor, major, or life‐threatening bleeding as defined by the VARC‐2 (Valve Academic Research Consortium) criteria (Figure 3B, Table 2, and Table S1). Importantly, there was no significant difference with respect to postprocedural pneumonia (3/72 patients in the GA group and 10/199 patients in the DS group, P=0.77) (Figure 4A). Interestingly, the postprocedural leukocyte (white blood cell) count showed a significant difference between groups (9±3×109/L in the GA group versus 8±3×109/L in the DS group, P=0.018) (Figure 4B), which might be explained by higher catecholamine doses in the GA group. We observed no significant difference in C‐reactive protein levels between the groups (361±351 nmol/L in the GA group and 278±239 nmol/L in the DS group, P=0.10 [not significant (ns)]) (Figure 4C). All patients were given an antibiotic prophylactic treatment for 48 hours post intervention, which consisted of a cephalosporin or, in case of known intolerance, a lincosamide. There was no difference in the frequency of antibiotic treatment that was not given for prophylaxis in the DS and GA groups (Table S2). A total of 3 patients experienced postprocedural stroke/transient ischemic attack, ie, 1% in each group (1/72 patients in the GA and 2/199 patients in the DS group, P=0.79 [ns]) (Table 2). A significantly smaller fraction of patients in the DS than in the GA group needed a postprocedural ICU stay (19% in the DS group versus 96% in the GA group, P<0.001) (Figure 5A). Accordingly, the mean length of postprocedural ICU stay was significantly decreased when the procedure was performed in DS (3±7 for the GA group versus 1±3 for the DS group, P<0.001) (Table 2). None of the patients had to be converted to open heart surgery. Only 3 patients (1%) were converted from DS to GA (Table 2). This was caused by a drop of oxygen saturation in one patient, severe epistaxis in one patient, and accidental bronchial intubation with the TEE probe in one patient. Detailed data on converted patients are given in Table S3.
Figure 3.
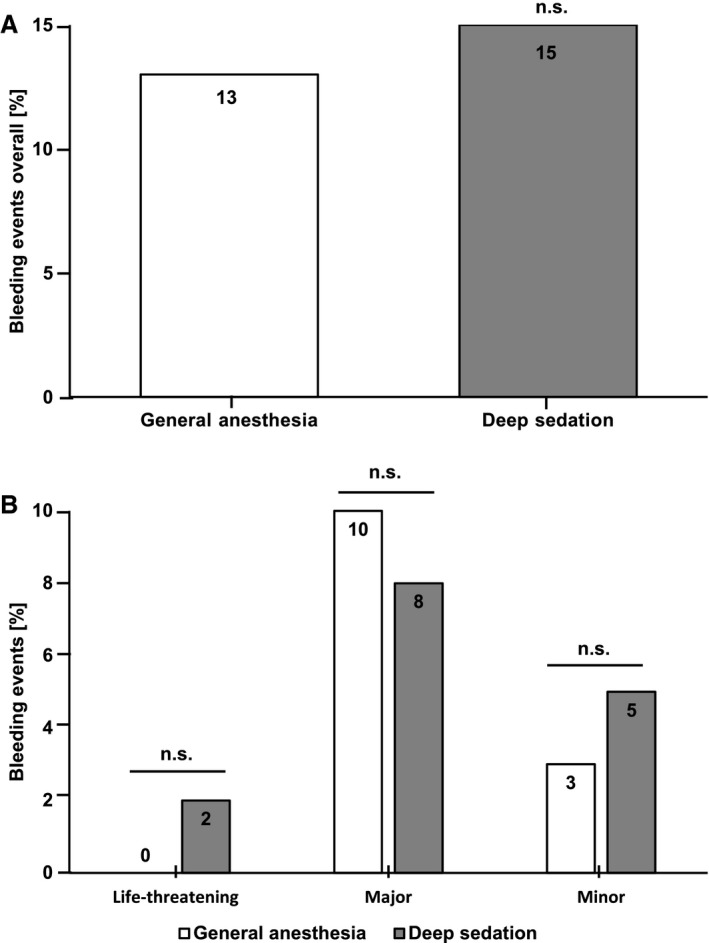
Comparison of postpercutaneous mitral valve repair procedure bleeding complications in the general anesthesia (GA) vs deep sedation (DS) groups. Bar diagrams showing the percentage of patients per group with an event. A, We observed no significant difference in the occurrence of overall bleeding complications during the first postprocedural week. No significant (n.s.) difference between the groups was observed, P=0.66. B, Similarly, distinguishing between life‐threatening (P=0.3), major (P=0.66), and minor (P=0.43) bleeding complications, there was no significant difference in the occurrence of bleeding complications during the first postprocedural week. No significant difference between the groups was observed.
Figure 4.
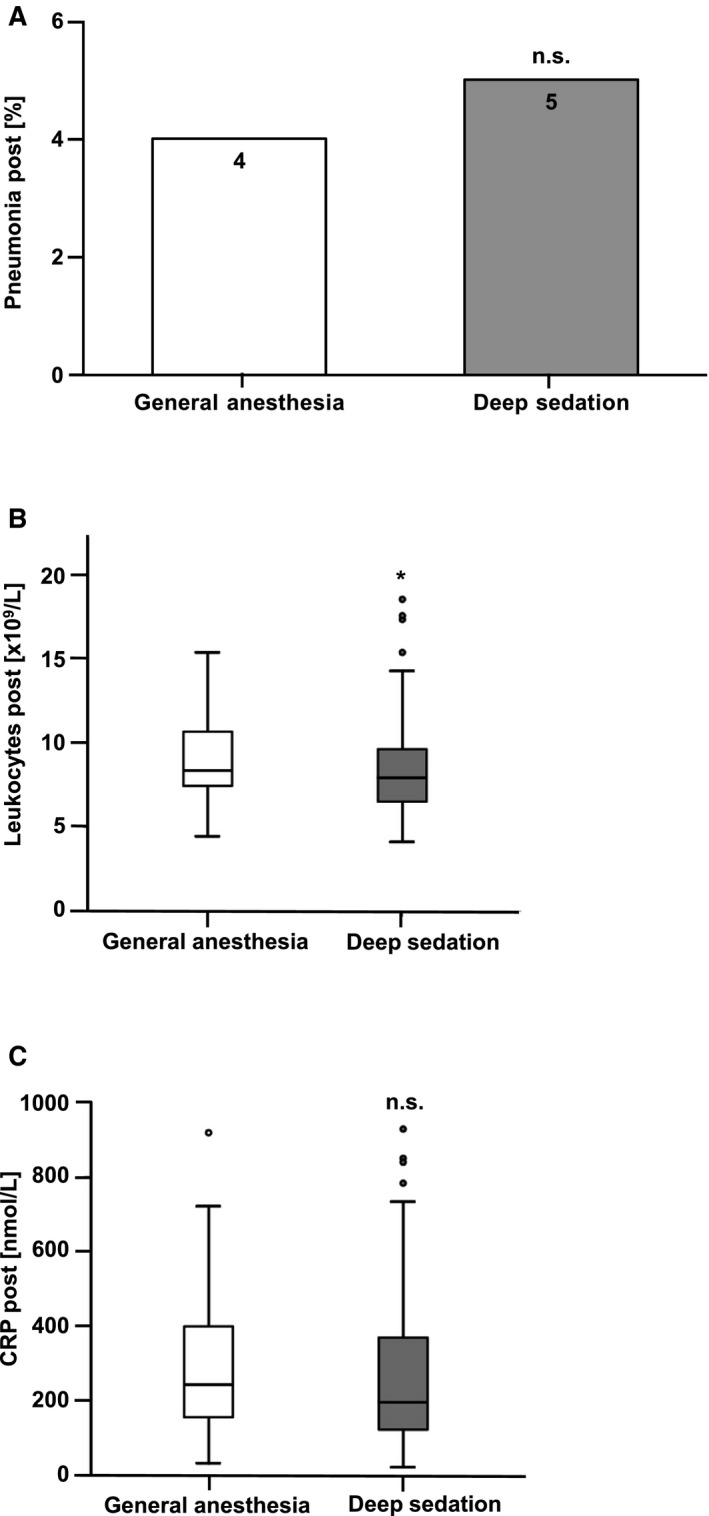
Comparison of pneumonia and inflammatory markers in the general anesthesia (GA) vs deep sedation (DS) groups. Bar diagram showing the percentage of patients per group with an event. A, The incidence of postprocedural pneumonia in patients undergoing percutaneous mitral valve repair (PMVR) using GA vs DS showed no significant differences between both groups. No significant (n.s.) difference between the groups was observed, P=0.77. B, Boxplot diagrams showing values in the DS and GA groups. The boxes consist of the 25% to the 75% quartiles of all measurements. The crossline marks the median of measurements (50% quartile). The whiskers mark the smallest and largest measurements. The circles are outliers. The blood leukocyte count was significantly lower after the PMVR procedure in the DS compared with the GA group, *P=0.018. C, Postprocedural C‐reactive protein (CRP) values were similar in the GA and the DS groups. No significant difference was observed between groups, P=0.1.
Figure 5.
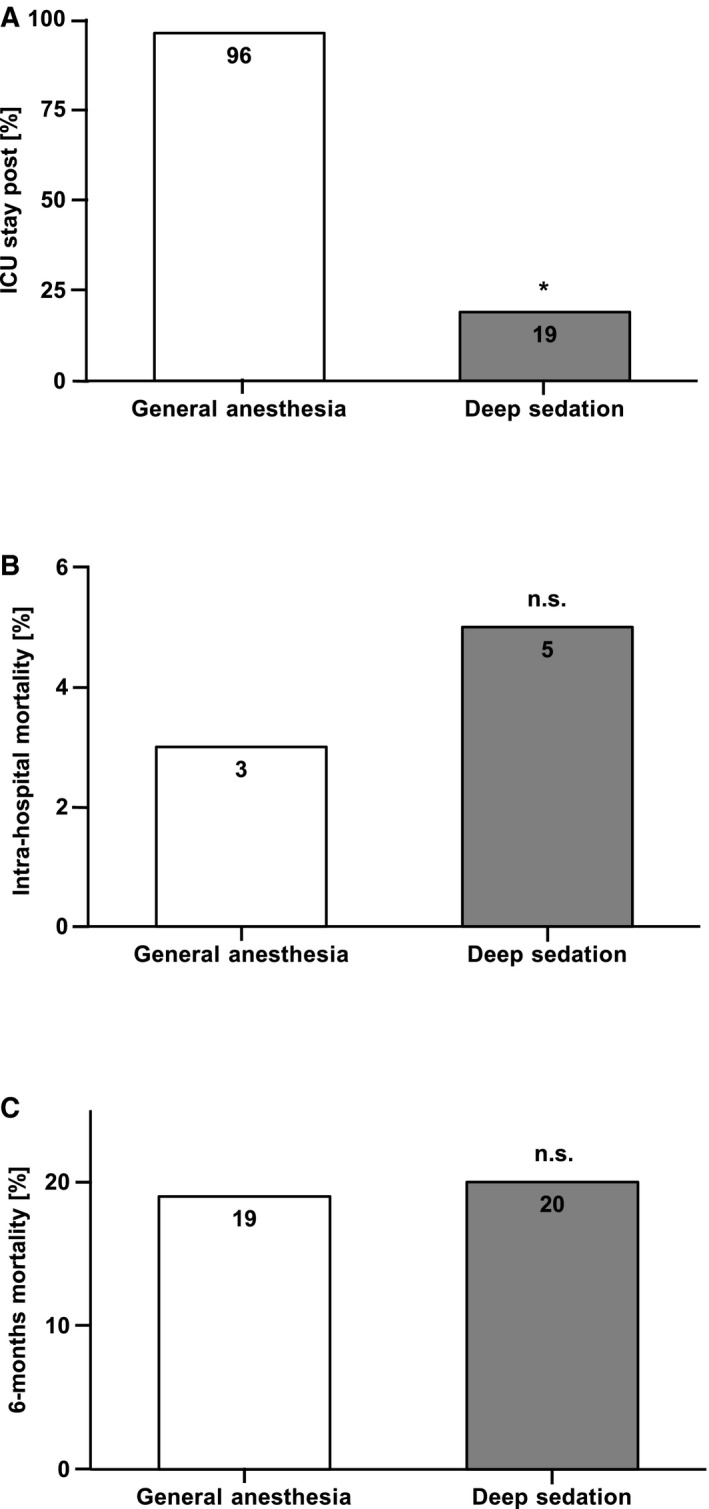
Length of intensive care unit (ICU) stay and intrahospital and 6‐month mortality in the general anesthesia (GA) vs deep sedation (DS) groups. A, Bar diagram showing the percentage of patients per group with an event. Percutaneous mitral valve repair in the DS group resulted in a reduced need for an ICU stay compared with the GA group, *P<0.001. B and C, Bar diagrams showing the percentage of patients per group with an event. Both the intrahospital (P=0.43) and the 6‐month mortality (P=0.77) showed no significant difference in the GA vs the DS group. No significant (n.s.) difference was observed between groups.
At follow‐up (8±5 months after PMVR), we observed a significant improvement in exercise capacity using the 6MWT compared with the preprocedural evaluation in both groups, with no significant difference between the DS and GA groups (Table 3). Preprocedural scores in the 6MWT were 160±116 meters, whereas the postprocedural scores were 267±132 meters (P<0.001). The need for rehospitalization because of heart failure was 12% in the GA group and 14% in the DS group (P=0.73, ns). Furthermore, New York Heart Association grade (grade 2±1 in each group, P=0.34 [ns]) did not significantly differ between the DS and GA groups (Table 3). Most importantly, intrahospital mortality (3% in the GA group versus 5% in the DS group, P=0.43 [ns]) (Figure 5B) and 6‐month mortality (19% in the GA group versus 20% in the DS group, P=0.77 [ns]) (Figure 5C) did not differ between the DS and GA groups. In summary, there was no significant difference in clinical end points between the groups at follow‐up.
Table 3.
Outcome at Follow‐Up
| All Patients (N=271) | GA (n=72) | DS (n=199) | P Value | |
|---|---|---|---|---|
| NYHA mean (grade) | 2±1 (159) | 2±1 (50) | 2±1 (109) | 0.34 |
| Need for rehospitalization because of heart failure | 14 (23/168) | 12 (6/49) | 14 (17/119) | 0.73 |
| Average No. of rehospitalizations | 0.2±0.4 | 0.2±0.5 | 0.2±0.4 | 0.74 |
| 6‐mo mortality | 20 (45/228) | 19 (13/70) | 20 (32/158) | 0.77 |
| Preprocedural 6‐min walk test, m | 160±116 (92) | 162±118 (29) | 159±116 (63) | 0.92 |
| Follow‐up 6‐min walk test, m | 267±132 (92) | 248±118 (29) | 276±138 (63) | 0.35 |
DS indicates deep sedation; GA, general anesthesia; NYHA, New York Heart Association.
Discussion
PMVR is beneficial for patients with mitral regurgitation not suitable for conventional surgery, particularly when they have serious comorbidities, severely reduced ejection fraction, increased perioperative risk, or a combination of these factors.
PMVR has been shown to increase functional capacitiy as measured by the 6MWT. In a study by Reichenspurner et al,18 outcomes in 117 patients with degenerative mitral regurgitation were examined. This study found a significant increase in 6MWT by a mean of 77.4 meters. In another study by Schäfer et al17 examining the impact of preprocedural LV ejection fraction on the outcome of MitraClip therapy in 393 patients with functional mitral regurgitation, the authors found a significant increase in 6MWT, which was most pronounced in those with an LV ejection fraction >40% (+69.8±103.3 meters) followed by those with an LV ejection fraction of 10% to 20% (61.1±150.3 meters). In a meta‐analysis of 2980 patients, Vakil et al19 found an increase in 6MWT from 261±14 meters at baseline to 360±25 meters at follow‐up. In our study, 6MWT increased from 160±116 to 267±132 meters. Our patient cohort had a high percentage (49%) of patients with severely reduced LV function of ≤35%. Cardiac decompensation was present in a considerable fraction of patients. This might in part explain the strong increase in 6MWT at follow‐up. For these frail patients, any cardiac stress may provoke additional cardiac damage with potentially serious complications. PMVR using the MitraClip system requires precise TEE guidance throughout the procedure to achieve exact positioning of the clip.20 Thus, PMVR procedures are commonly performed under GA because of the prolonged TEE time.4, 5 Furthermore, ventilation maneuvers can be performed facilitating the procedure in patients with challenging anatomies.12 In contrast, other percutaneous interventional approaches including transcatheter aortic valve replacement are now increasingly performed under conscious sedation or DS.6 Recent studies imply that DS can be an alternative to GA also in PMVR because the procedural outcome is as safe and as effective.21 We found that the procedure time, fluoroscopy time, and dose area product were significantly decreased in DS. The aforementioned differences were observed in our retrospective analysis and, thus, can be hypothesis generating only. We cannot exclude a bias by learning curve effects and the results should be confirmed in a prospective study design. Indeed, the shortened procedure time in DS is in contrast to the existing literature. Horn et al21 observed no significant difference in procedure time between the GA and DS groups, while Ledwoch et al22 found significantly longer procedure times when comparing conscious sedation with GA in a smaller study. In that study, the longer procedure times were explained by a larger proportion of patients receiving ≥2 clips. Also in our study, the group with shorter procedure times (DS) was the group with a smaller proportion of patients receiving ≥2 clips, which might partially explain the respective difference. Previous studies observed a reduction in postprocedural ICU stay.22, 23 In line with these findings, we found a significantly shorter postprocedural length of ICU stay in the DS group and a significantly reduced procedure time, fluoroscopy time, and dose area product. Importantly, there was no difference in procedural or clinical outcomes between groups immediately after the procedure and at follow‐up of 8±5 months.
Interestingly, we observed significantly lower catecholamine doses in the DS group compared with the GA group. This difference might be explained by reduced doses of anesthetic drugs, which have vasodilatory effects that cause BP reduction and thus increased need for counter‐regulation with catecholamine vasopressors. GA can cause complications in elder and frail patients,24 which represents the classical patient collective undergoing MitraClip therapy. On the other hand, in theory, DS might be associated with an increased risk for aspiration, particularly because patients undergo prolonged TEE. Importantly, we observed no significant differences regarding the development of pneumonia or other complications between GA and DS. In line with that, the inflammatory serum marker C‐reactive protein showed no significant difference between both groups (361±351 nmol/L in the GA group and 278±239 nmol/L in the DS group, P=0.1), whereas the blood leukocyte count was even significantly lower in the DS group (9±3×109/L in the GA group and 8±3×109/L in the DS group, P=0.018). The latter observation may be attributable to a reduced stress level in DS versus GA. It has previously been demonstrated that catecholamines induce lymphocyte recruitment and leukocytosis.25 As the GA group received significantly higher doses of norepinephrine, the higher leukocyte count could also be a consequence of catecholamine treatment rather than an increase in inflammation. Intravenous antibiotics (a cephalosporin or a lincosamide antibiotic in case of cephalosporin intolerance) were applied in all patients in the preinterventional and postinterventional period.
Limitations
We have to acknowledge that our study was not prospective and that it had a limited number of patients. However, the patient number seemed reasonable considering the complex nature of this intervention. To the best of our knowledge this is the largest study to date to compare DS and GA in PMVR. In this study, we addressed only the MitraClip system applied for interventional PMVR and our findings in patients with DS cannot necessarily be transferred to other systems currently tested in clinical trials such as the MitraLign or the CardioBand systems. Further studies providing long‐term follow‐up data beyond 1 year and prospective studies will be needed. In particular, randomized data including more patients than the only existing randomized study26 are warranted.
Conclusions
Our data suggest that PMVR in DS is safe and feasible. PMVR with a DS approach may simplify aspects of the procedure and the postprocedural hospital stay. Future prospective trials will have to further scrutinize the question of whether DS can be an equivalent alternative to GA in patients undergoing PMVR.
Sources of Funding
This study was supported by grants from the German Research Foundation (KFO 274), the Volkswagen Foundation (Lichtenberg program), and the German Heart Foundation. Ulrich is supported by a research grant of the German Cardiac Society (DGK).
Disclosures
Dr Langer and Dr Seizer were reimbursed by Abbott Vascular for training courses in the percutaneous mitral valve repair procedure. The remaining authors have no disclosures to report.
Supporting information
Table S1. Cause of Bleeding Complications
Table S2. Data on Nonprophylactic Antibiotic Treatment
Table S3. Data of Patients Converted From DS to GA
Figure S1. Mitral valve regurgitation (MR) at baseline (A), postintervention (B), and follow‐up (C) is depicted.
(J Am Heart Assoc. 2017;6:e007485 DOI: 10.1161/JAHA.117.007485.)29197832
Contributor Information
Peter Seizer, Email: peter.seizer@med.uni-tuebingen.de.
Harald F. Langer, Email: harald.langer@med.uni-tuebingen.de.
References
- 1. Levine RA, Hagege AA, Judge DP, Padala M, Dal‐Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia‐Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez‐Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Merot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Puceat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH; Leducq Mitral Transatlantic N . Mitral valve disease—morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mirabel M, Iung B, Baron G, Messika‐Zeitoun D, Detaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365. [DOI] [PubMed] [Google Scholar]
- 3. Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L; EVEREST II Investigators . Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5‐year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–2854. [DOI] [PubMed] [Google Scholar]
- 4. Boekstegers P, Hausleiter J, Baldus S, von Bardeleben RS, Beucher H, Butter C, Franzen O, Hoffmann R, Ince H, Kuck KH, Rudolph V, Schafer U, Schillinger W, Wunderlich N; Germany Society of Cardiology Working Group on Interventional Cardiology Focus Group on Interventional Mitral Valve T . Percutaneous interventional mitral regurgitation treatment using the Mitra‐Clip system. Clin Res Cardiol. 2014;103:85–96. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease—current management and future challenges. Lancet. 2016;387:1324–1334. [DOI] [PubMed] [Google Scholar]
- 6. Jensen HA, Condado JF, Devireddy C, Binongo J, Leshnower BG, Babaliaros V, Sarin EL, Lerakis S, Guyton RA, Stewart JP, Syed AQ, Mavromatis K, Kaebnick B, Rajaei MH, Tsai LL, Rahman A, Simone A, Keegan P, Block PC, Thourani VH. Minimalist transcatheter aortic valve replacement: the new standard for surgeons and cardiologists using transfemoral access? J Thorac Cardiovasc Surg. 2015;150:833–839. [DOI] [PubMed] [Google Scholar]
- 7. Kothandan H, Vui KH, Khung KY, Nian CH. Anesthesia management for MitraClip device implantation. Ann Card Anaesth. 2014;17:17–22. [DOI] [PubMed] [Google Scholar]
- 8. Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remiao F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem. 2011;18:2272–2314. [DOI] [PubMed] [Google Scholar]
- 9. Petsiti A, Tassoudis V, Vretzakis G, Zacharoulis D, Tepetes K, Ganeli G, Karanikolas M. Depth of anesthesia as a risk factor for perioperative morbidity. Anesthesiol Res Pract. 2015;2015:829151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teufel T, Steinberg DH, Wunderlich N, Doss M, Fichtlscherer S, Ledwoch J, Herholz T, Hofmann I, Sievert H. Percutaneous mitral valve repair with the MitraClip(R) system under deep sedation and local anaesthesia. EuroIntervention. 2012;8:587–590. [DOI] [PubMed] [Google Scholar]
- 11. Ussia GP, Barbanti M, Tamburino C. Feasibility of percutaneous transcatheter mitral valve repair with the MitraClip system using conscious sedation. Catheter Cardiovasc Interv. 2010;75:1137–1140. [DOI] [PubMed] [Google Scholar]
- 12. Patzelt J, Zhang Y, Seizer P, Magunia H, Henning A, Riemlova V, Patzelt TA, Hansen M, Haap M, Riessen R, Lausberg H, Walker T, Reutershan J, Schlensak C, Grasshoff C, Simon DI, Rosenberger P, Schreieck J, Gawaz M, Langer HF. Effects of mechanical ventilation on heart geometry and mitral valve leaflet coaptation during percutaneous edge‐to‐edge mitral valve repair. JACC Cardiovasc Interv. 2016;9:151–159. [DOI] [PubMed] [Google Scholar]
- 13. Patzelt J, Zhang Y, Magunia H, Jorbenadze R, Droppa M, Ulrich M, Cai S, Lausberg H, Walker T, Wengenmayer T, Rosenberger P, Schreieck J, Seizer P, Gawaz M, Langer HF. Immediate increase of cardiac output after percutaneous mitral valve repair (PMVR) determined by echocardiographic and invasive parameters: Patzelt: increase of cardiac output after PMVR. Int J Cardiol. 2017;236:356–362. [DOI] [PubMed] [Google Scholar]
- 14. Feldman T, Young A. Percutaneous approaches to valve repair for mitral regurgitation. J Am Coll Cardiol. 2014;63:2057–2068. [DOI] [PubMed] [Google Scholar]
- 15. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schäfer U, Maisano F, Butter C, Franzen O, Baldus S, Hausleiter J, Ussia GP, Sievert H, Geist V, Widder JD, Moccetti T, Schillinger W. Impact of preprocedural left ventricular ejection fraction on 1‐year outcomes after MitraClip implantation (from the ACCESS‐EU phase I, a prospective, multicenter, nonrandomized postapproval study of the MitraClip Therapy in Europe). Am J Cardiol. 2016;118:873–880. [DOI] [PubMed] [Google Scholar]
- 18. Reichenspurner H, Schillinger W, Baldus S, Hausleiter J, Butter C, Schaefer U, Pedrazzini G, Maisano F; Investigators A‐EPI . Clinical outcomes through 12 months in patients with degenerative mitral regurgitation treated with the MitraClip(R) device in the ACCESS‐EUrope Phase I trial. Eur J Cardiothorac Surg. 2013;44:e280–e288. [DOI] [PubMed] [Google Scholar]
- 19. Vakil K, Roukoz H, Sarraf M, Krishnan B, Reisman M, Levy WC, Adabag S. Safety and efficacy of the MitraClip(R) system for severe mitral regurgitation: a systematic review. Catheter Cardiovasc Interv. 2014;84:129–136. [DOI] [PubMed] [Google Scholar]
- 20. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L; EVEREST II Investigators . Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. [DOI] [PubMed] [Google Scholar]
- 21. Horn P, Hellhammer K, Minier M, Stenzel MA, Veulemans V, Rassaf T, Luedike P, Pohl J, Balzer J, Zeus T, Kelm M, Westenfeld R. Deep sedation Vs. general anesthesia in 232 patients undergoing percutaneous mitral valve repair using the MitraClip(R) system. Catheter Cardiovasc Interv. 2017. Jan 23 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Ledwoch J, Matic P, Franke J, Gafoor S, Bertog S, Reinartz M, Vaskelyte L, Hofmann I, Sievert H. Transcatheter mitral valve repair with the MitraClip((R)) can be performed without general anesthesia and without conscious sedation. Clin Res Cardiol. 2016;105:297–306. [DOI] [PubMed] [Google Scholar]
- 23. de Waha S, Seeburger J, Ender J, Desch S, Eitel I, Reinhardt A, Poss J, Fuernau G, Noack T, Merk DR, Schuler G, Sievers HH, Mohr FW, Thiele H. Deep sedation versus general anesthesia in percutaneous edge‐to‐edge mitral valve reconstruction using the MitraClip system. Clin Res Cardiol. 2016;105:535–543. [DOI] [PubMed] [Google Scholar]
- 24. Pakpirom J, Kraithep J, Pattaravit N. Length of postanesthetic care unit stay in elderly patients after general anesthesia: a randomized controlled trial comparing desflurane and sevoflurane. J Clin Anesth. 2016;32:294–299. [DOI] [PubMed] [Google Scholar]
- 25. Schulze J, Vogelgesang A, Dressel A. Catecholamines, steroids and immune alterations in ischemic stroke and other acute diseases. Aging Dis. 2014;5:327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rassaf T, Balzer J, Zeus T, Rammos C, Shayganfar S, Hall SV, Wagstaff R, Kelm M. Safety and efficacy of deep sedation as compared to general anaesthesia in percutaneous mitral valve repair using the MitraClip system. Catheter Cardiovasc Interv. 2014;84:E38–E42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cause of Bleeding Complications
Table S2. Data on Nonprophylactic Antibiotic Treatment
Table S3. Data of Patients Converted From DS to GA
Figure S1. Mitral valve regurgitation (MR) at baseline (A), postintervention (B), and follow‐up (C) is depicted.


