Abstract
Background
Morning blood pressure (BP) surge (MS), defined by the MS amplitude, is an independent prognostic factor of cardiovascular outcomes in some, but not all, populations.
Method and Results
We enrolled 2020 participants (1029 men; aged 30–79 years) with 24‐hour ambulatory BP data. During a median 19.7‐year follow‐up, 607 deaths (182 by cardiovascular causes) were confirmed from the National Death Registry. The amplitude of sleep‐trough MS (STMS) was derived from the difference between morning systolic BP (SBP) and lowest nighttime SBP. The rate of STMS was derived as the slope of linear regression of sequential SBP measures on time intervals within the STMS period. Thresholds for high STMS amplitude and rate were determined by the 95th percentiles (43.7 mm Hg and 11.3 mm Hg/h, respectively). Multivariable Cox models, adjusting for age, sex, body mass index, smoking, alcohol, low‐density lipoprotein cholesterol, 24‐hour SBP, night:day SBP ratio, and antihypertensive treatment, revealed that a high STMS rate (hazard ratio, 1.666; 95% confidence interval, 1.185–2.341), but not STMS amplitude (hazard ratio, 1.245; 95% confidence interval, 0.984–1.843), was significantly associated with a greater mortality risk. Similarly, STMS rate (hazard ratio, 2.608; 95% confidence interval, 1.554–4.375), but not STMS amplitude, was significantly associated with the risk of cardiovascular mortality (hazard ratio, 0.966; 95% confidence interval, 0.535–1.747). Moreover, the prognostic values of STMS rate were comparable in subjects with or without morning and nocturnal hypertension (P>0.05 for interaction for all). In simulation studies, STMS rate was less susceptible to measurement errors of the sleep‐trough SBP than STMS amplitude.
Conclusions
STMS rate could independently help identify subjects with an increased cardiovascular risk.
Keywords: ambulatory blood pressure monitoring, blood pressure, cardiovascular mortality, cardiovascular outcomes, morning blood pressure surge
Subject Categories: Epidemiology, Risk Factors, High Blood Pressure, Hypertension
Clinical Perspective
What Is New?
Conventional sleep‐trough morning blood pressure (BP) surge (STMS) focused only on the amplitude rather than the rate of BP increase.
We calculated the STMS rate as the slope of linear regression of sequential systolic BP measures on time intervals within the STMS period.
The reproducibility of the STMS rate was better than that of the STMS amplitude. The STMS rate was also a more sensitive and reliable prognostic factor for long‐term mortality than the STMS amplitude.
The prognostic values of the STMS rate were comparable in subjects with or without morning and nocturnal hypertension.
What Are the Clinical Implications?
Because the STMS rate can be easily calculated from the ambulatory BP monitoring data, it has the potential to become a routine parameter in daily practice for subjects receiving ambulatory BP monitoring, in conjunction with other routinely derived parameters, including STMS amplitude.
Blood pressure (BP) values vary markedly from day to night and from night to day; a nocturnal BP decrease is followed by a morning BP increase.1 Alterations in the short‐term (within 24 hours) circadian physiological BP variation may have an independent prognostic value and may be relevant for clinical detection and intervention.1 A large BP increase in the early morning has coincided with the fact that morning is a vulnerable period during which more cardiovascular events occur than at later times in the day.2 Ambulatory BP monitoring (ABPM), by tracking changes in the BP level over 24 hours, provides the opportunity to test the hypothesis that the high incidence of cardiovascular complications in the morning is linked to an exaggerated night‐to‐day BP elevation.3 Indeed, the morning BP surge (MS) has been demonstrated to be an important prognostic factor of cardiovascular end points in Japanese elderly hypertensive patients4 and in many other population‐based cohorts.5, 6 In a meta‐analysis of 5645 subjects from 8 cohorts, sleep‐trough MS (STMS) significantly and independently associated with cardiovascular outcomes.5 However, the prognostic value of MS for cardiovascular disease was not confirmed by recent prospective studies.7, 8, 9
The inconsistent results on the clinical significance of MS may indicate the need to investigate the different aspects of MS.6 Previous studies, however, only assessed the prognostic value of the STMS magnitude and the level of achieved morning BP, and they neglected the contribution of the rate at which morning BP increases to incur risk. To fully characterize the influence of STMS on the early morning risk of cardiovascular events, it is warranted to consider not only the amplitude but also the rate of STMS. We hypothesized that the rate of a BP increase carries valuable prognostic information in association with cardiovascular outcomes beyond the amplitude of STMS. Moreover, subjects with morning and nocturnal hypertension are known to have increased risks for cardiovascular events and total mortality.10, 11 Whether the presence of morning or nocturnal hypertension could modify the prognostic value of MS is largely unknown.10 Therefore, the present study aimed to investigate the independent prognostic significance of MS parameters in predicting long‐term all‐cause and cardiovascular mortalities in a community‐based cohort.
Moreover, considering that frequent BP measurements during sleep may disturb sleep and reduce nocturnal BP decrease, and less frequent BP measurements may not capture the true sleep‐trough BP, we therefore conducted a series of simulation studies of possible scenarios to investigate the influences of measurement errors on the rate and amplitude of STMS.
Methods
Study Population
The data that support the findings of this study are available from the corresponding author on reasonable request. The study population consisted of 2020 participants (overall, 1029 men; aged 30–79 years) with complete demographic and 24‐hour ABPM data drawn from a community‐based survey conducted from Sep 3, 1991 to June 16, 1993.12 Subjects having taken antihypertensive agents were also included. As such, the sample size of the present study was larger than that in our previous study.13 Only subjects without nighttime BP data were excluded, and the all‐inclusive manner allowed all unedited ambulatory BP data for analysis, which resembles clinical practice. All participants received a baseline comprehensive cardiovascular evaluation, including complete medical history, physical examination, and blood chemistry analysis from overnight fasting serum and plasma samples.13, 14 None of the participants had a previous history of diabetes mellitus, angina pectoris, or peripheral vascular disease, and none had clinical or echocardiographic evidence of other significant cardiac diseases. The study was approved by Taipei Veterans General Hospital (Taipei, Taiwan) and the institutional review board at Johns Hopkins University (Baltimore, MD), with the exemption of informed consent.
Twenty‐Four‐Hour Ambulatory BPs and MS
The office BP measurements were taken in a special clinic established at the local health station for 3 to 5 months to examine the eligible subjects in the selected 3 regions in Taiwan.12 Office systolic BP (SBP) and diastolic BP (DBP) levels were measured manually by experienced cardiologists using a mercury sphygmomanometer. Two or more measurements separated by at least 5 minutes were taken from the right arm of participants after they had been seated for at least 5 minutes. The average of at least 2 consecutive measurements was then recorded and used for analysis.
Descriptions of the conduct of the field study and ABPM measurements have been detailed elsewhere.12, 14 In brief, after instructions, all study subjects received the examination of 24‐hour ABPM on a usual working day. The recorder (model 90217; Spacelabs Inc, Redmond, WA) was programmed to measure ambulatory BP (full inflation, followed by deflation, in steps of 8 mm Hg) at 20‐minute intervals during the daytime (ie, from 6 am to 10 pm) and at 60‐minute intervals during the nighttime (ie, from 10 pm to 6 am). The morning period was defined as the interval in the first 2 hours of the daytime, the evening period was defined as the last 2 hours before nighttime, and a preawakening period was defined as the last 2 hours of the nighttime. Sleep‐trough SBP was defined as the lowest nighttime reading. MS can, therefore, be described as follows (Figure 1): (1) STMS, defined as the morning SBP (average of the SBP readings during the morning period) minus the sleep‐trough SBP; (2) preawakening surge, defined as the morning SBP minus the preawakening SBP (average of the SBP readings during the preawakening period); (3) morning‐evening SBP difference, defined as the difference between morning and evening SBP (average of the SBP readings during the evening period); and (4) morning‐night SBP difference, defined as the difference between morning and nighttime SBP (average of the SBP readings during the nighttime). SBP night:day ratio was calculated as the ratio of nighttime SBP:daytime SBP (average of the SBP readings during the daytime). For each individual subject, the rate of STMS was derived as the slope of the linear regression of successive SBP measures on time intervals within the time frame of STMS. Morning hypertension was defined as BP of at least 135/85 mm Hg for ambulatory readings within the first 2 hours of daytime.10 Nocturnal hypertension was defined by nighttime SBP ≥120 mm Hg or DBP ≥70 mm Hg.15
Figure 1.
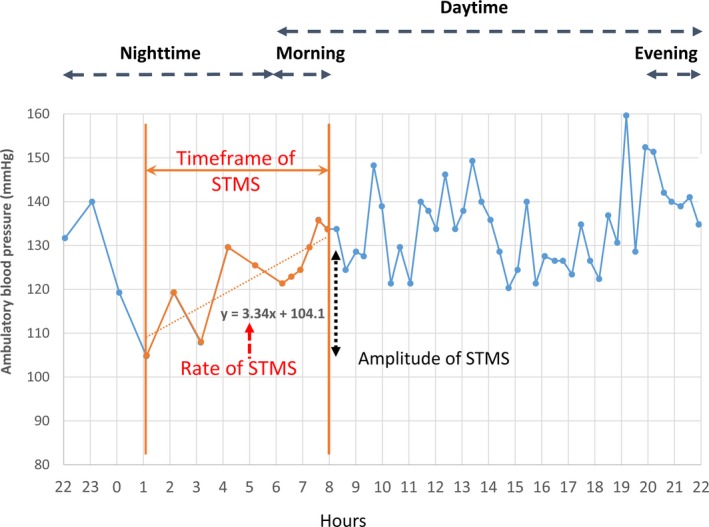
Illustration of parameters describing the sleep‐trough morning blood pressure surge (STMS). After identification of nighttime blood pressure (BP) trough and morning BP peak, linear regression analysis between BP (y axis) and time (x axis) was conducted. The slope of the BP increase is the STMS rate.
To avoid interobserver and intraobserver variations, we performed a fully automated batch analysis for the calculation of 24‐hour BP readings from ABPM using a custom‐designed routine in Matlab, versions 4.2 and 7.0 (The MathWorks, Inc).
Reproducibility
We conducted the reproducibility analysis on the basis of a Japanese reproducibility study16 for MS parameters.
Follow‐Up
We ascertained the causes and dates of death for all participants deceased before December 31, 2013, by linking our database with the National Death Registry through a unique personal identification number given to every Taiwanese citizen. The National Death Registry holds valid information based on the certified death certificates, coded according to the International Classification of Disease, Ninth and Tenth Revisions (ICD‐9 and ICD‐10, respectively). The ICD‐9 and ICD‐10 codes representing cardiovascular deaths were 390 to 459 and I00 to I99, respectively. The accuracy of cause‐of‐death coding in Taiwan's National Death Registry database has been validated.17
Statistical Analysis
All data are expressed as proportions (percentages) or means and SDs. Associations of the STMS rate and amplitude with all‐cause and cardiovascular mortalities over a median follow‐up of 20 years were examined by the Cox proportional hazard regression analysis. We first evaluated the hazard ratios (HRs) of these parameters in quintiles and >95th percentile for all‐cause and cardiovascular mortalities. The baseline multivariable Cox model was constructed on the basis of significant results from the univariate analyses for sex, age, height, alcohol, dyslipidemia, smoking, hypertension, total cholesterol, low‐ and high‐density lipoprotein cholesterol, triglycerides, heart rate, office SBP, 24‐hour SBP, 24‐hour DBP, daytime SBP, nighttime SBP, morning SBP, and a previous systematic review.5 We determined the threshold for high status of the morning surge parameters by identifying the 95th percentile. To investigate the relationship between MS and long‐term all‐cause and cardiovascular mortalities in different conditions, we conducted a series of sensitivity analyses. A 2‐tailed P<0.05 was considered statistically significant. The analyses were performed using SAS, version 9.3, and the software package Comprehensive Meta‐Analysis, version 2 (Bio Stat, Englewood, NJ).18
Simulation Studies
A higher sampling rate at night may help capture a lower sleep‐trough SBP but may also interfere with subjects’ sleep quality and the extent of nocturnal BP decrease. We, therefore, conducted a series of simulation studies (Data S1) described as follows to investigate the effect of measurement errors of true sleep‐trough SBP on the STMS rate and amplitude and the corresponding regression dilution analysis.
In brief, in simulation study 1 (N=2020; present cohort), we simulated the true sleep‐trough SBP as if the true lowest sleep SBP was before or after the originally identified one (Figure S1); in simulation study 2 (N=42; Japanese reproducibility study), we conducted the simulation by reducing the nighttime sampling frequency of ABPM on the basis of a previously published study population.16 In addition, the calculation of morning SBP may also be affected by the awakening time. Therefore, in simulation study 3 (N=2020; present cohort), we simulated the true awakening time before or after the original setting.
Results
Characteristics of the study population are shown in Table 1. Compared with subjects with normal STMS rates, those with high rates had greater body weight, body mass index, total and low‐density lipoprotein cholesterol levels, 24‐hour SBP and DBP, and morning SBP. Office SBP and DBP were comparable.
Table 1.
Baseline Characteristics of the Study Population and Subjects With Normal or High Rate of MS Based on the Cutoff of 11.33 mm Hg/h
| Characteristics | Total Population | Normal Rate of STMS | High Rate of STMS | P Value |
|---|---|---|---|---|
| (N=2020) | (n=1919) | (n=101) | ||
| Sex (male) | 1029 (51.4) | 973 (51.2) | 56 (55.4) | 0.4127 |
| Age, y | 55.2±13.1 | 55.2±13.2 | 55.1±12.1 | 0.9083 |
| Height, cm | 158.8±8.5 | 158.8±8.5 | 159.5±9.1 | 0.4368 |
| Weight, kg | 63.1±11.1 | 63.0±11.1 | 65.7±10.9 | 0.0191 |
| Body mass index, kg/m2 | 25.0±3.7 | 24.9±3.7 | 25.8±3.7 | 0.0230 |
| Alcohol | 243 (12.1) | 226 (11.9) | 17 (16.8) | 0.1400 |
| Dyslipidemia | 325 (17.9) | 296 (17.2) | 29 (30.8) | 0.0008 |
| Smoking | 511 (25.5) | 479 (25.2) | 32 (31.6) | 0.1479 |
| Hypertension | 861 (42.6) | 824 (42.9) | 37 (36.6) | 0.2117 |
| Antihypertensive agents | 861 (42.6) | 824 (42.9) | 37 (36.6) | 0.2117 |
| Total cholesterol, mg/dL | 200.8±38.9 | 200.0±38.3 | 215.6±45.6 | 0.0015 |
| LDL‐C, mg/dL | 124.6±34.9 | 124.1±34.5 | 133.6±41.6 | 0.0341 |
| HDL‐C, mg/dL | 49.5±13.0 | 49.5±13.0 | 49.8±12.6 | 0.8079 |
| Triglycerides | 138.9±115.6 | 137.3±112.8 | 168.5±158.2 | 0.0613 |
| Heart rate, beats/min | 70.5±13.7 | 70.6±13.9 | 69.7±9.9 | 0.3921 |
| Office SBP, mm Hg | 142.7±25.1 | 142.5±25.0 | 147.7±26.1 | 0.0429 |
| Office DBP, mm Hg | 89.4±23.4 | 89.3±23.8 | 91.7±14.9 | 0.1415 |
| 24‐h SBP | 128.5±17.5 | 128.4±17.4 | 133.0±19.1 | 0.0090 |
| 24‐h DBP | 81.3±11.7 | 81.2±11.6 | 84.4±12.3 | 0.0073 |
| Daytime SBP, mm Hg | 130.3±17.7 | 130.0±17.6 | 136.1±20.2 | 0.0035 |
| Nighttime SBP, mm Hg | 122.3±18.2 | 122.4±18.3 | 121.7±17.0 | 0.6908 |
| Morning SBP, mm Hg | 130.4±19.3 | 129.9±19.1 | 140.0±19.2 | <0.0001 |
| Amplitude of STMS, mm Hg | 21.7±12.3 | 21.0±12.0 | 35.0±11.7 | <0.0001 |
| Rate of STMS, mm Hg/h | 3.3±4.1 | 2.7±3.1 | 14.7±3.5 | <0.0001 |
| Preawakening surge, mm Hg | 8.6±14.5 | 7.4±13.6 | 31.8±10.8 | <0.0001 |
| ME difference, mm Hg | 1.8±13.5 | 1.7±13.6 | 3.4±12.0 | 0.2323 |
| MN difference, mm Hg | 7.9±10.6 | 7.4±10.4 | 17.9±9.7 | <0.0001 |
| Trough SBP, mm Hg | 106.6±17.3 | 106.9±17.4 | 101.0±15.4 | 0.001 |
Data are given as number (percentage) or mean±SD. DBP indicates diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; ME difference, difference between morning and evening SBP; MN difference, difference between morning and night SBP; MS, morning blood pressure surge; SBP, systolic blood pressure; and STMS, sleep‐trough MS.
During a median 19.7‐year follow‐up, there were 607 deaths (182 with cardiovascular causes). Univariate analysis of predictors of cardiovascular and all‐cause mortalities in the continuous scale are shown in Table S1. Among the MS parameters of STMS, preawakening surge, morning‐evening SBP difference, and morning‐night SBP difference were significantly associated with all‐cause mortality, and only rate of STMS and morning‐night SBP difference were significantly associated with cardiovascular mortality. In multivariable analysis adjusting for age, sex, antihypertensive treatment, body mass index, low‐density lipoprotein cholesterol, smoking, alcohol drinking, 24‐hour SBP, and the SBP night:day ratio, only rate of STMS was significantly associated with all‐cause mortality (Table S2).
The relationships between mortality risk and STMS amplitude or rate in quintiles and above the 95th percentile (threshold for high status) were evaluated by multivariable Cox models and are shown in Figures 2 and 3, respectively. Subjects with STMS amplitude ≥43.7 mm Hg (95th percentile; Figure 2) did not show a significant increase in all‐cause or cardiovascular mortalities compared with subjects with a normal STMS amplitude (all‐cause mortality: HR, 1.245; 95% confidence interval, 0.911–1.701; P=0.1691; cardiovascular mortality: HR, 0.966; 95% confidence interval, 0.535–1.747; P=0.9101; Table 2). In contrast, subjects with an STMS rate ≥11.3 mm Hg/h (95th percentile; Figure 3) had a significantly increased risk of all‐cause and cardiovascular mortalities compared with subjects with an STMS rate <11.3 mm Hg/h (all‐cause mortality: HR, 1.666; 95% confidence interval, 1.185–2.341; P=0.0033; cardiovascular mortality: HR, 2.608; 95% confidence interval, 1.554–4.375; P=0.0003; Table 2). A categorical analysis, which classified subjects into high and normal MS using thresholds for defining abnormally high MS parameters at the 95th percentile (Table 2), a high MS defined by the rate of STMS was significantly associated with total and cardiovascular mortalities. Similar trends were observed when the cut point of 90th percentile was used (Table S3). The sensitivity analysis demonstrated consistent results by including only records with 80% successful measurements for the analysis and at least 8 BP measurements within the time frame from sleep‐trough through to the morning period (Table S4) and by stratifying patients on the basis of subjects receiving antihypertensive agents or not (Table S5).
Figure 2.
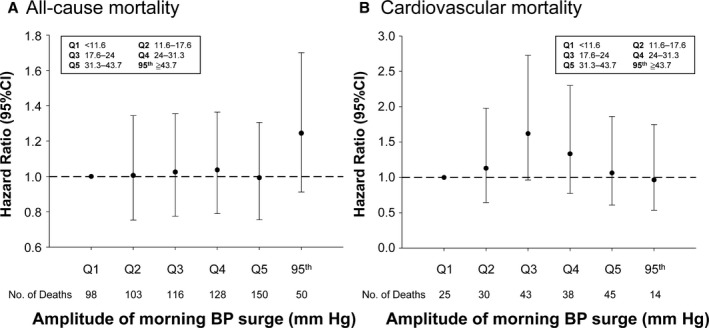
Multivariable model of the associations of amplitude of sleep‐trough morning blood pressure (BP) surge with all‐cause mortality (A) and cardiovascular mortality (B), with adjustment for sex, age, antihypertensive treatment, body mass index, low‐density lipoprotein cholesterol, smoking, alcohol drinking, 24‐hour systolic BP, and systolic BP night:day ratio. Quintile 1 (Q1) was the reference. 95th indicates 95th percentile; and CI, confidence interval.
Figure 3.
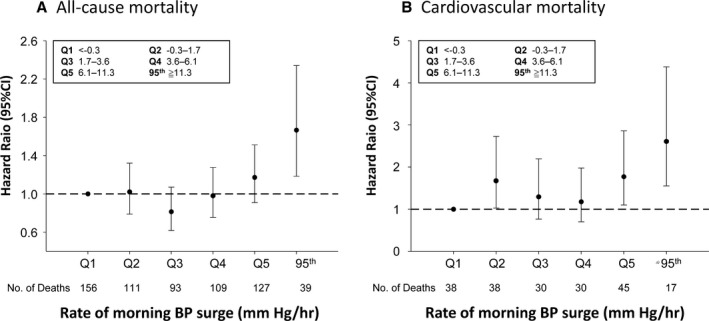
Multivariable model of the associations of the rate of sleep‐trough morning blood pressure (BP) surge with all‐cause mortality (A) and cardiovascular mortality (B), with adjustment for sex, age, antihypertensive treatment, body mass index, low‐density lipoprotein cholesterol, smoking, alcohol drinking, 24‐hour systolic BP, and systolic BP night:day ratio. Quintile 1 (Q1) was the reference. 95th indicates 95th percentile; and CI, confidence interval.
Table 2.
Multivariable Analysis for the Prognostic Values of Various Parameters Describing Night‐to‐Morning SBP Changes With All‐Cause and Cardiovascular Mortality
| Parameters | Thresholdsa | All‐Cause Mortality (Event=607) | Cardiovascular Mortality (Event=182) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | Statistical Power for All‐Cause Deathb | HR | 95% CI | P Value | Statistical Power for Cardiovascular Deathb | ||
| Amplitude of STMS, mm Hg | 43.67 | 1.245 | 0.911–1.701 | 0.16 | 0.25 | 0.966 | 0.535–1.747 | 0.9101 | 0.05 |
| Rate of STMS, mm Hg/h | 11.33 | 1.666 | 1.185–2.341 | 0.0033c | 0.84c | 2.608 | 1.554–4.375 | 0.0003c | 0.86c |
| Preawakening surge, mm Hg | 32.75 | 0.826 | 0.534–1.277 | 0.39 | 0.2 | 1.380 | 0.3048–2.555 | 0.3048 | 0.18 |
| ME difference, mm Hg | 25.73 | 1.24 | 0.916–1.679 | 0.16 | 0.24 | 1.183 | 0.664–2.108 | 0.5692 | 0.08 |
| MN difference, mm Hg | 25.7 | 1.114 | 0.767–1.620 | 0.57 | 0.1 | 0.728 | 0.343–1.545 | 0.4086 | 0.17 |
Each morning blood pressure surge parameter was 1‐by‐1 tested in a multivariable Cox proportional hazard model. Models were adjusted for sex, age, antihypertensive treatment, body mass index, low‐density lipoprotein cholesterol, smoking, alcohol drinking, 24‐hour SBP, and the SBP night:day ratio. CI indicates confidence interval; HR, hazard ratio; ME difference, difference between morning and evening SBP; MN difference, difference between morning and night SBP; SBP, systolic blood pressure; and STMS, sleep‐trough morning blood pressure surge.
The thresholds were determined by the 95th percentile of parameters describing the night‐to‐morning SBP change.
For both all‐cause and cardiovascular deaths, a significant increase of risk for normal vs high STMS rate is practically meaningful. There is 84% power to detect a 66% increase of risk for all‐cause death, given a sample size of 2020 patients, a hazard ratio of 1.666, an overall probability of death of 0.3475, a ratio of patients in the group of high STMS rate as 5%, and a type I error rate of 5%. Similarly, for the power for cardiovascular death, with the previously described conditions, except for hazard ratio of 2.608, the overall probability of cardiovascular death is 0.1042 and there is 86% power to detect a 160% increase of risk.
Given the present sample size, the explanation of the p values and statistical power for all‐cause and cardiovascular mortalities were illustrated as above.
Survival curves stratified according to high or normal STMS amplitude and rate are presented in Figure 4A and 4B. We then stratified subjects into subgroups with or without morning hypertension or nocturnal hypertension. As shown in Figure 5, in multivariable Cox proportional hazard models accounting for sex, age, antihypertensive treatment, body mass index, low‐density lipoprotein cholesterol, smoking, alcohol drinking, and 24‐hour SBP, the prognostic values of a high STMS rate in these subgroups were comparable with all P>0.05 for interaction, suggesting its independent prognostic role in these subgroups.
Figure 4.
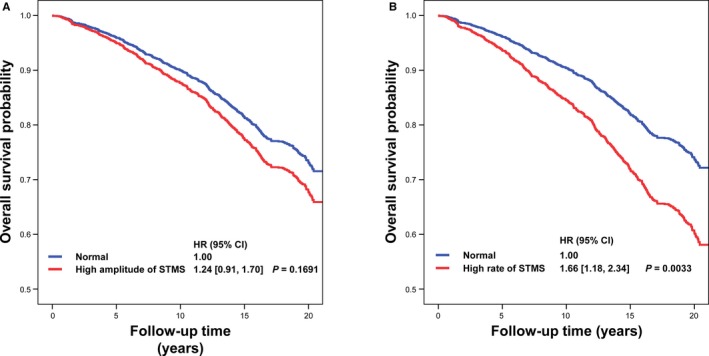
Survival curves of subjects stratified according to high or normal amplitude of sleep‐trough morning blood pressure surge (STMS; A) and high or normal rate of STMS (B). CI indicates confidence interval; and HR, hazard ratio.
Figure 5.
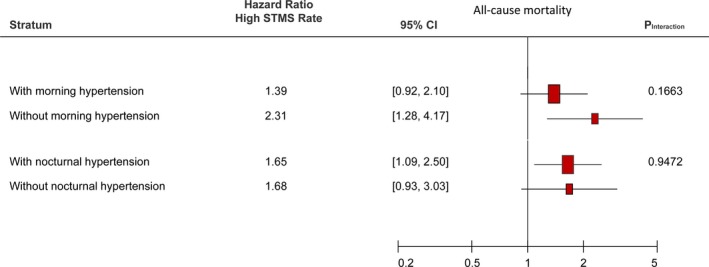
The prognostic values of high vs normal rate of sleep‐trough morning blood pressure surge (STMS rate) in subgroups with or without morning hypertension (defined by morning systolic blood pressure >135 mm Hg or diastolic blood pressure >85 mm Hg)10 and with or without nocturnal hypertension (defined by nighttime systolic blood pressure >120 mm Hg or diastolic blood pressure >70 mm Hg).15
The 3 simulation studies compared the relative sensitivity of STMS rate and amplitude with measurement errors of true sleep‐trough SBP, reduced nighttime sampling rate, and deviation from the true awakening time. In all simulation studies, the standardized mean differences between the simulated values and original measured values were consistently lower for the STMS rate than for the STMS amplitude (Table S6).
Results of the reproducibility study are shown in Table S7. The intraclass coefficients between 2 repeated measurements for the same individual were 0.31 and 0.21 for the STMS rate and amplitude, respectively.
Discussion
Although a large and rapid BP increase in the early morning has been associated with a poor prognosis, previous studies focused only on the amplitude rather than the rate of BP increase.5 BP is normally low at night while sleeping and starts to increase a few hours before waking up, exhibiting a physiological diurnal variation. Only an extreme morning BP increase is considered hazardous to the cardiovascular system.19 However, only the STMS rate was significantly associated with mortalities of our study subjects. Therefore, the STMS rate appears to be a more sensitive prognostic factor than the STMS amplitude, derived from the 24‐hour ABPM data in association with cardiovascular outcomes. These findings could also be supported by the largest statistical power of STMS rate than all other MS parameters, as demonstrated in Table 2.
A previous meta‐analysis based on individual participant data demonstrated that individuals with isolated nocturnal hypertension were associated with a significantly higher risk of total mortality and all cardiovascular events than normotensive individuals.11 In comparison with MS parameters, morning hypertension may be an easier target to manage because both ABPM and home BP monitoring can provide morning BP measurements.20 However, both a high nocturnal BP and an exaggerated MS can contribute to morning hypertension. More important, as shown in Figure 5, the prognostic values in subject with or without morning or nocturnal hypertension were comparable. This important finding may suggest the indispensable role of ABPM in measuring nocturnal BP and MS parameters and clearly supports the important prognostic utility of MS parameters.
A higher nighttime sampling rate could have captured a lower sleep‐trough SBP and a larger STMS as a result, but could also be offset by a poorer sleep quality and an incomplete nocturnal BP decrease. Therefore, the simulation studies may be valuable in quantifying the impact of such technical constraints of cuff‐based BP measurement. In our simulation studies, we evaluated the impact of the measurement errors resulting from the identification of sleep‐trough SBP, a lower sampling rate, and inaccurate preawakening time on the estimated STMS amplitude and rate. We clearly demonstrated that the STMS rate was less susceptible to the measurement errors than the STMS amplitude. Moreover, the observed HRs for mortality with STMS rate or amplitude could have been underestimated because of the effect of regression dilution resulting from the measurement error. Therefore, the STMS rate may be a more reliable estimate to describe the adverse effect of the MS. These findings are imperative to clinical practice, which may help to explain the previous inconsistent prognostic values of STMS.6
In a previous meta‐analysis of subjects from 8 populations,5 STMS amplitude >90th percentile was an independent risk factor of mortality and cardiovascular events after adjusting for the same covariables as in our study. Given the relatively small sample size and fewer end points in our study (607 all‐cause deaths), the STMS amplitude only reached borderline prognostic significance, whereas all other MS parameters were of no significant prognostic value. In contrast, STMS rate was significantly associated with both cardiovascular and total mortalities in our community‐based cohort.
Morning BP increase is a well‐recognized physiological phenomenon,2, 21, 22 but few studies have investigated the prognostic value of the rate of BP increase. Staessen et al reported, in 1999, that a higher sleeping‐to‐waking morning BP increase rate (slope), indirectly calculated by fitting a regression line from 4 to 10 am, was associated with a lower risk of cardiovascular events.23 In contrast to the fixed interval method adopted by Staessen et al,23 the rate of STMS in the present analysis was calculated by first identifying the nighttime SBP trough and then fitting a regression over the trough‐to‐morning period. In addition, only subjects with STMS rates >95th percentile had a significantly increased risk of mortality, which suggests that only an extreme condition of a well‐recognized physiological phenomenon can be associated with a harmful effect.
The amplitude of STMS in the present study had only borderline prognostic significance for mortalities, which may be explained by several reasons. First, compared with the previous seminal study investigating the prognostic value of STMS in elderly hypertensive outpatients, our population‐based study subjects were relatively younger, with a lower mean 24‐hour SBP level.4 The characteristics of our study population may dilute the impact of the amplitude of STMS on cardiovascular mortality, which may be modulated by age and 24‐hour SBP level. Second, with the long follow‐up period, confounding factors, such as the initiation of antihypertensive therapy, may reduce the risk of baseline MS, especially in the younger patients. Third, the issue of measurement errors, especially during nighttime, which has been comprehensively investigated in our study, might also result in the regression dilution effect. However, even with the previously described confounding factors, which may reduce the true impact of MS and STMS amplitude, the rate of STMS could still significantly associate with all‐cause and cardiovascular mortalities. Further studies should be conducted to evaluate whether the STMS rate in different age populations confers differential impacts and has comparable manageability in response to medications.24
Although BP measurement every 15 to 30 minutes during asleep hours for ABPM was formally recommended in 2005,25 the adequacy has never been scientifically proved. In our simulation studies, we have demonstrated clearly that rate of STMS is less susceptible to measurement error than the amplitude by involving more BP measurements within the time frame between the nighttime trough and morning. Compared with the measurement of the amplitude of MS, the calculation of the rate of STMS involves many more BP measurements; however, it is a trade‐off decision between the frequency of nighttime BP measurement and sleep quality. In addition, the STMS rate was less susceptible to errors than STMS amplitude when a fixed circadian window was used, as shown in simulation study 3.
Because the STMS rate can be easily calculated from the ambulatory BP data, it could become a routine parameter in daily practice for subjects receiving ABPM in conjunction with other routinely derived parameters, including STMS amplitude. Further clinical application is anticipated if similar results are found in other cohorts. In our reproducibility analysis on a Japanese population16 (Table S7), the reproducibility for the STMS rate was better than the reproducibility for the STMS amplitude. Future novel technology, such as cuff‐less BP monitoring,26 which can substantially reduce the interference resulting from the cuff inflation and deflation of oscillometric BP measurements, may have potential in increasing the reproducibility of the MS parameters and more accurately determining the STMS amplitude and rate.
Limitations
Nonfatal cardiovascular events were not considered in the present study. Therefore, the prognostic value of MS parameters for all cardiovascular events may have been underestimated. The definitions of daytime and nighttime were arbitrarily determined by the research team 25 years ago. We, therefore, conducted simulation study 1 to account for such an inaccuracy. History of drug therapy during the follow‐up period was not available. Thus, we only accounted for the impact of baseline antihypertensive medications on the prognostic values in the multivariable models, as done in a previous meta‐analysis.5 The use of antihypertensive treatment as a time‐dependent variable is infeasible because the field study was conducted long before the commencement of the national health insurance program, and there were no relevant records for analysis. We did, however, analyze the possible modulating effect of antihypertensive agents on the relationship between MS and cardiovascular and all‐cause mortalities. As shown in the stratified analysis based on taking or not taking antihypertensive agents in Table S5, all P values for interaction were insignificant, suggesting that antihypertensive agents had trivial influence on the prognostic role of MS. In fact, given that the use of antihypertensive agents could probably ameliorate the association between STMS and cardiovascular outcomes, the original prognostic value of STMS rate may be even more pronounced, considering the possibility of regression dilution, if there is any. The information of sleep apnea syndrome was not obtainable 25 years ago. However, sleep apnea syndrome is associated with an increased cardiovascular risk and linked with elevated nocturnal BP. On the basis of the equation for calculating STMS amplitude, the elevated nocturnal BP is related to a lower STMS amplitude. Therefore, sleep apnea could be a significant confounder that might dilute the prognostic value of MS. Even with the possible diluting effects of sleep apnea syndrome, our study still demonstrated the independent prognostic value of MS for 20‐year all‐cause and cardiovascular mortalities. As shown in Figure 5, exaggerated MS had a comparable prognostic value in subjects with or without morning or nocturnal hypertension. The results suggest the independent role of both morning or nocturnal hypertension and STMS, which may have significant clinical implication for the care of hypertensive patients.
Sources of Funding
This work was supported in part by grants from the National Science Council (NSC 99‐2314‐B‐010‐034‐MY3, MOST 103‐2314‐B‐010‐050‐MY2, and MOST 106‐2314‐B‐075‐051‐MY3), an intramural grant from the Taipei Veterans General Hospital (grant V104C‐140), Research and Development contract NO1‐AG‐1‐2118, grants from the Ministry of Health and Welfare (MOHW104‐TDU‐B‐211‐113‐003, MOHW106‐TDU‐B‐211‐113001), and the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Disclosures
None.
Supporting information
Data S1. Simulation studies.
Table S1. Univariate Analysis of the Associations of Various Parameters Describing Night‐to‐Morning Systolic Blood Pressure Changes in Continuous Scale With All‐Cause and Cardiovascular Mortality
Table S2. Multivariable Analysis of the Associations of Various Parameters Describing Night‐to‐Morning Systolic Blood Pressure Changes in Continuous Scale With All‐Cause and Cardiovascular Mortality
Table S3. Sensitivity Analysis by the Multivariable Analysis of Various Parameters Describing Night‐to‐Morning Systolic Blood Pressure Changes Using the 90th Percentile as the Thresholds for Predicting All‐Cause and Cardiovascular Mortality
Table S4. Sensitivity Analysis by Including Only Records With 80% Successful Measurements for the Analysis and at Least 8 BP Measurements Within the Time Interval From Sleep‐Trough Through to the Morning Period for Multivariable Cox Proportional Hazard Model
Table S5. Sensitivity Analysis by Multivariable Analysis Stratified According to Subjects Receiving Antihypertensive Agents or Not: Relationship With All‐Cause Mortality
Table S6. Results of Simulation Studies
Table S7. Reproducibility Analysis Based on a Previous Published Study Population6 (N=42)
Figure S1. The construction of the 6 simulated scenarios by assuming different measurement errors for the true sleep‐trough systolic blood pressure (SBP) in simulation study 1.
(J Am Heart Assoc. 2017;6:e007667 DOI: 10.1161/JAHA.117.007667.)29223957
Contributor Information
Hao‐Min Cheng, Email: hmcheng@vghtpe.gov.tw.
Chen‐Huan Chen, Email: chench@vghtpe.gov.tw.
References
- 1. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 2. Argentino C, Toni D, Rasura M, Violi F, Sacchetti ML, Allegretta A, Balsano F, Fieschi C. Circadian variation in the frequency of ischemic stroke. Stroke. 1990;21:387–389. [DOI] [PubMed] [Google Scholar]
- 3. Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H; Hypertension Working Group. Ambulatory blood pressure: normality and comparison with other measurements. Hypertension. 1999;34:818–825. [DOI] [PubMed] [Google Scholar]
- 4. Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, Ohkubo T, Torp‐Pedersen C, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Sandoya E, Kawecka‐Jaszcz K, Ibsen H, Imai Y, Wang J, Staessen JA; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators . Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. [DOI] [PubMed] [Google Scholar]
- 6. Sheppard JP, Hodgkinson J, Riley R, Martin U, Bayliss S, McManus RJ. Prognostic significance of the morning blood pressure surge in clinical practice: a systematic review. Am J Hypertens. 2015;28:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: the Ohasama study. Hypertension. 2006;47:149–154. [DOI] [PubMed] [Google Scholar]
- 8. Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, Ambrosio G, Reboldi G. Day‐night dip and early‐morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012;60:34–42. [DOI] [PubMed] [Google Scholar]
- 9. Israel S, Israel A, Ben‐Dov IZ, Bursztyn M. The morning blood pressure surge and all‐cause mortality in patients referred for ambulatory blood pressure monitoring. Am J Hypertens. 2011;24:796–801. [DOI] [PubMed] [Google Scholar]
- 10. Wang JG, Kario K, Park JB, Chen CH. Morning blood pressure monitoring in the management of hypertension. J Hypertens. 2017;35:1554–1563. [DOI] [PubMed] [Google Scholar]
- 11. Fan H‐Q, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, Björklund‐Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Dolan E, Kuznetsova T, Stolarz‐Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka‐Jaszcz K, Imai Y, Ibsen H, O'Brien E, Wang J, Staessen JA; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators . Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. [DOI] [PubMed] [Google Scholar]
- 12. Chen CH, Ting CT, Lin SJ, Hsu TL, Chou P, Kuo HS, Wang SP, Yin FC, Chang MS. Relation between diurnal variation of blood pressure and left ventricular mass in a Chinese population. Am J Cardiol. 1995;75:1239–1243. [PubMed] [Google Scholar]
- 13. Sung SH, Cheng HM, Wang KL, Yu WC, Chuang SY, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. White coat hypertension is more risky than prehypertension: important role of arterial wave reflections. Hypertension. 2013;61:1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen CH, Ting CT, Lin SJ, Hsu TL, Ho SJ, Chou P, Chang MS, O'Connor F, Spurgeon H, Lakatta E, Yin FC. Which arterial and cardiac parameters best predict left ventricular mass? Circulation. 1998;98:422–428. [DOI] [PubMed] [Google Scholar]
- 15. Kikuya M, Hansen TW, Thijs L, Bjorklund‐Bodegard K, Kuznetsova T, Ohkubo T, Richart T, Torp‐Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA; International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators . Diagnostic thresholds for ambulatory blood pressure monitoring based on 10‐year cardiovascular risk. Circulation. 2007;115:2145–2152. [DOI] [PubMed] [Google Scholar]
- 16. Eguchi K, Hoshide S, Hoshide Y, Ishikawa S, Shimada K, Kario K. Reproducibility of ambulatory blood pressure in treated and untreated hypertensive patients. J Hypertens. 2010;28:918–924. [DOI] [PubMed] [Google Scholar]
- 17. Lu TH, Lee MC, Chou MC. Accuracy of cause‐of‐death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000;29:336–343. [DOI] [PubMed] [Google Scholar]
- 18. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta‐Analysis Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 19. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. [DOI] [PubMed] [Google Scholar]
- 20. Kario K. Time for focus on morning hypertension: pitfall of current antihypertensive medication. Am J Hypertens. 2005;18:149–151. [DOI] [PubMed] [Google Scholar]
- 21. Weber MA. The 24‐hour blood pressure pattern: does it have implications for morbidity and mortality? Am J Cardiol. 2002;89:27a–33a. [DOI] [PubMed] [Google Scholar]
- 22. Kaplan NM. Morning surge in blood pressure. Circulation. 2003;107:1347. [DOI] [PubMed] [Google Scholar]
- 23. Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J; Systolic Hypertension in Europe Trial Investigators. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA. 1999;282:539–546. [DOI] [PubMed] [Google Scholar]
- 24. Kario K. Is vascular morning blood pressure surge in the elderly resistant to antihypertensives and more risky? Hypertension. 2012;60:e16; author reply e17–e18. [DOI] [PubMed] [Google Scholar]
- 25. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals, part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 26. Gao M, Cheng HM, Sung SH, Chen CH, Olivier NB, Mukkamala R. Estimation of pulse transit time as a function of blood pressure using a nonlinear arterial tube‐load model. IEEE Trans Biomed Eng. 2017;64:1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Simulation studies.
Table S1. Univariate Analysis of the Associations of Various Parameters Describing Night‐to‐Morning Systolic Blood Pressure Changes in Continuous Scale With All‐Cause and Cardiovascular Mortality
Table S2. Multivariable Analysis of the Associations of Various Parameters Describing Night‐to‐Morning Systolic Blood Pressure Changes in Continuous Scale With All‐Cause and Cardiovascular Mortality
Table S3. Sensitivity Analysis by the Multivariable Analysis of Various Parameters Describing Night‐to‐Morning Systolic Blood Pressure Changes Using the 90th Percentile as the Thresholds for Predicting All‐Cause and Cardiovascular Mortality
Table S4. Sensitivity Analysis by Including Only Records With 80% Successful Measurements for the Analysis and at Least 8 BP Measurements Within the Time Interval From Sleep‐Trough Through to the Morning Period for Multivariable Cox Proportional Hazard Model
Table S5. Sensitivity Analysis by Multivariable Analysis Stratified According to Subjects Receiving Antihypertensive Agents or Not: Relationship With All‐Cause Mortality
Table S6. Results of Simulation Studies
Table S7. Reproducibility Analysis Based on a Previous Published Study Population6 (N=42)
Figure S1. The construction of the 6 simulated scenarios by assuming different measurement errors for the true sleep‐trough systolic blood pressure (SBP) in simulation study 1.


