Abstract
Background
The cardiovascular complications of cancer therapeutics are the focus of the burgeoning field of cardio‐oncology. A common challenge in this field is the impact of cancer drugs on cardiac repolarization (ie, QT prolongation) and the potential risk for the life‐threatening arrhythmia torsades de pointes. Although QT prolongation is not a perfect marker of arrhythmia risk, this has become a primary safety metric among oncologists. Cardiologists caring for patients receiving cancer treatment should become familiar with the drugs associated with QT prolongation, its incidence, and appropriate management strategies to provide meaningful consultation in this complex clinical scenario.
Methods and Results
In this article, we performed a systematic review (using Preferred Reporting Items of Systematic Reviews and Meta‐Analyses (PRISMA) guidelines) of commonly used cancer drugs to determine the incidence of QT prolongation and clinically relevant arrhythmias. We calculated summary estimates of the incidence of all and clinically relevant QT prolongation as well as arrhythmias and sudden cardiac death. We then describe strategies to prevent, identify, and manage QT prolongation in patients receiving cancer therapy. We identified a total of 173 relevant publications. The weighted incidence of any corrected QT (QTc) prolongation in our systematic review in patients treated with conventional therapies (eg, anthracyclines) ranged from 0% to 22%, although QTc >500 ms, arrhythmias, or sudden cardiac death was extremely rare. The risk of QTc prolongation with targeted therapies (eg, small molecular tyrosine kinase inhibitors) ranged between 0% and 22.7% with severe prolongation (QTc >500 ms) reported in 0% to 5.2% of the patients. Arrhythmias and sudden cardiac death were rare.
Conclusions
Our systematic review demonstrates that there is variability in the incidence of QTc prolongation of various cancer drugs; however, the clinical consequence, as defined by arrhythmias or sudden cardiac death, remains rare.
Keywords: cancer therapy, cardiac arrhythmia, cardio‐oncology, ECG, oncology, QT interval electrocardiography, sudden death, tyrosine kinase inhibitors, torsade de pointes
Subject Categories: Electrophysiology, Arrhythmias, Electrocardiology (ECG)
Clinical Perspective
What Is New?
We provide a systematic review of the available literature on corrected QT (QTc) prolongation attributable to cancer therapy.
Any QTc prolongation is common with both conventional and targeted cancer therapy; however, the incidence of significant QTc prolongation (to >500 ms) is more common with targeted therapy.
The reported incidence of arrhythmias and sudden cardiac death attributable to QTc prolongation from cancer therapy is extremely rare in the literature.
What Are the Clinical Implications?
Our systematic review provides a reliable estimate of the risk of developing QTc prolongation for many cancer drugs that can be used to educate physicians and patients.
When using drugs that are associated with an elevated incidence of QTc prolongation, careful monitoring is required during treatment.
Careful evaluation with a rigorous measurement of the QT interval is an important strategy to prevent unnecessary cessation of cancer therapy and to minimize the risk of arrhythmias.
Prompt treatment of severe QTc prolongation is needed and should be regarded as an emergency if linked with arrhythmic events or cardiac symptoms, such as syncope.
The advances in treatment of cancer have led to significant improvement in cancer‐related mortality.1 Although many of the conventional drugs, such as the anthracyclines, continue to be used widely, there are many efficacious targeted therapies that are introduced into the market. An important off‐target effect of some of these drugs includes abnormalities in cardiac repolarization resulting in QT prolongation. QT prolongation has been linked to an increased risk of life‐threatening ventricular arrhythmia and reports of sudden cardiac death (SCD).2 Therefore, the management of the effects of cancer therapeutics on cardiac repolarization necessitates collaboration between oncologists and cardiologists. This systematic review of the literature of conventional and targeted anticancer therapies is intended to help the clinicians do the following: (1) appreciate the QT prolongation and arrhythmia potential of the many commonly used cancer drugs; (2) recognize the need for careful evaluation of the QT changes, especially in the context of other underlying ECG or cardiac abnormalities; and (3) understand strategies to investigate and manage patients with cancer therapy–induced QT prolongation, such that the risk of SCD is not increased and potentially lifesaving cancer therapy is not withheld inappropriately.
Methods
Systematic Search of Cancer Therapy‐Induced QT Prolongation
Search strategy
Our search adhered to the Preferred Reporting Items of Systematic Reviews and Meta‐Analyses (PRISMA) statement for systematic reviews.3 We performed a literature search with 3 databases: EMBASE, MEDLINE, and Cochrane Central Register of Controlled Trials databases (1974–December, 2015) using 3 concepts: (1) clinical trials, (2) individual anticancer drugs, and (3) cardiotoxicity. Only English‐language articles were reviewed. Reference lists of individual publications and review articles were searched manually for additional studies, and drug labels of individual drugs were reviewed.
Study selection
Phase 1 trials evaluating the effects on QT interval of individual cancer drugs at different doses, phase 2 trials, phase 3 randomized controlled trials, and phase 4 postmarketing studies with systematic monitoring and reporting of ECG data and cardiovascular safety were considered. Prospective cohort studies were included if systematic ECG monitoring was performed. Episodes of arrhythmia or SCD were not taken into account if there was an alternative explanation for their occurrence or if there was no ECG monitoring during the study. Older clinical trials without relevant information on the QT or nonsystematic collection of ECG data were excluded (Figure 1).
Figure 1.
Flow chart of articles: summary of the systematic review. CCRCT indicates Cochrane Central Register of Controlled Trials.
Data Management and Analysis: Data extraction
All data were extracted by 2 cardiologists (A.P.‐S. and C.G.) using predefined electronic data extraction forms, including number of subjects treated, number of subjects experiencing QT prolongation (any Common Terminology Cancer Adverse Events (CTCAE) scale version 3 grading), number of subjects experiencing grade III or more QT prolongation (ie, QT corrected prolongation of >60 ms from baseline or >500 ms), arrhythmia episodes, and SCD. No data on other cardiotoxic effects of the drugs were collected.
Data synthesis
The proportion of patients with QT prolongation or arrhythmia events or cases of SCD was calculated for every study. For each drug, a weighted average of the proportion of patients experiencing QT prolongation was calculated from all the studies using the number of patients treated in each study as the weighting factor.
Results
Our systematic search yielded 5263 articles; of those articles, 1189 full‐text articles were reviewed, and 173 were finally included (Figure 1). Summary of the various cancer drugs and the incidence of QT prolongation based on our systematic review is presented in Table 1, and a classification based on incidence of corrected QT (QTc) prolongation is provided in Table 2. Additional description of commonly used agents and their impact on QTc is summarized below.
Table 1.
Cancer Drugs and Their Effects on QTc Prolongation Identified From the Systematic Review
| Drug Type | Drug | No. of Studies | Total No. | Range of Patients With QTc Increase, %a | Weighted Average of Patients With QTc Increase, %a | Weighted Average of Patients With QTc >500 ms, % | Arrhythmia/SCD, No. |
|---|---|---|---|---|---|---|---|
| Antimetabolites4, 5 | Fluorouracil | 1 | 102 | 0 | 0 | 0 | 0/0 |
| Capecitabine | 1 | 52 | 19 | 19 | 0 | 0/0 | |
| Purine analogs6 | Fludarabine | 1 | 56 | 0 | 0 | 0 | 0/0 |
| Antimicrotubule agents7, 8 | Paclitaxel | 3 | 290 | 1–4 | 2.4 | 0 | 0/0 |
| Tyrosine kinase inhibitors9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 | Afatinib | 1 | 60 | 0 | 0 | 0 | 0/0 |
| Aflibercept | 1 | 43 | 4.6 | 4.6 | 0 | 0/0 | |
| Bosutinib | 2 | 87 | 0–37 | 11.5 | 0 | 0/0 | |
| Ceritinib | 1 | 130 | 0.7 | 0.7 | 0.7 | 0/0 | |
| Crizotinib | 2 | 101 | 0 | 0.9 | 0.9 | 0/0 | |
| Dasatinib | 10 (1 with paclitaxel, 1 with ixabepilone, 1 with cetuximab) | 611 | 1.6–73 | 8.0 | 1.0 | 1/0 | |
| Dovitinib | 2 | 49 | 3–15 | 8.1 | 4.1 | 0/0 | |
| Imatinib | 5 | 897 | <0.5–6.9 | 3.1 | 0.02 | 0/0 | |
| Lapatinib | 2 (with trastuzumab+paclitaxel) | 117 | 1.7 | 1.7 | 1.7 | 0/0 | |
| Lenvatinib | 2 | 319 | 0–8.1 | 6.5 | 1.2 | 0/0 | |
| Nilotinib | 13 | 3076 | 0–24 | 2.7 | 0.2 | 0/5 | |
| Nintedanib | 2 | 94 | 0–3.3 | 1.1 | 1.1 | 0/0 | |
| Pazopanib | 3 | 99 | 0–5.9 | 1.0 | 0 | 0/1 | |
| Ponatinib | 2 | 120 | 0–3.7 | 2.5 | 1.7 | 1/0 | |
| Sorafenib/sunitinib | 6 | 280 | 0–17.8 | 8.5 | 1.9 | 0/0 | |
| Vandetanib | 32 | 2567 | 0–66.7 | 8.5 | 2.7 | 1/0 | |
| Histone deacetylase inhibitors82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98 | Belinostat | 3 | 195 | 0–36.0 | 8.7 | 4.1 | 1/0 |
| Panobinostat | 10 (2 with bevacizumab, 1 with everolimus) | 654 | 0–31.4 | 4.4 | 0.7 | 0/0 | |
| Romidepsin | 2 | 112 | 0–2.1 | 1.8 | 0 | 0/0 | |
| Vorinostat | 6 | 189 | 0–35.7 | 12.2 | 3.2 | 0/0 | |
| Proteasome inhibitor99, 100 | Bortezomib | 2 | 22 | 0–10 | 4.5 | 4.5 | 0/0 |
| Vascular endothelial growth factor inhibitors101, 102, 103, 104 | Cediranib | 4 (1 with FOLFOX) | 127 | 7.7–20.5 | 14.2 | 2.4 | 0/0 |
| Antiangiogenic105, 106, 107, 108, 109 | Combretastatin (CA4P) | 3 | 110 | 6.5–72 | 22.7 | 0.9 | 0/0 |
| Vadimezan (ASA404) | 4 | 77 | 0–100 | 20.8 | 5.2 | 0/0 | |
| Protein kinase C inhibitor110, 111, 112, 113, 114 | Enzastaurin | 5 | 135 | 6–24 | 11.8 | 2 | 0/0 |
| Monoclonal antibodies115, 116, 117, 118 | Trastuzumab and Pertuzumab | 4 | 167 | 0 | 0 | 0 | 0/0 |
| B‐Raf inhibitor119, 120 | Vemurafenib | 2 | 3597 | 0–6.5 | 2.2 | 1.8 | 2/0 |
| Other121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136 | Arsenic trioxide | 15 | 533 | 0–38 | 22.0 | 5.8 | 24/1 |
FOLFOX indicates folinic acid, fluorouracil, and oxaliplatin; QTc, corrected QT; and SCD, sudden cardiac death.
Common terminology cancer adverse events scale grade ≥I.
Table 2.
Classification of the QTc Prolongation Potential Cancer Drugs Based on Our Systematic Review
| Classification | Drug |
|---|---|
| High risk (>10% incidence) |
Arsenic trioxide Bosutinib Capecitabine Cediranib Combretastatin (CA4P) Enzastaurin Vadimezan Vorinostat |
| Moderate risk (5%–10% incidence) |
Belinostat Dasatinib Dovitinib Lenvatinib Sorafenib Sunitinib Vandetanib |
| Low risk (1%–5% incidence) |
Aflibercept Imatinib Lapatinib Nilotinib Nintedanib Paclitaxel Panobinostat Ponatinib Romidepsin Vemurafenib |
| Very low risk (<1% incidence) |
Anthracyclines Fluorouracil Afatinib Ceritinib Crizotinib Fludarabine Pazopanib Pertuzumab Trastuzumab |
QTc indicates corrected QT.
Nontargeted Cancer Therapy
Arsenic trioxide
Arsenic trioxide is used in the treatment of refractory or relapsed acute promyelocytic leukemia. The package insert reports that QTc prolongation >500 ms occurs in up to 40% of patients.121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136 Individual cases of SCD have been described.137 The severity of the clinical presentation is variable, with some patients experiencing marked QTc prolongation, but QTc prolongation is not seen with oral administration.138 Therefore, a baseline 12‐lead ECG and electrolyte levels are recommended. Concomitant QT‐prolonging agents should be discontinued, and electrolytes should be normal before and during treatment.133 If QTc increases to >500 ms, continuation of arsenic trioxide should be carefully evaluated. QTc should be monitored at least weekly with a 12‐lead ECG or with any symptoms. If a patient develops torsades de pointes (TdP) and the treatment needs to be resumed, it should be administered in a monitored unit.
Anthracyclines (eg, doxorubicin, idarubicin, epirubicin)
Anthracyclines are used in many common cancers. ECG monitoring was common in initial trials with only rare instances of QTc prolongation.139, 140, 141, 142, 143, 144, 145 QTc prolongation in the short‐term setting (10–20 ms) has been reported, with case reports and case series of patients presenting with acute cardiotoxicity and heart failure coupled with QTc prolongation. However, these were always in the setting of other coexisting conditions and use of other QTc‐prolonging drugs.146, 147 Despite the widespread use of anthracyclines, there does not appear to be an increased incidence of TdP.148, 149
Antimetabolites
Fluorouracil is used in many common malignancies, such as breast and colon cancer. Its cardiotoxicity manifests as angina and coronary vasospasm. Its proarrhythmic effect is linked with ischemia. Careful assessment in 102 patients receiving fluorouracil demonstrated a mild increase in QTc (mean, 15 ms)4, 150 and ventricular premature complexes on Holter monitoring. Capecitabine is a prodrug to fluorouracil. There are some isolated cases of ventricular arrhythmia related to ischemia. QTc prolongation appears to be more frequent in patients with previously known left ventricular dysfunction, previous irradiation, or trastuzumab therapy, but no QTc prolongation–related arrhythmias have been documented.5
Alkylating and alkylating‐like agents and purine analogs
Cyclophosphamide is a widely used agent with no clearly demonstrated arrhythmogenicity. Average QTc prolongation of 20 ms after high‐dose cyclophosphamide added to other drugs before autologous bone marrow transplantation has been reported in a small study of patients with non‐Hodgkin lymphoma.151 However, no arrhythmias were seen. Incidence of QTc prolongation with cisplatin, carboplatin, or oxaliplatin has not been reported. Effects of fludarabine were reported to be null in a series of patients.6
Antimicrotubule agents
Paclitaxel is used in many malignancies, including breast, lung, and ovarian cancer. Despite a consistent bradycardic effect and orthostatic hypotension in taxane‐treated patients, only mild and infrequent QTc prolongation has been reported.7, 8, 152
Targeted Cancer Therapies
Small‐molecule tyrosine kinase inhibitors
Small‐molecule tyrosine kinase inhibitors (TKIs) are used in the treatment of hematological malignancies and solid tumors, such as renal cell carcinoma and gastrointestinal tumors. The effects of TKIs on QTc are different between agents. QTc prolongation was frequent (>5% of patients experiencing CTCAE scale grade I QTc prolongation) in patients treated with dasatinib, vandetanib, sorafenib, or sunitinib. Dasatinib is used to treat hematological malignancies and has been associated with QTc prolongation in 8% of treated patients (range, 1%–70%), but QTc >500 ms was seen only in <1%.13, 18, 20, 21, 22, 25, 32, 40, 42, 75
Vandetanib is used to treat symptomatic or progressive medullary thyroid cancer and has a dose‐dependent effect on QTc prolongation,46 affecting 15% to 20% of patients.153 Reduction of dose reverses QTc prolongation.52 A meta‐analysis of 9 randomized trials with 4813 patients estimated a risk ratio for QTc prolongation versus control of 7.90 (95% confidence interval, 4.03–15.50).154 In our review, the weighted incidence of any vandetanib‐related QTc prolongation was 8.6%, with QTc >500 ms in 2.6%.43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 155, 156, 157 Because of its long half‐life (19 days), special care is needed when monitoring patients with QTc prolongation. Because of its clinical efficacy, vandetanib was approved by the Food and Drug Administration for human use in 2012, but with safe prescription strategies that include obtaining a baseline ECG and at 2 to 4 and 8 to 12 weeks after starting the treatment and every 3 months thereafter.158
Sorafenib and sunitinib are used for the treatment of advanced renal cell carcinoma, unresectable hepatocellular carcinoma, and gastrointestinal stromal tumors. QTc prolongation of <10 ms was observed in a small study with sorafenib.41 A carefully performed study of cardiac safety in patients receiving sorafenib and sunitinib showed an incidence of QTc prolongation of any degree of 9.5% among 86 patients.34, 159 No episodes of TdP were reported. Sunitinib has a dose‐dependent effect on QTc prolongation. Subsequent trials with sunitinib with cardiac safety monitoring have reported an average incidence of QTc prolongation of any degree of 8.5% and a QTc >500 ms of 1.7% without any arrhythmia.15, 23, 28, 37
Nilotinib is approved for chronic myelogenous leukemia (Philadelphia chromosome positive). It is known to prolong QTc, with 5 cases of SCD reported in 867 patients treated in initial trials leading to a warning in the Food and Drug Administration labeling. Two subsequent phase 2 studies and one retrospective analysis showed that 2% to 8% of patients have a >60‐ms increase in QTc from baseline, with 1.2% developing a QTc of >500 ms,27, 160 without any cases of SCD. The weighted proportion of QTc prolongation of any grade with nilotinib was 2.7% with QTc of >500 ms, seen in 0.3% of cases. Caution and periodic ECG monitoring are advised when using nilotinib.71, 73, 74, 76, 77, 79, 80 QTc prolongation was common (37%) in patients with hepatic impairment treated with bosutinib,10 but this was not seen in other smaller studies.11, 12 QTc prolongation was infrequent or absent with afatinib, crizotinib, ceritinib, dovitinib, imatinib, lapatinib, lenvatinib, nintedanib, pazopanib, and ponatinib.9, 14, 16, 17, 19, 24, 26, 27, 29, 30, 31, 32, 33, 35, 36, 38, 39, 72, 78, 81, 161, 162, 163, 164
Monoclonal antibody–based TKIs
For trastuzumab, despite its known effects on left ventricular ejection fraction, no relevant changes to QTc have been documented.117, 118, 165 Similarly, pertuzumab has not shown QTc effects.115, 116 Bevacizumab has been used alone or in combination with other TKI and other chemotherapeutic agents without causing QTc prolongation, despite its cardiotoxicity potential.166, 167, 168, 169
Histone deacetylase inhibitors
This class of drug is used in the treatment of hematological malignancies, such as T‐cell lymphomas and multiple myeloma. The prevalence of QTc prolongation is frequent (10%–15%) in patients treated with vorinostat94, 95, 96, 97, 98, 170 and belinostat.171, 172, 173 Other histone deacetylase inhibitors, including panobinostat (used for multiple myeloma)82, 83, 84, 85, 86, 87, 88, 89, 90, 91 and romidepsin,92, 93, 174 have a lower incidence of QTc prolongation (≈1%).
Proteasome inhibitors
Bortezomib used in the treatment of multiple myeloma is the only proteasome inhibitor that has been associated with QTc prolongation of >500 ms in 1 patient in a pooled analysis of 2 studies involving 22 patients treated with bortezomib in combination with other chemotherapy.99, 100
Vascular endothelial growth factor (VEGF) inhibitors and vascular disruptors
These drugs are used in the treatment of various solid malignancies. Among the identified studies, QTc prolongation was seen in 14% of patients treated with cediranib101, 102, 103, 104 and 21% of patients treated with vadimezan (ASA404).108, 109, 175 Caution and periodic ECG monitoring is advised during the treatment with these agents. Aflibercept was also associated with a small proportion of QTc prolongation (5%) in one study.29 The effect of QTc prolongation of combretastatin‐A4 has been consistently shown in the literature, but no TdP events have been reported.106, 107 The incidence of QTc prolongation during infusion seems to be dose dependent and seems to affect virtually all patients treated, with increases of as much as 37 ms in patients treated at higher doses (80 mg/m2).105, 176, 177 However, this drug has not been approved for therapeutic use at present.
Protein kinase C inhibitors
Enzastaurin is a serine/threonine kinase inhibitor that targets protein kinase C and protein kinase B pathways. It is now in phase 3 trials and has antitumoral activity in non–small‐cell lung cancer. On the basis of phase 1 and 2 studies, QTc prolongation occurs in 12% of treated patients. No reports about arrhythmia have been published, but careful use is recommended with periodic monitoring of ECGs.110, 111, 112, 113, 114
BRAF inhibitors
Data from patients with metastatic melanoma treated with vemurafenib show QTc prolongations in 3.2% on average and QTc >500 ms in 2.3%, with only 0.06% incidence of arrhythmias.119, 120
Mechanisms of Drug‐Induced QTc Prolongation
The molecular mechanisms of QTc prolongation with many cancer drugs are not known. Interaction with the normal function of one of the potassium channel proteins of the cardiomyocytes (human Ether‐a‐go‐go (hERG)) seems to be the cause of QTc prolongation for arsenic trioxide and TKIs.178 For histone deacetylase inhibitors, whether the QTc prolongation is attributable to inhibition of hERG or other mechanisms is unknown. Concomitant use of drugs that inhibit the metabolism of the cancer drugs can also prolong QTc (eg, inhibitors of cytochrome P450 3A4 (CYP3A4) enzymes [herbal products, azole fungals, macrolides, and certain HIV medications] and CYP2D6 enzymes [eg, fluoxetine]). Alternatively, conditions that prevent elimination pathways of cancer drugs can prolong QTc (eg, renal and liver failure). There is also a potential for genetic predisposition to drug‐induced QTc prolongation,179 although specific associations have not been established for cancer therapeutics.
Discussion: Management of Patients at Risk or With Cancer Therapy–Related QT Prolongation
On the basis of our systematic review and our clinical experience, a suggested management approach for patients scheduled to start potential QT‐prolonging cancer therapy or for patients with QT prolongation during cancer treatment is described later and summarized in Figure 2.
Figure 2.
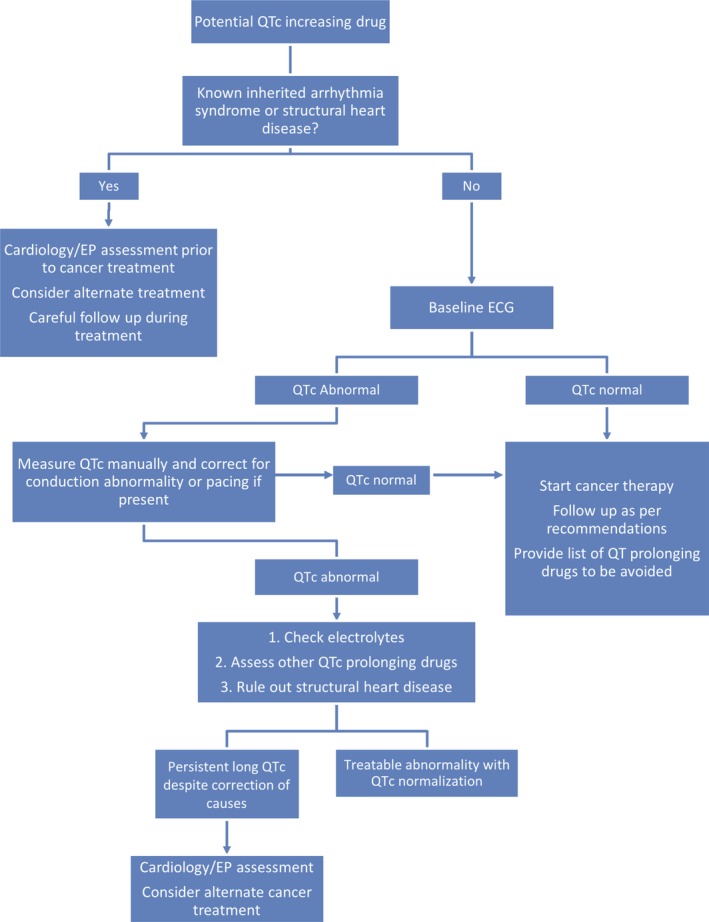
Algorithm of assessment of patients at risk of corrected QT (QTc) prolongation or with QTc prolongation before or during cancer treatment. EP (Electrophysiology)
Precancer Treatment Assessment and Prevention
In patients scheduled to receive potential QT‐prolonging cancer drugs, a complete medical and medication history (including nonprescription, recreational, and complementary/alternative medicines) should be obtained. In cancer patients being evaluated for clinical trials (before the start of cancer therapy), the prevalence of prolonged QTc has been reported to be ≈6%.180, 181, 182 Therefore, a pretreatment ECG should be performed to document QTc values. A risk score to identify individual patient‐specific risk of QTc prolongation during cancer therapy does not exist.
Appropriate measurement of QTc
Measurement of the QT should be based on leads that normally show the earliest QRS onset and the latest end of the T wave (T‐wave offset), which are II and V5. The end of the QT interval is the point at which the T wave reaches the isoelectric line. A normal QTc interval is >350 and <450 ms in adult men and >360 and <460 ms in adult women. Because the QT interval is inversely proportional to heart rate (HR), different formulas have been described to correct the QT interval for the HR. The objective of calculating the QTc is to obtain a patient's QT corrected to an HR of 60 beats per minute (bpm) which equals to an R interval to R interval (RR) time of 1000ms. The Bazett formula and the Fridericia formula () are based on the assumption of an exponential relationship between QT and the beat to beat interval (RR interval). This relationship is less precise for fast HRs and, hence, other formulas were suggested as alternatives, especially for faster HR (>90 bpm): the Framingham formula183 (), assuming a linear relationship, and the Hodges formula ().184 The Bazett and Fridericia formulas are used most commonly, but evidence supports correcting QT with the Hodges formula to be more accurate, especially at an HR >90 bpm.185 When an intraventricular conduction delay, left bundle branch block, right bundle branch block, or paced rhythm (usually adopting left bundle branch block–like morphological features) is present, a modified QT interval can be calculated by subtracting 48.5% of the duration of the QRS from the measured QT () and then correcting it for HR with conventional formulas or by taking a QTc of >550 ms as abnormal without any substraction.186 Subtracting the QRS duration from the QT measurement (ie, calculating the so‐called JT interval) and using a cutoff of >360 ms is an alternative to the modified QT interval calculation.187 Most ECG machines automatically report a QT interval by calculating the time between the earliest QRS onset of all leads and the latest offset of the T wave. As a result, the automatic QT interval is often longer than the QT interval from any individual lead. Also, automated measurements have not been validated in conduction abnormalities (eg, left bundle branch block) and, hence, manual measurement is the only option. Figure 3 provides useful examples of QT measurement and corrections in several ECG scenarios. It is our suggestion that the QTc calculation can be performed accurately with HR between 60 and 90 bpm with both Bazett and Fridericia formulas and that for HR >90 bpm, the Hodges correction is the most widely accepted. When a broad QRS of >120 ms (bundle branch block or conduction delay) is present using a QTc of >550 ms as a cutoff for abnormality is acceptable, but if baseline QTc is at the upper end of normal or for QRS that is wide but <120 ms, it is our advice to use the modified QT interval (see above) for a more precise and reproducible measurement.
Figure 3.
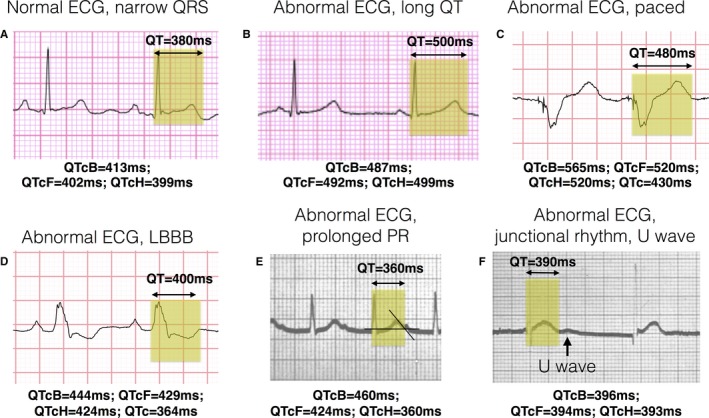
Examples of QT measurement and correction (QTc). ECG strips from lead II recorded at 25 mm/s and at 1 mm/mV with the measurement of the QT interval highlighted and calculations of different corrected measures: Bazett formula (QTcB), Hodges formula (QTcH), and Fridericia formula (QTcF). A correction with the Hodges formula is exemplified here: QTcH=QT+1.75×[heart rate (HR)−60]. A, A normal ECG with narrow QRS and a normal QT interval [QTcH=380+1.75×(71−60)=399 ms]. B, A narrow QRS with prolonged QT interval [QTcH=500+1.75×(57−60)=495 ms]. C, An example of a wide QRS because of a biventricular paced rhythm (note 2 small pacing spikes preceding the QRS) that falsely prolongs QT [QTcH=480+1.75(83−60)=520 ms, final QTc=QTcH−180×0.5=430 ms]. D, A wide QRS as a result of a left bundle branch block [LBBB; QTcH=400+1.75×(74−60)=424 ms, final QTc=QTcH−120×0.5=364 ms]. E, A patient with a prolonged PR interval of 360 ms with the P wave overlapping with the T‐wave recording; drawing an imaginary line following the downslope of the T wave is the accepted way of calculating the T‐wave offset and, thus, the end of the QT interval [QTcH=360+1.75×(98−60)=426 ms]. F, A patient with junctional bradycardia, where the T wave is followed by a subsequent wave (U wave) that should not be included in the QT measurement [QTcH=390+1.75×(62−60)=393 ms].
Identifying causes and risk for QT prolongation
If patients have QTc prolongation, correctable causes should be identified. First, electrolytes should be measured and any abnormalities (hypokalemia, hypomagnesemia, hypocalcemia) should be corrected before treatment. Patients with cancer specifically are at risk for electrolyte imbalance, especially during cancer treatment, because of the following: (1) poor oral intake and conditions that promote loss of potassium and/or magnesium, like salt‐losing nephropathy secondary to platinum salts; (2) diarrhea or emesis from mucositis; (3) fever with sweating; (4) treatment with laxatives; (5) alcohol abuse; and (6) treatment with corticosteroids. Drugs with potentially synergistic effects with cancer therapy to prolong QTc should be identified and modified or stopped. Table 3 provides a selective list of commonly used noncancer treatment drugs that prolong QTc and some safer alternatives, and an exhaustive list can be obtained from http://crediblemeds.org and is updated frequently.188 Other causes of baseline QTc prolongation include structural heart disease189 and genetic inherited arrhythmias, including Brugada syndrome, congenital long QT syndrome, and catecholaminergic polymorphic ventricular tachycardia. These patients should be evaluated by a cardiologist or cardiac electrophysiologist before cancer therapy with potential QT‐prolonging drugs. A comprehensive review of all medications that need to be avoided or the strategy for monitoring during the treatment should be defined carefully in these patients before cancer therapy.190
Table 3.
Noncancer Drugs Known to Cause QTc Prolongation
| Risk | Drug Categories | ||||
|---|---|---|---|---|---|
| Antiarrhythmic Drugs | Common Antibacterial and Antifungal Drugs | Prokinetic and Antiemetic Drugs | Antipsychotics | Antidepressants | |
| Known risk |
Amiodarone Disopyramide Dofetilide Dronedarone Flecainide Ibutilide Procainamide Quinidine Sotalol |
Moxifloxacin Levofloxacin Ciprofloxacin Clarithromycin Erythromycin Azithromycin Fluconazole Pentamidine |
Domperidone Chlorpromazine Ondansetron Droperidol |
Haloperidol Mesoridazine Thioridazine Pimozide |
Escitalopram Citalopram |
| Possible risk |
Telavancin Telithromycin Gemifloxacin Norfloxacin Ofloxacin |
Dolasetron Granisetron Promethazine Tropisetron |
Lithium Clozapine Paliperidone Risperidone Promethazine Perphenazine Pimavanserin Iloperidone Aripiprazole Asenapine |
Clomipramine Desipramine Imipramine Mirtazapine Nortriptyline Trimipramine Venlafaxine |
|
| Conditional risk | Ivabradine |
Amphotericin B Itraconazole Ketoconazole Metronidazole Posaconazole Voriconazole Cotrimoxazole (avoid in congenital long QT syndrome) |
Metoclopramide |
Quetiapine Olanzapine Ziprasidone |
Amitriptyline Doxepin Fluoxetine Fluvoxamine Paroxetine Setraline Trazodone |
| Alternatives |
Penicillin Cephalosporins Doxycycline Anidulafungin |
Aprepitant Fosaprepitant Palonosetron |
Brexpiprazole |
Desvenlafaxine Bupropion (except in supratherapeutic dose) Vortioxetine Vilazodone Levomilnacipran Milnacipran |
|
Known risk of torsades de pointes (TdP): These drugs prolong the QT interval and are clearly associated with a known risk of TdP, even when taken as recommended. Possible risk of TdP: These drugs can cause QT prolongation but lack evidence for a risk of TdP when taken as recommended. Conditional risk of TdP: These drugs could cause TdP only under certain conditions, such as excessive dosing, electrolyte imbalance, and interacting with other drugs that can cause TdP. Alternatives: Drugs that at this point have not been linked to clinically significant QTc prolongation.188 (Please see http://crediblemeds.org for an exhaustive list.) QTc indicates corrected QT.
Patient counseling and monitoring
Patients should be counseled about worrisome cardiac signs after starting the treatment (syncope, presyncope, fainting, rapid palpitations, or dizzy spells) that should prompt them to seek medical evaluation. With respect to early detection, ECGs should be repeated during treatment, as per drug labels. ECG monitoring after a change in dose of a QTc‐prolonging drug is recommended.191 However, because experience with some of the newer cancer drugs is limited, if concern for QTc prolongation exists, an ECG should be performed after every treatment cycle and when the plasma concentration of the drug reaches a steady state (eg, 5 half‐lives). The duration of monitoring will depend on the half‐life of all implicated drugs and the impairment in the elimination pathways. In hospitalized patients, if available, a QT‐alert system could be used to identify those who prolong their QTc during cancer treatment. This has been shown to be effective in the detection of patients (cancer and noncancer) at risk of TdP.192 Strategies to minimize the risk of cancer therapy–related QTc prolongation are summarized in Table 4.
Table 4.
Summary of Strategies to Minimize Cancer Therapy–Related QTc Prolongation and Risk of TdP190
| 1. Avoid use of QTc‐prolonging drugs in patients with pretreatment QTc >450 ms |
| 2. Discontinue QTc‐prolonging drug(s) if QTc interval prolongs to >500 or >550 ms if a baseline widening of QRS is present (>120 ms secondary to pacing or bundle branch block) |
| 3. Reduce dose or discontinue QTc‐prolonging drug(s) if the QTc increases ≥60 ms from pretreatment value |
| 4. Maintain electrolytes (serum potassium, magnesium, and calcium) concentration within normal range |
| 5. Avoid important known drug interactions |
| 6. Adjust doses of renally eliminated QTc‐prolonging drugs in patients with acute kidney injury or chronic kidney disease |
| 7. Avoid rapid intravenous administration of QTc‐prolonging drugs |
| 8. Administration of >1 drug with the potential to prolong the QT interval should be avoided |
| 9. Avoid use of QTc‐prolonging drugs in patients with a history of drug‐induced TdP or those who have previously been resuscitated from an episode of SCD |
| 10. Avoid use of QTc interval–prolonging drugs in patients who have been diagnosed as having one of the congenital long QT syndromes |
| 11. Monitor ECG with frequency, depending on ongoing therapy, drug concentration, and dose changes of QTc‐prolonging therapy |
QTc indicates corrected QT; SCD, sudden cardiac death; and TdP, torsades de pointes.
Management of QTc Prolongation
When a prolonged QTc interval is detected (>500 ms or an increase of >60 ms longer than baseline), the patient should be evaluated carefully, with discontinuation of all offending drugs immediately (if appropriate). Strategies previously discussed to identify causes of QTc prolongation should be considered. Patients who experience associated syncope or presyncope, palpitations, or QTc >500 ms and/or bradycardia (HR, <60 bpm) should be evaluated immediately in a monitored setting. An ECG should be repeated every 24 hours until resolution of QTc prolongation is confirmed. A prolonged QTc increases the risk of a potentially lethal ventricular arrhythmia called TdP (Figure 4A). Most of the data on the risk of TdP in relation to QTc prolongation are from large registries of patients with congenital long QT syndromes.193, 194 These data show that each 10‐ms increase in QTc contributes approximately a 5% to 7% increase in risk for cardiac events, including syncope, cardiac arrest, and/or death. Other risk factors for TdP beyond QTc have been described in settings outside of cancer therapy (Table 5) and, thus, the extrapolation to the cancer population is less clear.195 Although some medications are associated with QTc prolongation, not all drugs that prolong the QTc cause TdP. Therefore, the risk assessment of TdP attributable to drugs should not only be based on QTc alone but considered in the context of other predisposing TdP risk factors.196 Recurrence of TdP is frequent and, hence, the occurrence of a single event mandates urgent clinical evaluation and monitoring.
Figure 4.
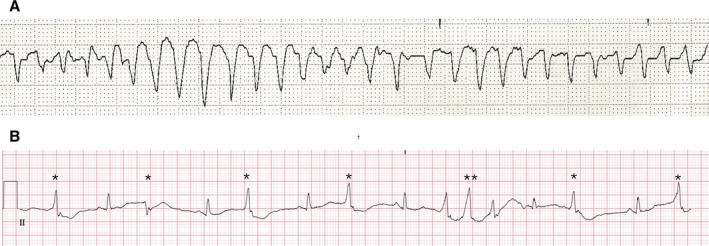
Torsades de pointes (TdP) and premonitory signs of TdP. A, Rhythm strip of a prolonged episode of TdP in a patient with congenital long QT syndrome and hypomagnesemia. B, Rhythm strip of a patient with prolonged corrected QT of 580 ms with frequent ventricular complexes of different morphological features (*) and triplets (**) indicating electrical instability and high risk of developing TdP.
Table 5.
Clinical Risk Factors of TdP
| Categories | Examples |
|---|---|
| Congenital | Congenital long QT syndrome |
| Physiological | Female sex, bradycardia, baseline QT prolongation |
| Structural heart disease | Myocardial ischemia, congestive heart failure, hypertrophic cardiomyopathy |
| Electrolytes | Hypokalemia, hypomagnesemia, hypocalcemia |
| Drugs | Digitalis therapy, other noncancer QT‐prolonging drugs (Table 3) |
| Arrhythmias | Recent conversion to sinus rhythm from atrial fibrillation with a QT‐prolonging drug (eg, amiodarone or dofetilide) |
| Other | Liver or renal dysfunction, hypothyroidism, hospitalization, intensive care unit stay |
TdP indicates torsades de pointes.
Drugs for correcting prolonged QTc should be started if worrisome ECG signs of TdP exist (eg, frequent ventricular premature beats or short runs of nonsustained ventricular tachycardia) or if TdP develops (asymptomatic or symptomatic). Premonitory and worrisome signs for TdP are prolonged QTc (>500 ms), severe aberration of the T‐U segment, beat‐to‐beat instability (more marked aberration of the T wave after a long R‐R interval), and/or frequent ventricular premature beats (Figure 4B). Patients with such abnormalities should be admitted to a cardiac care unit. The first‐line treatment is magnesium sulfate, given intravenously with repeated doses if signs of electric instability persist. Next is the initiation of a β‐adrenergic drug, such as isoproterenol, titrated to obtain an HR of >100 bpm with careful evaluation of the response, because patients with inherited congenital long QT syndrome could experience worsening symptoms. The role of antiarrhythmic therapies is less well established, but in case of refractory TdP, lidocaine infusion can be considered.197
Temporary ventricular or atrial pacing at 100 to 120 bpm should be considered if the patient is refractory to the previous measures. If the patient has a preexisting pacemaker or implantable cardioverter defibrillator system, changes in the lower rates can have the same protective effects. This measure leads to the disappearance of virtually all ventricular arrhythmias.198
Specialist Consultation
Cardiology and/or cardiac electrophysiology (EP) consultation is specifically advised in the following: (1) patients seen with a markedly prolonged QTc interval (>500 ms); (2) those receiving treatment with a known QTc‐prolonging drug, who experience symptoms suggestive of being of cardiac origin; and (3) those with known inherited arrhythmia disorders. Patients who experience associated syncope or presyncope suspected to be of cardiac origin, rapid palpitations, or QTc prolongation with new‐onset bradycardia (HR <60 bpm) and a high degree of heart block (second and third degree) are at high risk for repeated episodes and should be in a monitored setting with specialist consultation.199
Long‐Term Treatment
No recommendations on the role of cardiac implantable device insertion exist to date. Patients with severe bradycardia secondary to cancer therapy who are candidates for a QTc‐prolonging drug may benefit from a dual‐chamber pacemaker insertion to avoid symptomatic sinus bradycardia or sinus pauses that are risk factors for TdP. An implantable cardioverter defibrillator should be considered as follows: (1) if the life expectancy of the patient is >1 year, (2) if the patient has experienced resuscitated SCD, or (3) if the patient has experienced severe arrhythmia from a known QTc‐prolonging agent without any correctable cause and no alternative cancer treatment is available. These patients require careful individual evaluation and discussion in a multidisciplinary team to ensure that the risks and benefits of implantable cardioverter defibrillator therapy are considered. Also, the potential to turn off active implantable cardioverter defibrillator therapy if and when a patient reaches the palliative stage should be discussed.
Limitations
The translation of findings of QTc prolongation or associated arrhythmias from clinical trials from which our data are obtained to clinical practice is challenging. Most of the clinical trials of new cancer therapies in this review excluded patients with a baseline pre‐treatment QTc >450 ms, carefully followed up patients with repeated ECGs, or avoided the use of concomitant QTc‐prolonging drugs. Therefore, findings from such trials may not be generalizable to clinical practice, where such meticulous follow‐up may not be offered. Therefore, care must be taken in patients receiving potentially QTc‐prolonging drugs in routine clinical practice. Because our search was exhaustive, cancer therapies that are not listed in Table 1 are unlikely to cause clinically relevant QTc prolongation. However, because of the rapid pace of new drug discovery in the field of oncology, it is possible that new therapies causing QTc prolongation have been introduced into the market during the preparation of this article. Regardless, the concepts of diagnosis and management of QTc prolongation remain the same.
Conclusions
Patients with cancer receiving treatment are prone to QTc prolongation because of many risk factors and comorbidities. The true incidence of QTc prolongation and TdP from the multitude of cancer drugs is challenging to determine. In patients treated with conventional cancer drugs, the weighted incidence of any QTc prolongation in our systematic review varied between 0% and 22%, although QTc >500 ms, arrhythmias, or SCD was extremely rare. The risk of QTc prolongation with targeted therapies was also variable (0%–22.7%), with severe prolongation (QTc >500 ms) reported in 0% to 5.2% of the patients. However, arrhythmias and SCD were rare. Strategies to prevent QTc prolongation and the risk of subsequent TdP involve identification of potential drug interactions, correction of underlying electrolyte abnormalities, careful ECG monitoring, and patient education.
Sources of Funding
Dr. P Thavendiranathan is supported by the Canadian Institutes of Health Research New Investigator Award (FRN 147814).
Disclosures
None.
(J Am Heart Assoc. 2017;6:e007724 DOI: 10.1161/JAHA.117.007724.)29217664
References
- 1. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074–1077. [DOI] [PubMed] [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 4. Oztop I, Gencer M, Okan T, Yaren A, Altekin E, Turker S, Yilmaz U. Evaluation of cardiotoxicity of a combined bolus plus infusional 5‐fluorouracil/folinic acid treatment by echocardiography, plasma troponin I level, QT interval and dispersion in patients with gastrointestinal system cancers. Jpn J Clin Oncol. 2004;34:262–268. [DOI] [PubMed] [Google Scholar]
- 5. Koca D, Salman T, Unek IT, Oztop I, Ellidokuz H, Eren M, Yilmaz U. Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy. 2011;57:381–387. [DOI] [PubMed] [Google Scholar]
- 6. Yin W, Karyagina EV, Lundberg AS, Greenblatt DJ, Lister‐James J. Pharmacokinetics, bioavailability and effects on electrocardiographic parameters of oral fludarabine phosphate. Biopharm Drug Dispos. 2010;31:72–81. [DOI] [PubMed] [Google Scholar]
- 7. Conte PF, Gennari A. Anthracyclines‐paclitaxel combinations in the treatment of breast cancer. Ann Oncol. 1997;8:939–943. [DOI] [PubMed] [Google Scholar]
- 8. Kamineni P, Prakasa K, Hasan SP, Ravi A, Dawkins F. Cardiotoxicities of paclitaxel in African Americans. J Natl Med Assoc. 2004;96:995. [PMC free article] [PubMed] [Google Scholar]
- 9. Molife LR, Rudman SM, Alam S, Tan DS, Kristeleit H, Middleton G, Propper D, Bent L, Stopfer P, Uttenreuther‐Fischer M, Wallenstein G, de Bono J, Spicer J. Phase II, open‐label trial to assess QTcF effects, pharmacokinetics and antitumor activity of afatinib in patients with relapsed or refractory solid tumors. Cancer Chemother Pharmacol. 2013;72:1213–1222. [DOI] [PubMed] [Google Scholar]
- 10. Abbas R, Chalon S, Leister C, El Gaaloul M, Sonnichsen D. Evaluation of the pharmacokinetics and safety of bosutinib in patients with chronic hepatic impairment and matched healthy subjects. Cancer Chemother Pharmacol. 2013;71:123–132. [DOI] [PubMed] [Google Scholar]
- 11. Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D. Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol. 2011;51:1721–1727. [DOI] [PubMed] [Google Scholar]
- 12. Abbas R, Hug BA, Leister C, Sonnichsen D. A randomized, crossover, placebo‐ and moxifloxacin‐controlled study to evaluate the effects of bosutinib (SKI‐606), a dual Src/Abl tyrosine kinase inhibitor, on cardiac repolarization in healthy adult subjects. Int J Cancer. 2012;131:E304–E311. [DOI] [PubMed] [Google Scholar]
- 13. Algazi AP, Weber JS, Andrews SC, Urbas P, Munster PN, Deconti RC, Hwang J, Sondak VK, Messina JL, McCalmont T, Daud AI. Phase I clinical trial of the Src inhibitor dasatinib with dacarbazine in metastatic melanoma. Br J Cancer. 2012;106:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angevin E, Lopez‐Martin JA, Lin CC, Gschwend JE, Harzstark A, Castellano D, Soria JC, Sen P, Chang J, Shi M, Kay A, Escudier B. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19:1257–1268. [DOI] [PubMed] [Google Scholar]
- 15. Bergh J, Mariani G, Cardoso F, Liljegren A, Awada A, Vigano L, Huang X, Verkh L, Kern KA, Giorgetti C, Gianni L. Clinical and pharmacokinetic study of sunitinib and docetaxel in women with advanced breast cancer. Breast. 2012;21:507–513. [DOI] [PubMed] [Google Scholar]
- 16. Deininger MW, Kopecky KJ, Radich JP, Kamel‐Reid S, Stock W, Paietta E, Emanuel PD, Tallman M, Wadleigh M, Larson RA, Lipton JH, Slovak ML, Appelbaum FR, Druker BJ. Imatinib 800 mg daily induces deeper molecular responses than imatinib 400 mg daily: results of SWOG S0325, an intergroup randomized PHASE II trial in newly diagnosed chronic phase chronic myeloid leukaemia. Br J Haematol. 2014;164:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eisen T, Shparyk Y, Macleod N, Jones R, Wallenstein G, Temple G, Khder Y, Dallinger C, Studeny M, Loembe AB, Bondarenko I. Effect of small angiokinase inhibitor nintedanib (BIBF 1120) on QT interval in patients with previously untreated, advanced renal cell cancer in an open‐label, phase II study. Invest New Drugs. 2013;31:1283–1293. [DOI] [PubMed] [Google Scholar]
- 18. Fornier MN, Morris PG, Abbruzzi A, D'Andrea G, Gilewski T, Bromberg J, Dang C, Dickler M, Modi S, Seidman AD, Sklarin N, Chang J, Norton L, Hudis CA. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann Oncol. 2011;22:2575–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fumoleau P, Koch KM, Brain E, Lokiec F, Rezai K, Awada A, Hayward L, Werutsky G, Bogaerts J, Marreaud S, Cardoso F. A phase I pharmacokinetics study of lapatinib and tamoxifen in metastatic breast cancer (EORTC 10053 Lapatam study). Breast. 2014;23:663–669. [DOI] [PubMed] [Google Scholar]
- 20. Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, Antonia S, Gray J, Litschauer S, Tetteh L, Neuger A, Song L, Rawal B, Schell MJ, Bepler G. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbolsheimer P, Kapoor R, Smith KL, Perry D, Verma N, Veytsman I, Jelinek J, Swain SM. Phase I trial of dasatinib and ixabepilone in patients with solid tumors. Invest New Drugs. 2013;31:92–98. [DOI] [PubMed] [Google Scholar]
- 22. Johnson FM, Bekele BN, Feng L, Wistuba I, Tang XM, Tran HT, Erasmus JJ, Hwang LL, Takebe N, Blumenschein GR, Lippman SM, Stewart DJ. Phase II study of dasatinib in patients with advanced non‐small‐cell lung cancer. J Clin Oncol. 2010;28:4609–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, Lassman AB, Nolan CP, De Angelis LM, Gavrilovic I, Norden A, Drappatz J, Lee EQ, Purow B, Plotkin SR, Batchelor T, Abrey LE, Omuro A. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang YK, Yoo C, Ryoo BY, Lee JJ, Tan E, Park I, Park JH, Choi YJ, Jo J, Ryu JS, Ryu MH. Phase II study of dovitinib in patients with metastatic and/or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib. Br J Cancer. 2013;109:2309–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, Nakamae H, Huguet F, Boque C, Chuah C, Bleickardt E, Bradley‐Garelik MB, Zhu C, Szatrowski T, Shapiro D, Baccarani M. Dasatinib versus imatinib in newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. [DOI] [PubMed] [Google Scholar]
- 26. Kantarjian HM, Giles FJ, Bhalla KN, Pinilla‐Ibarz J, Larson RA, Gattermann N, Ottmann OG, Hochhaus A, Radich JP, Saglio G, Hughes TP, Martinelli G, Kim DW, Shou Y, Gallagher NJ, Blakesley R, Baccarani M, Cortes J, Le Coutre PD. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24‐month follow‐up results. Blood. 2011;117:1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kantarjian HM, Hochhaus A, Saglio G, Souza CD, Flinn IW, Stenke L, Goh YT, Rosti G, Nakamae H, Gallagher NJ, Hoenekopp A, Blakesley RE, Larson RA, Hughes TP. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome‐positive, chronic myeloid leukaemia: 24‐month minimum follow‐up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–851. [DOI] [PubMed] [Google Scholar]
- 28. Koeberle D, Montemurro M, Samaras P, Majno P, Simcock M, Limacher A, Lerch S, Katalin K, Inauen R, Hess V, Saletti P, Borner M, Roth A, Bodoky G. Continuous Sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06). Oncologist. 2010;15:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maison‐Blanche P, Vermorken JB, Goksel T, Machiels JP, Agarwala S, Rottey S, Daugaard G, Volovat C, Scheulen M, Sengelov L, Grecea D, Eniu A, Jager E, Meiri E, Cascinu S, Strumberg D, Demir G, Clemens M, Pinotti G, Nardi M, Guthrie T, Boelle E, Magherini E. A randomized, double blind, placebo‐controlled study to assess QTc interval prolongation of standard dose aflibercept in cancer patients treated with docetaxel. J Cardiovasc Pharmacol. 2013;61:495–504. [DOI] [PubMed] [Google Scholar]
- 30. Marcolino MS, Boersma E, Clementino NC, Nunes M, Barbosa MM, Silva MHC, Geleijnse ML, Ribeiro AL. The duration of the use of imatinib mesylate is only weakly related to elevated BNP levels in chronic myeloid leukaemia patients. Hematol Oncol. 2011;29:124–130. [DOI] [PubMed] [Google Scholar]
- 31. Ou SH, Tong WP, Azada M, Siwak‐Tapp C, Dy J, Stiber JA. Heart rate decrease during crizotinib treatment and potential correlation to clinical response. Cancer. 2013;119:1969–1975. [DOI] [PubMed] [Google Scholar]
- 32. Radich JP, Kopecky KJ, Appelbaum FR, Kamel‐Reid S, Stock W, Malnassy G, Paietta E, Wadleigh M, Larson RA, Emanuel P, Tallman M, Lipton J, Turner AR, Deininger M, Druker BJ. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic‐phase chronic myeloid leukemia. Blood. 2012;120:3898–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, De Las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine‐refractory thyroid cancer. N Engl J Med. 2015;372:621–630. [DOI] [PubMed] [Google Scholar]
- 34. Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. [DOI] [PubMed] [Google Scholar]
- 35. Shaw AT, Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, Vansteenkiste J, Sharma S, De Pas T, Riely GJ, Solomon BJ, Wolf J, Thomas M, Schuler M, Liu G, Santoro A, Lau YY, Goldwasser M, Boral AL, Engelman JA. Ceritinib in ALK‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;370:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaw AT, Ou SHI, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella‐Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, Zheng ZL, Tan WW, Stephenson P, Shreeve SM, Tye LM, Christensen JG, Wilner KD, Clark JW, Iafrate AJ. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shirao K, Nishida T, Doi T, Komatsu Y, Muro K, Li Y, Ueda E, Ohtsu A. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs. 2010;28:866–875. [DOI] [PubMed] [Google Scholar]
- 38. Shumaker RC, Zhou M, Ren M, Fan J, Martinez G, Aluri J, Darpo B. Effect of lenvatinib (E7080) on the QTc interval: results from a thorough QT study in healthy volunteers. Cancer Chemother Pharmacol. 2014;73:1109–1117. [DOI] [PubMed] [Google Scholar]
- 39. Sonnichsen D, Dorer DJ, Cortes J, Talpaz M, Deininger MW, Shah NP, Kantarjian HM, Bixby D, Mauro MJ, Flinn IW, Litwin J, Turner CD, Haluska FG. Analysis of the potential effect of ponatinib on the QTc interval in patients with refractory hematological malignancies. Cancer Chemother Pharmacol. 2013;71:1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi S, Miyazaki M, Okamoto I, Ito Y, Ueda K, Seriu T, Nakagawa K, Hatake K. Phase I study of dasatinib (BMS‐354825) in Japanese patients with solid tumors. Cancer Sci. 2011;102:2058–2064. [DOI] [PubMed] [Google Scholar]
- 41. Tolcher AW, Appleman LJ, Shapiro GI, Mita AC, Cihon F, Mazzu A, Sundaresan PR. A phase I open‐label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67:751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, Morris MJ, Hudes G, Calabro F, Cheng S, Trudel GC, Paliwal P, Sternberg CN. Phase II study of dasatinib in patients with metastatic castration‐resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Annunziata CM, Walker AJ, Minasian L, Yu M, Kotz H, Wood BJ, Calvo K, Choyke P, Kimm D, Steinberg SM, Kohn EC. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010;16:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arnold AM, Seymour L, Smylie M, Ding K, Ung Y, Findlay B, Lee CW, Djurfeldt M, Whitehead M, Ellis P, Goss G, Chan A, Meharchand J, Alam Y, Gregg R, Butts C, Langmuir P, Shepherd F; National Cancer Institute of Canada Clinical Trials Group Study BR . Phase II study of vandetanib or placebo in small‐cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25:4278–4284. [DOI] [PubMed] [Google Scholar]
- 45. Azad AA, Beardsley EK, Hotte SJ, Ellard SL, Klotz L, Chin J, Kollmannsberger C, Mukherjee SD, Chi KN. A randomized phase II efficacy and safety study of vandetanib (ZD6474) in combination with bicalutamide versus bicalutamide alone in patients with chemotherapy naive castration‐resistant prostate cancer. Invest New Drugs. 2014;32:746–752. [DOI] [PubMed] [Google Scholar]
- 46. Blackhall FH, O'Brien M, Schmid P, Nicolson M, Taylor P, Milenkova T, Kennedy SJ, Thatcher N. A phase I study of vandetanib in combination with vinorelbine/cisplatin or gemcitabine/cisplatin as first‐line treatment for advanced non‐small cell lung cancer. J Thorac Oncol. 2010;5:1285–1288. [DOI] [PubMed] [Google Scholar]
- 47. Broniscer A, Baker JN, Tagen M, Onar‐Thomas A, Gilbertson RJ, Davidoff AM, Panandiker AP, Leung W, Chin TK, Stewart CF, Kocak M, Rowland C, Merchant TE, Kaste SC, Gajjar A. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol. 2010;28:4762–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Broniscer A, Baker SD, Wetmore C, Pai Panandiker AS, Huang J, Davidoff AM, Onar‐Thomas A, Panetta JC, Chin TK, Merchant TE, Baker JN, Kaste SC, Gajjar A, Stewart CF. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res. 2013;19:3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chheda MG, Wen PY, Hochberg FH, Chi AS, Drappatz J, Eichler AF, Yang D, Beroukhim R, Norden AD, Gerstner ER, Betensky RA, Batchelor TT. Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study. J Neurooncol. 2015;121:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coleman RL, Moon J, Sood AK, Hu W, Delmore JE, Bonebrake AJ, Anderson GL, Chambers SK, Markman M. Randomised phase II study of docetaxel plus vandetanib versus docetaxel followed by vandetanib in patients with persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: SWOG S0904. Eur J Cancer. 2014;50:1638–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Boer R, Humblet Y, Wolf J, Nogova L, Ruffert K, Milenkova T, Smith R, Godwood A, Vansteenkiste J. An open‐label study of vandetanib with pemetrexed in patients with previously treated non‐small‐cell lung cancer. Ann Oncol. 2009;20:486–491. [DOI] [PubMed] [Google Scholar]
- 52. De Boer RH, Arrieta O, Yang CH, Gottfried M, Chan V, Raats J, De Marinis F, Abratt RP, Wolf J, Blackhall FH, Langmuir P, Milenkova T, Read J, Vansteenkiste JF. Vandetanib plus pemetrexed for the second‐line treatment of advanced non‐small‐cell lung cancer: a randomized, double‐blind phase III trial. J Clin Oncol. 2011;29:1067–1074. [DOI] [PubMed] [Google Scholar]
- 53. Fields EC, Damek D, Gaspar LE, Liu AK, Kavanagh BD, Waziri A, Lillehei K, Chen C. Phase I dose escalation trial of vandetanib with fractionated radiosurgery in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82:51–57. [DOI] [PubMed] [Google Scholar]
- 54. Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SM, Wells SA, Balis FM. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19:4239–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Halmos B, Jia Y, Bokar JA, Fu P, Adelstein DJ, Juergens R, Rodal MB, Dowlati A. A phase I study of the combination of oxaliplatin/docetaxel and vandetanib for the treatment of advanced gastroesophageal cancer. Invest New Drugs. 2013;31:1244–1250. [DOI] [PubMed] [Google Scholar]
- 56. Herbst RS, Sun Y, Eberhardt WEE, Germonpre P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE. Vandetanib plus docetaxel versus docetaxel as second‐line treatment for patients with advanced non‐small‐cell lung cancer (ZODIAC): a double‐blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heymach JV, Johnson BE, Prager D, Csada E, Roubec J, Pesek M, Spasova I, Belani CP, Bodrogi I, Gadgeel S, Kennedy SJ, Hou J, Herbst RS. Randomized, placebo‐controlled phase II study of vandetanib plus docetaxel in previously treated non small‐cell lung cancer. J Clin Oncol. 2007;25:4270–4277. [DOI] [PubMed] [Google Scholar]
- 58. Heymach JV, Paz‐Ares L, De Braud F, Sebastian M, Stewart DJ, Eberhardt WEE, Ranade AA, Cohen G, Trigo JM, Sandler AB, Bonomi PD, Herbst RS, Krebs AD, Vasselli J, Johnson BE. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first‐line treatment for advanced non‐small‐cell lung cancer. J Clin Oncol. 2008;26:5407–5415. [DOI] [PubMed] [Google Scholar]
- 59. Kiura K, Nakagawa K, Shinkai T, Eguchi K, Ohe Y, Yamamoto N, Tsuboi M, Yokota S, Seto T, Jiang H, Nishio K, Saijo N, Fukuoka M. A randomized, double‐blind, phase IIa dose‐finding study of vandetanib (ZD6474) in Japanese patients with non‐small cell lung cancer. J Thorac Oncol. 2008;3:386–393. [DOI] [PubMed] [Google Scholar]
- 60. Kreisl TN, McNeill KA, Sul J, Iwamoto FM, Shih J, Fine HA. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neuro Oncol. 2012;14:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kummar S, Gutierrez ME, Chen A, Turkbey IB, Allen D, Horneffer YR, Juwara L, Cao L, Yu Y, Kim YS, Trepel J, Chen H, Choyke P, Melillo G, Murgo AJ, Collins J, Doroshow JH. Phase I trial of vandetanib and bevacizumab evaluating the VEGF and EGF signal transduction pathways in adults with solid tumours and lymphomas. Eur J Cancer. 2011;47:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, Gomez JM, Bonichon F, Leenhardt L, Soufflet C, Licour M, Schlumberger MJ. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double‐blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. [DOI] [PubMed] [Google Scholar]
- 63. Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng RP, Chen YM, Emerson L, Langmuir P, Manegold C. Vandetanib versus placebo in patients with advanced non‐small‐cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double‐blind phase III trial (ZEPHYR). J Clin Oncol. 2012;30:1114–1121. [DOI] [PubMed] [Google Scholar]
- 64. Mayer EL, Isakoff SJ, Klement G, Downing SR, Chen WY, Hannagan K, Gelman R, Winer EP, Burstein HJ. Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res Treat. 2012;136:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meyerhardt JA, Ancukiewicz M, Abrams TA, Schrag D, Enzinger PC, Chan JA, Kulke MH, Wolpin BM, Goldstein M, Blaszkowsky L, Zhu AX, Elliott M, Regan E, Jain RK, Duda DG. Phase I study of cetuximab, irinotecan, and vandetanib (ZD6474) as therapy for patients with previously treated metastastic colorectal cancer. PLoS One. 2012;7:e38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Michael M, Gibbs P, Smith R, Godwood A, Oliver S, Tebbutt N. Open‐label phase I trial of vandetanib in combination with mFOLFOX6 in patients with advanced colorectal cancer. Invest New Drugs. 2009;27:253–261. [DOI] [PubMed] [Google Scholar]
- 67. Natale RB, Bodkin D, Govindan R, Sleckman BG, Rizvi NA, Capo A, Germonpre P, Eberhardt WEE, Stockman PK, Kennedy SJ, Ranson M. Vandetanib versus gefitinib in patients with advanced non‐small‐cell lung cancer: results from a two‐part, double‐blind, randomized phase II study. J Clin Oncol. 2009;27:2523–2529. [DOI] [PubMed] [Google Scholar]
- 68. Saletti P, Sessa C, De Dosso S, Cerny T, Renggli V, Koeberle D. Phase I dose‐finding study of vandetanib in combination with gemcitabine in locally advanced unresectable or metastatic pancreatic adenocarcinoma. Oncology. 2011;81:50–54. [DOI] [PubMed] [Google Scholar]
- 69. Saunders MP, Wilson R, Peeters M, Smith R, Godwood A, Oliver S, Van Cutsem E. Vandetanib with FOLFIRI in patients with advanced colorectal adenocarcinoma: results from an open‐label, multicentre phase I study. Cancer Chemother Pharmacol. 2009;64:665–672. [DOI] [PubMed] [Google Scholar]
- 70. Zhang L, Li S, Zhang Y, Zhan J, Zou BY, Smith R, Martin PD, Jiang Y, Liao H, Guan Z. Pharmacokinetics and tolerability of vandetanib in Chinese patients with solid, malignant tumors: an open‐label, phase I, rising multiple‐dose study. Clin Ther. 2011;33:315–327. [DOI] [PubMed] [Google Scholar]
- 71. Tojo A, Usuki K, Urabe A, Maeda Y, Kobayashi Y, Jinnai I, Ohyashiki K, Nishimura M, Kawaguchi T, Tanaka H, Miyamura K, Miyazaki Y, Hughes T, Branford S, Okamoto S, Ishikawa J, Okada M, Usui N, Tanii H, Amagasaki T, Natori H, Naoe T. A phase I/II study of nilotinib in Japanese patients with imatinib‐resistant or ‐intolerant Ph+ CML or relapsed/refractory Ph+ ALL. Int J Hematol. 2009;89:679–688. [DOI] [PubMed] [Google Scholar]
- 72. Dang C, Lin N, Moy B, Come S, Sugarman S, Morris P, Abbruzzi A, Chen C, Steingart R, Patil S, Norton L, Winer E, Hudis C. Dose‐dense doxorubicin and cyclophosphamide followed by weekly paclitaxel with trastuzumab and lapatinib in HER2/neu‐overexpressed/amplified breast cancer is not feasible because of excessive diarrhea. J Clin Oncol. 2010;28:2982–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koren‐Michowitz M, le Coutre P, Duyster J, Scheid C, Panayiotidis P, Prejzner W, Rowe JM, Schwarz M, Goldschmidt N, Nagler A. Activity and tolerability of nilotinib: a retrospective multicenter analysis of chronic myeloid leukemia patients who are imatinib resistant or intolerant. Cancer. 2010;116:4564–4572. [DOI] [PubMed] [Google Scholar]
- 74. Sawaki A, Nishida T, Doi T, Yamada Y, Komatsu Y, Kanda T, Kakeji Y, Onozawa Y, Yamasaki M, Ohtsu A. Phase 2 study of nilotinib as third‐line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011;117:4633–4641. [DOI] [PubMed] [Google Scholar]
- 75. Argiris A, Feinstein TM, Wang L, Yang T, Agrawal S, Appleman LJ, Stoller RG, Grandis JR, Egloff AM. Phase I and pharmacokinetic study of dasatinib and cetuximab in patients with advanced solid malignancies. Invest New Drugs. 2012;30:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim TD, le Coutre P, Schwarz M, Grille P, Levitin M, Fateh‐Moghadam S, Giles FJ, Dorken B, Haverkamp W, Kohncke C. Clinical cardiac safety profile of nilotinib. Haematologica. 2012;97:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nicolini FE, Turkina A, Shen ZX, Gallagher N, Jootar S, Powell BL, De Souza C, Zheng M, Szczudlo T, le Coutre P. Expanding nilotinib access in clinical trials (ENACT): an open‐label, multicenter study of oral nilotinib in adult patients with imatinib‐resistant or imatinib‐intolerant Philadelphia chromosome‐positive chronic myeloid leukemia in the chronic phase. Cancer. 2012;118:118–126. [DOI] [PubMed] [Google Scholar]
- 78. Inada‐Inoue M, Ando Y, Kawada K, Mitsuma A, Sawaki M, Yokoyama T, Sunakawa Y, Ishida H, Araki K, Yamashita K, Mizuno K, Nagashima F, Takekura A, Nagamatsu K, Sasaki Y. Phase 1 study of pazopanib alone or combined with lapatinib in Japanese patients with solid tumors. Cancer Chemother Pharmacol. 2014;73:673–683. [DOI] [PubMed] [Google Scholar]
- 79. Gordon JK, Martyanov V, Magro C, Wildman HF, Wood TA, Huang WT, Crow MK, Whitfield ML, Spiera RF. Nilotinib (Tasigna) in the treatment of early diffuse systemic sclerosis: an open‐label, pilot clinical trial. Arthritis Res Ther. 2015;17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nicolini FE, Etienne G, Dubruille V, Roy L, Huguet F, Legros L, Giraudier S, Coiteux V, Guerci‐Bresler A, Lenain P, Cony‐Makhoul P, Gardembas M, Hermet E, Rousselot P, Ame S, Gagnieu MC, Pivot C, Hayette S, Maguer‐Satta V, Etienne M, Dulucq S, Rea D, Mahon FX. Nilotinib and peginterferon alfa‐2a for newly diagnosed chronic‐phase chronic myeloid leukaemia (NiloPeg): a multicentre, non‐randomised, open‐label phase 2 study. Lancet Haematol. 2015;2:e37–e46. [DOI] [PubMed] [Google Scholar]
- 81. Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib‐resistant CML and Philadelphia chromosome–positive ALL. N Engl J Med. 2006;354:2542–2551. [DOI] [PubMed] [Google Scholar]
- 82. Drappatz J, Lee EQ, Hammond S, Grimm SA, Norden AD, Beroukhim R, Gerard M, Schiff D, Chi AS, Batchelor TT, Doherty LM, Ciampa AS, LaFrankie DC, Ruland S, Snodgrass SM, Raizer JJ, Wen PY. Phase I study of panobinostat in combination with bevacizumab for recurrent high‐grade glioma. J Neurooncol. 2012;107:133–138. [DOI] [PubMed] [Google Scholar]
- 83. Jones SF, Infante JR, Thompson DS, Mohyuddin A, Bendell JC, Yardley DA, Burris HA III. A phase I trial of oral administration of panobinostat in combination with paclitaxel and carboplatin in patients with solid tumors. Cancer Chemother Pharmacol. 2012;70:471–475. [DOI] [PubMed] [Google Scholar]
- 84. Oki Y, Buglio D, Fanale M, Fayad L, Copeland A, Romaguera J, Kwak LW, Pro B, De Castro Faria S, Neelapu S, Fowler N, Hagemeister F, Zhang J, Zhou S, Feng L, Younes A. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin Cancer Res. 2013;19:6882–6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rathkopf DE, Picus J, Hussain A, Ellard S, Chi KN, Nydam T, Allen‐Freda E, Mishra KK, Porro MG, Scher HI, Wilding G. A phase 2 study of intravenous panobinostat in patients with castration‐resistant prostate cancer. Cancer Chemother Pharmacol. 2013;72:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sharma S, Beck J, Mita M, Paul S, Woo MM, Squier M, Gadbaw B, Prince HM. A phase I dose‐escalation study of intravenous panobinostat in patients with lymphoma and solid tumors. Invest New Drugs. 2013;31:974–985. [DOI] [PubMed] [Google Scholar]
- 87. Tarhini AA, Zahoor H, McLaughlin B, Gooding WE, Schmitz JC, Siegfried JM, Socinski MA, Argiris A. Phase I trial of carboplatin and etoposide in combination with panobinostat in patients with lung cancer. Anticancer Res. 2013;33:4475–4482. [PMC free article] [PubMed] [Google Scholar]
- 88. Gray JE, Haura E, Chiappori A, Tanvetyanon T, Williams CC, Pinder‐Schenck M, Kish JA, Kreahling J, Lush R, Neuger A, Tetteh L, Akar A, Zhao X, Schell MJ, Bepler G, Altiok S. A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC inhibitor, combined with erlotinib in patients with advanced aerodigestive tract tumors. Clin Cancer Res. 2014;20:1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. San‐Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Gunther A, Nakorn TN, Siritanaratkul N, Corradini P, Chuncharunee S, Lee JJ, Schlossman RL, Shelekhova T, Yong K, Tan D, Numbenjapon T, Cavenagh JD, Hou J, LeBlanc R, Nahi H, Qiu L, Salwender H, Pulini S, Moreau P, Warzocha K, White D, Blade J, Chen W, de la Rubia J, Gimsing P, Lonial S, Kaufman JL, Ocio EM, Veskovski L, Sohn SK, Wang MC, Lee JH, Einsele H, Sopala M, Corrado C, Bengoudifa BR, Binlich F, Richardson PG. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double‐blind phase 3 trial. Lancet Oncol. 2014;15:1195–1206. [DOI] [PubMed] [Google Scholar]
- 90. Lee EQ, Reardon DA, Schiff D, Drappatz J, Muzikansky A, Grimm SA, Norden AD, Nayak L, Beroukhim R, Rinne ML, Chi AS, Batchelor TT, Hempfling K, McCluskey C, Smith KH, Gaffey SC, Wrigley B, Ligon KL, Raizer JJ, Wen PY. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015;17:862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sharma S, Witteveen PO, Lolkema MP, Hess D, Gelderblom H, Hussain SA, Porro MG, Waldron E, Valera SZ, Mu S. A phase I, open‐label, multicenter study to evaluate the pharmacokinetics and safety of oral panobinostat in patients with advanced solid tumors and varying degrees of renal function. Cancer Chemother Pharmacol. 2015;75:87–95. [DOI] [PubMed] [Google Scholar]
- 92. Otterson GA, Hodgson L, Pang H, Vokes EE; Cancer, Leukemia Group B . Phase II study of the histone deacetylase inhibitor Romidepsin in relapsed small cell lung cancer (Cancer and Leukemia Group B 30304). J Thorac Oncol. 2010;5:1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, Scarisbrick J, Reddy S, Robak T, Becker JC, Samtsov A, McCulloch W, Kim YH. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T‐cell lymphoma. J Clin Oncol. 2010;28:4485–4491. [DOI] [PubMed] [Google Scholar]
- 94. Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, Harris C, Zwiebel J, Wright JJ, Espinoza‐Delgado I, Baer MR, Holleran JL, Egorin MJ, Grant S. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Holkova B, Supko JG, Ames MM, Reid JM, Shapiro GI, Perkins EB, Ramakrishnan V, Tombes MB, Honeycutt C, McGovern RM, Kmieciak M, Shrader E, Wellons MD, Sankala H, Doyle A, Wright J, Roberts JD, Grant S. A phase I trial of vorinostat and alvocidib in patients with relapsed, refractory, or poor prognosis acute leukemia, or refractory anemia with excess blasts‐2. Clin Cancer Res. 2013;19:1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kirschbaum M, Gojo I, Goldberg SL, Bredeson C, Kujawski LA, Yang A, Marks P, Frankel P, Sun X, Tosolini A, Eid JE, Lubiniecki GM, Issa JP. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167:185–193. [DOI] [PubMed] [Google Scholar]
- 97. Tu Y, Hershman DL, Bhalla K, Fiskus W, Pellegrino CM, Andreopoulou E, Makower D, Kalinsky K, Fehn K, Fineberg S, Negassa A, Montgomery LL, Wiechmann LS, Alpaugh RK, Huang M, Sparano JA. A phase I‐II study of the histone deacetylase inhibitor vorinostat plus sequential weekly paclitaxel and doxorubicin‐cyclophosphamide in locally advanced breast cancer. Breast Cancer Res Treat. 2014;146:145–152. [DOI] [PubMed] [Google Scholar]
- 98. Han JY, Lee SH, Lee GK, Yun T, Lee YJ, Hwang KH, Kim JY, Kim HT. Phase I/II study of gefitinib (Iressa((R))) and vorinostat (IVORI) in previously treated patients with advanced non‐small cell lung cancer. Cancer Chemother Pharmacol. 2015;75:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lu S, Wang J, Xu X, Ni X, Huang C, Qiu H, Hu X, Yang J. Bortezomib in combination with epirubicin, dexamethasone and thalidomide is a highly effective regimen in the treatment of multiple myeloma: a single‐center experience. Int J Hematol. 2009;89:34–38. [DOI] [PubMed] [Google Scholar]
- 100. Messinger Y, Gaynon P, Raetz E, Hutchinson R, DuBois S, Glade‐Bender J, Sposto R, Van Der Giessen J, Eckroth E, Bostrom BC. Phase I study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr Blood Cancer. 2010;55:254–259. [DOI] [PubMed] [Google Scholar]
- 101. Fox E, Aplenc R, Bagatell R, Chuk MK, Dombi E, Goodspeed W, Goodwin A, Kromplewski M, Jayaprakash N, Marotti M, Brown KH, Wenrich B, Adamson PC, Widemann BC, Balis FM. A phase 1 trial and pharmacokinetic study of cediranib, an orally bioavailable pan‐vascular endothelial growth factor receptor inhibitor, in children and adolescents with refractory solid tumors. J Clin Oncol. 2010;28:5174–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Satoh T, Yamaguchi K, Boku N, Okamoto W, Shimamura T, Yamazaki K, Shi X, Mishima H. Phase I results from a two‐part phase I/II study of cediranib in combination with mFOLFOX6 in Japanese patients with metastatic colorectal cancer. Invest New Drugs. 2012;30:1511–1518. [DOI] [PubMed] [Google Scholar]
- 103. Trarbach T, Schultheis B, Gauler TC, Schneider V, Strumberg D, Eberhardt WEE, Le Scouiller S, Marotti M, Brown KH, Drevs J. Phase I open‐label study of cediranib, an oral inhibitor of VEGF signalling, in combination with the oral Src inhibitor saracatinib in patients with advanced solid tumours. Invest New Drugs. 2012;30:1962–1971. [DOI] [PubMed] [Google Scholar]
- 104. Dahut WL, Madan RA, Karakunnel JJ, Adelberg D, Gulley JL, Turkbey IB, Chau CH, Spencer SD, Mulquin M, Wright J, Parnes HL, Steinberg SM, Choyke PL, Figg WD. Phase II clinical trial of cediranib in patients with metastatic castration‐resistant prostate cancer. BJU Int. 2013;111:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cooney MM, Radivoyevitch T, Dowlati A, Overmoyer B, Levitan N, Robertson K, Levine SL, DeCaro K, Buchter C, Taylor A, Stambler BS, Remick SC. Cardiovascular safety profile of combretastatin A4 phosphate in a single‐dose phase I study in patients with advanced cancer. Clin Cancer Res. 2004;10:96–100. [DOI] [PubMed] [Google Scholar]
- 106. Ng QS, Mandeville H, Goh V, Alonzi R, Milner J, Carnell D, Meer K, Padhani AR, Saunders MI, Hoskin PJ. Phase Ib trial of radiotherapy in combination with combretastatin‐A4‐phosphate in patients with non‐small‐cell lung cancer, prostate adenocarcinoma, and squamous cell carcinoma of the head and neck. Ann Oncol. 2012;23:231–237. [DOI] [PubMed] [Google Scholar]
- 107. Rustin GJ, Shreeves G, Nathan PD, Gaya A, Ganesan TS, Wang D, Boxall J, Poupard L, Chaplin DJ, Stratford MR, Balkissoon J, Zweifel M. A phase Ib trial of CA4P (combretastatin A‐4 phosphate), carboplatin, and paclitaxel in patients with advanced cancer. Br J Cancer. 2010;102:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hida T, Tamiya M, Nishio M, Yamamoto N, Hirashima T, Horai T, Tanii H, Shi MM, Kobayashi K, Horio Y. Phase I study of intravenous ASA404 (vadimezan) administered in combination with paclitaxel and carboplatin in Japanese patients with non‐small cell lung cancer. Cancer Sci. 2011;102:845–851. [DOI] [PubMed] [Google Scholar]
- 109. Pili R, Rosenthal MA, Mainwaring PN, Van Hazel G, Srinivas S, Dreicer R, Goel S, Leach J, Wong S, Clingan P. Phase II study on the addition of ASA404 (vadimezan; 5,6‐dimethylxanthenone‐4‐acetic acid) to docetaxel in CRMPC. Clin Cancer Res. 2010;16:2906–2914. [DOI] [PubMed] [Google Scholar]
- 110. Carducci MA, Musib L, Kies MS, Pili R, Truong M, Brahmer JR, Cole P, Sullivan R, Riddle J, Schmidt J, Enas N, Sinha V, Thornton DE, Herbst RS. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24:4092–4099. [DOI] [PubMed] [Google Scholar]
- 111. Glimelius B, Lahn M, Gawande S, Cleverly A, Darstein C, Musib L, Liu Y, Spindler KL, Frodin JE, Berglund A, Bystrom P, Qvortrup C, Jakobsen A, Pfeiffer P. A window of opportunity phase II study of enzastaurin in chemonaive patients with asymptomatic metastatic colorectal cancer. Ann Oncol. 2010;21:1020–1026. [DOI] [PubMed] [Google Scholar]
- 112. Jourdan E, Leblond V, Maisonneuve H, Benhadji KA, Hossain AM, Nguyen TS, Wooldridge JE, Moreau P. A multicenter phase II study of single‐agent enzastaurin in previously treated multiple myeloma. Leuk Lymphoma. 2014;55:2013–2017. [DOI] [PubMed] [Google Scholar]
- 113. Rademaker‐Lakhai JM, Beerepoot LV, Mehra N, Radema SA, van Maanen R, Vermaat JS, Witteveen EO, Visseren‐Grul CM, Musib L, Enas N, van Hal G, Beijnen JH, Schellens JH, Voest EE. Phase I pharmacokinetic and pharmacodynamic study of the oral protein kinase C beta‐inhibitor enzastaurin in combination with gemcitabine and cisplatin in patients with advanced cancer. Clin Cancer Res. 2007;13:4474–4481. [DOI] [PubMed] [Google Scholar]
- 114. Tanai C, Ohe Y, Takahashi T, Kunitoh H, Murakami H, Yamamoto N, Nakamura Y, Nokihara H, Shukuya T, Baldwin JR, Koshiji M, Tamura T. A phase I study of enzastaurin combined with pemetrexed in advanced non‐small cell lung cancer. J Thorac Oncol. 2010;5:1068–1074. [DOI] [PubMed] [Google Scholar]
- 115. Garg A, Li J, Clark E, Knott A, Carrothers TJ, Marier JF, Cortes J, Brewster M, Visich J, Lum B. Exposure‐response analysis of pertuzumab in HER2‐positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol. 2013;72:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gupta M, Wang B, Carrothers TJ, LoRusso PM, Chu YW, Shih T, Loecke D, Joshi A, Saad O, Yi JH, Girish S. Effects of trastuzumab emtansine (T‐DM1) on QT interval and safety of pertuzumab plus T‐DM1 in patients with previously treated human epidermal growth factor receptor 2‐positive metastatic breast cancer. Clin Pharmacol Drug Dev. 2013;2:11–24. [DOI] [PubMed] [Google Scholar]
- 117. Xu N, Redfern CH, Gordon M, Eppler S, Lum BL, Trudeau C. Trastuzumab, in combination with carboplatin and docetaxel, does not prolong the QT interval of patients with HER2‐positive metastatic or locally advanced inoperable solid tumors: results from a phase Ib study. Cancer Chemother Pharmacol. 2014;74:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yavas O, Yazici M, Eren O, Oyan B. The acute effect of trastuzumab infusion on ECG parameters in metastatic breast cancer patients. Swiss Med Wkly. 2007;137:556–558. [DOI] [PubMed] [Google Scholar]
- 119. Flaherty L, Hamid O, Linette G, Schuchter L, Hallmeyer S, Gonzalez R, Cowey CL, Pavlick A, Kudrik F, Curti B, Lawson D, Chapman PB, Margolin K, Ribas A, McDermott D, Flaherty K, Cranmer L, Hodi FS, Day BM, Linke R, Hainsworth J. A single‐arm, open‐label, expanded access study of vemurafenib in patients with metastatic melanoma in the United States. Cancer J. 2014;20:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Larkin J, Del Vecchio M, Ascierto PA, Krajsova I, Schachter J, Neyns B, Espinosa E, Garbe C, Sileni VC, Gogas H, Miller WH, Mandala M, Hospers GAP, Arance A, Queirolo P, Hauschild A, Brown MP, Mitchell L, Veronese L, Blank CU. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open‐label, multicentre, safety study. Lancet Oncol. 2014;15:436–444. [DOI] [PubMed] [Google Scholar]
- 121. Ardalan B, Subbarayan PR, Ramos Y, Gonzalez M, Fernandez A, Mezentsev D, Reis I, Duncan R, Podolsky L, Lee K, Lima M, Ganjei‐Azar P. A phase I study of 5‐fluorouracil/leucovorin and arsenic trioxide for patients with refractory/relapsed colorectal carcinoma. Clin Cancer Res. 2010;16:3019–3027. [DOI] [PubMed] [Google Scholar]
- 122. Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21:3609–3615. [DOI] [PubMed] [Google Scholar]
- 123. Beer TM, Tangen CM, Nichols CR, Margolin KA, Dreicer R, Stephenson WT, Quinn DI, Raghavan D, Crawford ED. Southwest Oncology Group phase II study of arsenic trioxide in patients with refractory germ cell malignancies. Cancer. 2006;106:2624–2629. [DOI] [PubMed] [Google Scholar]
- 124. Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007;13:1762–1768. [DOI] [PubMed] [Google Scholar]
- 125. Fox E, Razzouk BI, Widemann BC, Xiao S, O'Brien M, Goodspeed W, Reaman GH, Blaney SM, Murgo AJ, Balis FM, Adamson PC. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008;111:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Grimm SA, Marymont M, Chandler JP, Muro K, Newman SB, Levy RM, Jovanovic B, McCarthy K, Raizer JJ. Phase I study of arsenic trioxide and temozolomide in combination with radiation therapy in patients with malignant gliomas. J Neurooncol. 2012;110:237–243. [DOI] [PubMed] [Google Scholar]
- 127. Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A, Reynolds J, Di Iulio J, Tiley C, Taylor K, Filshie R, Seldon M, Taper J, Szer J, Moore J, Bashford J, Seymour JF, Australasian L, Lymphoma G. All‐trans‐retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120:1570–1580; quiz 1752 [DOI] [PubMed] [Google Scholar]
- 128. Kindler HL, Aklilu M, Nattam S, Vokes EE. Arsenic trioxide in patients with adenocarcinoma of the pancreas refractory to gemcitabine: a phase II trial of the University of Chicago Phase II Consortium. Am J Clin Oncol. 2008;31:553–556. [DOI] [PubMed] [Google Scholar]
- 129. Raffoux E, Rousselot P, Poupon J, Daniel MT, Cassinat B, Delarue R, Taksin AL, Rea D, Buzyn A, Tibi A, Lebbe G, Cimerman P, Chomienne C, Fermand JP, De The H, Degos L, Hermine O, Dombret H. Combined treatment with arsenic trioxide and all‐trans‐retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol. 2003;21:2326–2334. [DOI] [PubMed] [Google Scholar]
- 130. Sekeres MA, MacIejewski JP, Erba HP, Afable M, Englehaupt R, Sobecks R, Advani A, Seel S, Chan J, Kalaycio ME. A phase 2 study of combination therapy with arsenic trioxide and gemtuzumab ozogamicin in patients with myelodysplastic syndromes or secondary acute myeloid leukemia. Cancer. 2011;117:1253–1261. [DOI] [PubMed] [Google Scholar]
- 131. Shigeno K, Naito K, Sahara N, Kobayashi M, Nakamura S, Fujisawa S, Shinjo K, Takeshita A, Ohno R, Ohnishi K. Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: updated outcomes of the phase II study and postremission therapies. Int J Hematol. 2005;82:224–229. [DOI] [PubMed] [Google Scholar]
- 132. Siu CW, Au WY, Yung C, Kumana CR, Lau CP, Kwong YL, Tse HF. Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications for long‐term cardiac safety. Blood. 2006;108:103–106. [DOI] [PubMed] [Google Scholar]
- 133. Tarhini AA, Kirkwood JM, Tawbi H, Gooding WE, Islam MF, Agarwala SS. Safety and efficacy of arsenic trioxide for patients with advanced metastatic melanoma. Cancer. 2008;112:1131–1138. [DOI] [PubMed] [Google Scholar]
- 134. Yamazaki K, Terada H, Satoh H, Naito K, Takeshita A, Uehara A, Katoh H, Ohnishi K, Hayashi H. Arrhythmogenic effects of arsenic trioxide in patients with acute promyelocytic leukemia and an electrophysiological study in isolated guinea pig papillary muscles. Circ J. 2006;70:1407–1414. [DOI] [PubMed] [Google Scholar]
- 135. Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, Shinjo K, Fujita Y, Matsui H, Takeshita A, Sugiyama S, Satoh H, Terada H, Ohno R. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. 2000;133:881–885. [DOI] [PubMed] [Google Scholar]
- 136. Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutre S, Dahlberg S, Ellison R, Warrell RP Jr. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. [DOI] [PubMed] [Google Scholar]
- 137. Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, Luger SM, Ma MK, Ley TJ, DiPersio JF. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. 2001;98:266–271. [DOI] [PubMed] [Google Scholar]
- 138. Au WY, Kumana CR, Lee HK, Lin SY, Liu H, Yeung DY, Lau JS, Kwong YL. Oral arsenic trioxide‐based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10‐year follow‐up study. Blood. 2011;118:6535–6543. [DOI] [PubMed] [Google Scholar]
- 139. Lahtinen R, Kuikka J, Nousiainen T, Uusitupa M, Lansimies E. Cardiotoxicity of epirubicin and doxorubicin: a double‐blind randomized study. Eur J Haematol. 1991;46:301–305. [DOI] [PubMed] [Google Scholar]
- 140. Lopez M, Vici P, Carpano S, Natali M, Ganzina F, Conti EM, Di Lauro L. Combination chemotherapy with oral idarubicin and cyclophosphamide for metastatic breast cancer. J Cancer Res Clin Oncol. 1991;117:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Smit EF, Piers DA, Postmus PE. Phase II study of high‐dose epirubicin and etoposide in advanced non‐small cell lung cancer. Eur J Cancer. 1992;28A:1965–1967. [DOI] [PubMed] [Google Scholar]
- 142. Colozza M, Gori S, Mosconi AM, Anastasi P, Basurto C, Ludovini V, De Angelis V, Giansanti M, Tonato M. Salvage chemotherapy in metastatic breast cancer: an experience with the combination of mitoxantrone, 5‐fluorouracil, and L‐leucovorin. Breast Cancer Res Treat. 1996;38:277–282. [DOI] [PubMed] [Google Scholar]
- 143. Martoni A, Guaraldi M, Piana E, Strocchi E, Petralia A, Busutti L, Preti P, Robustelli G, Raimondi M, Ferrara G, Palomba G, Lelli G, Picece V, Recaldin E, Caffo O, Ambrosini G, Sarobba G, Farris A, Pannuti F. Multicenter randomized clinical trial on high‐dose epirubicin plus cis‐platinum versus vinorelbine plus cis‐platinum in advanced non small cell lung cancer. Lung Cancer. 1998;22:31–38. [DOI] [PubMed] [Google Scholar]
- 144. Morandi P, Ruffini PA, Benvenuto GM, La Vecchia L, Mezzena G, Raimondi R. Serum cardiac troponin I levels and ECG/Echo monitoring in breast cancer patients undergoing high‐dose (7 g/m(2)) cyclophosphamide. Bone Marrow Transplant. 2001;28:277–282. [DOI] [PubMed] [Google Scholar]
- 145. Hussein MA, Wood L, Hsi E, Srkalovic G, Karam M, Elson P, Bukowski RM. A phase II trial of pegylated liposomal doxorubicin, vincristine, and reduced‐dose dexamethasone combination therapy in newly diagnosed multiple myeloma patients. Cancer. 2002;95:2160–2168. [DOI] [PubMed] [Google Scholar]
- 146. Iwata N, Karasawa M, Omine M, Maekawa T, Suzuki T, Kawai Y. Aclarubicin‐associated QTc prolongation and ventricular fibrillation. Cancer Treat Rep. 1984;68:527–529. [PubMed] [Google Scholar]
- 147. Kishi S, Yoshida A, Yamauchi T, Tsutani H, Lee JD, Nakamura T, Naiki H, Ueda T. Torsade de pointes associated with hypokalemia after anthracycline treatment in a patient with acute lymphocytic leukemia. Int J Hematol. 2000;71:172–179. [PubMed] [Google Scholar]
- 148. Gennari A, De Tursi M, Carella C, Ricevuto E, Orlandini C, Frassoldati A, Conte P, Bruzzi P, Iacobelli S. Epirubicin plus low‐dose trastuzumab in HER2 positive metastatic breast cancer. Breast Cancer Res Treat. 2009;115:131–136. [DOI] [PubMed] [Google Scholar]
- 149. Schmid P, Krocker J, Kreienberg R, Klare P, Kittel K, Sommer H, Heinrich G, Steck T, Lichtenegger W, Elling D, Kummel S. Non‐pegylated liposomal doxorubicin and docetaxel in metastatic breast cancer: final results of a phase II trial. Cancer Chemother Pharmacol. 2009;64:401–406. [DOI] [PubMed] [Google Scholar]
- 150. Wacker A, Lersch C, Scherpinski U, Reindl L, Seyfarth M. High incidence of angina pectoris in patients treated with 5‐fluorouracil. Oncology. 2003;65:108–112. [DOI] [PubMed] [Google Scholar]
- 151. Kuittinen T, Jantunen E, Vanninen E, Mussalo H, Nousiainen T, Hartikainen J. Late potentials and QT dispersion after high‐dose chemotherapy in patients with non‐Hodgkin lymphoma. Clin Physiol Funct Imaging. 2010;30:175–180. [DOI] [PubMed] [Google Scholar]
- 152. Rowinsky EK, McGuire WP, Guarnieri T, Fisherman JS, Christian MC, Donehower RC. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9:1704–1712. [DOI] [PubMed] [Google Scholar]
- 153. Martin P, Oliver S, Kennedy SJ, Partridge E, Hutchison M, Clarke D, Giles P. Pharmacokinetics of vandetanib: three phase I studies in healthy subjects. Clin Ther. 2012;34:221–237. [DOI] [PubMed] [Google Scholar]
- 154. Liu Y, Liu Y, Fan ZW, Li J, Xu GG. Meta‐analysis of the risks of hypertension and QTc prolongation in patients with advanced non‐small cell lung cancer who were receiving vandetanib. Eur J Clin Pharmacol. 2015;71:541–547. [DOI] [PubMed] [Google Scholar]
- 155. Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16:1391–1397. [DOI] [PubMed] [Google Scholar]
- 156. Tamura T, Minami H, Yamada Y, Yamamoto N, Shimoyama T, Murakami H, Horiike A, Fujisaka Y, Shinkai T, Tahara M, Kawada K, Ebi H, Sasaki Y, Jiang H, Saijo N. A phase I dose‐escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1:1002–1009. [PubMed] [Google Scholar]
- 157. Kovacs MJ, Reece DE, Marcellus D, Meyer RM, Mathews S, Dong RP, Eisenhauer E. A phase II study of ZD6474 (Zactima, a selective inhibitor of VEGFR and EGFR tyrosine kinase in patients with relapsed multiple myeloma—NCIC CTG IND.145. Invest New Drugs. 2006;24:529–535. [DOI] [PubMed] [Google Scholar]
- 158. Anon. DailyMed — CAPRELSA—vandetanib tablet. National Library of Medicine. 2017. Retrieved from: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=e5721cb8-4185-47b9-bbb3-1c587e558a03&type=display. Accessed October 10, 2017.
- 159. Bello CL, Mulay M, Huang X, Patyna S, Dinolfo M, Levine S, Van Vugt A, Toh M, Baum C, Rosen L. Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic‐ pharmacodynamic evaluation of sunitinib. Clin Cancer Res. 2009;15:7045–7052. [DOI] [PubMed] [Google Scholar]
- 160. Larson RA, Yin OQP, Hochhaus A, Saglio G, Clark RE, Nakamae H, Gallagher NJ, Demirhan E, Hughes TP, Kantarjian HM, Le Coutre PD. Population pharmacokinetic and exposure‐response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase. Eur J Clin Pharmacol. 2012;68:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dogan E, Yorgun H, Petekkaya I, Ozer N, Altundag K, Ozisik Y. Evaluation of cardiac safety of lapatinib therapy for ErbB2‐positive metastatic breast cancer: a single center experience. Med Oncol. 2012;29:3232–3239. [DOI] [PubMed] [Google Scholar]
- 162. Valachis A, Nearchou A, Lind P, Mauri D. Lapatinib, trastuzumab or the combination added to preoperative chemotherapy for breast cancer: a meta‐analysis of randomized evidence. Breast Cancer Res Treat. 2012;135:655–662. [DOI] [PubMed] [Google Scholar]
- 163. de Braud F, Cascinu S, Spitaleri G, Pilz K, Clementi L, Liu D, Sikken P, De Pas T. A phase I, dose‐escalation study of volasertib combined with nintedanib in advanced solid tumors. Ann Oncol. 2015;26:2341–2346. [DOI] [PubMed] [Google Scholar]
- 164. Heath EI, Infante J, Lewis LD, Luu T, Stephenson J, Tan AR, Kasubhai S, LoRusso P, Ma B, Suttle AB, Kleha JF, Ball HA, Dar MM. A randomized, double‐blind, placebo‐controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tonyali O, Coskun U, Sener N, Inanc M, Akman T, Oksuzoglu B, Ozdemir NY, Yazilitas D, Benekli M, Uner A, Yamac D, Demirci U, Yildiz R, Karaca H, Unal OU, Bal O, Gumus M, Buyukberber S; Anatolian Society of Medical O . Nine‐week trastuzumab treatment versus 52‐week trastuzumab treatment for HER2‐positive early‐stage breast cancer. J Cancer Res Clin Oncol. 2012;138:2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Seymour JF, Pfreundschuh M, Trneny M, Sehn LH, Catalano J, Csinady E, Moore N, Coiffier B; Investigators MS . R‐CHOP with or without bevacizumab in patients with previously untreated diffuse large B‐cell lymphoma: final MAIN study outcomes. Haematologica. 2014;99:1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Altomare I, Bendell JC, Bullock KE, Uronis HE, Morse MA, Hsu SD, Zafar SY, Blobe GC, Pang H, Honeycutt W, Sutton L, Hurwitz HI. A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist. 2011;16:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yardley DA, Hart L, Waterhouse D, Whorf R, Drosick DR, Murphy P, Badarinath S, Daniel BR, Childs BH, Burris H. Addition of bevacizumab to three docetaxel regimens as adjuvant therapy for early stage breast cancer. Breast Cancer Res Treat. 2013;142:655–665. [DOI] [PubMed] [Google Scholar]
- 169. Okines AFC, Langley RE, Thompson LC, Stenning SP, Stevenson L, Falk S, Seymour M, Coxon F, Middleton GW, Smith D, Evans L, Slater S, Waters J, Ford D, Hall M, Iveson TJ, Petty RD, Plummer C, Allum WH, Blazeby JM, Griffin M, Cunningham D. Bevacizumab with peri‐operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro‐oesophageal adenocarcinoma: a safety report. Ann Oncol. 2013;24:702–709. [DOI] [PubMed] [Google Scholar]
- 170. Munster PN, Rubin EH, Van Belle S, Friedman E, Patterson JK, Van Dyck K, Li X, Comisar W, Chodakewitz JA, Wagner JA, Iwamoto M. A single supratherapeutic dose of vorinostat does not prolong the QTc interval in patients with advanced cancer. Clin Cancer Res. 2009;15:7077–7084. [DOI] [PubMed] [Google Scholar]
- 171. Giaccone G, Rajan A, Berman A, Kelly RJ, Szabo E, Lopez‐Chavez A, Trepel J, Lee MJ, Cao L, Espinoza‐Delgado I, Spittler J, Loehrer PJ Sr. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol. 2011;29:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, Bhat G, Choi MR, Walewski J, Savage K, Foss F, Allen LF, Shustov A. Belinostat in patients with relapsed or refractory peripheral T‐cell lymphoma: results of the pivotal phase II BELIEF (CLN‐19) study. J Clin Oncol. 2015;33:2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Thomas A, Rajan A, Szabo E, Tomita Y, Carter CA, Scepura B, Lopez‐Chavez A, Lee MJ, Redon CE, Frosch A, Peer CJ, Chen Y, Piekarz R, Steinberg SM, Trepel JB, Figg WD, Schrump DS, Giaccone G. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: a clinical and translational study. Clin Cancer Res. 2014;20:5392–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kim M, Thompson LA, Wenger SD, O'Bryant CL. Romidepsin: a histone deacetylase inhibitor for refractory cutaneous T‐cell lymphoma. Ann Pharmacother. 2012;46:1340–1348. [DOI] [PubMed] [Google Scholar]
- 175. McKeage MJ, Fong P, Jeffery M, Baguley BC, Kestell P, Ravic M, Jameson MB. 5,6‐dimethylxanthenone‐4‐acetic acid in the treatment of refractory tumors: a phase I safety study of a vascular disrupting agent. Clin Cancer Res. 2006;12:1776–1784. [DOI] [PubMed] [Google Scholar]
- 176. He X, Li S, Huang H, Li Z, Chen L, Ye S, Huang J, Zhan J, Lin T. A pharmacokinetic and safety study of single dose intravenous combretastatin A4 phosphate in Chinese patients with refractory solid tumours. Br J Clin Pharmacol. 2011;71:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ibrahim MA, Do DV, Sepah YJ, Shah SM, Van Anden E, Hafiz G, Donahue JK, Rivers R, Balkissoon J, Handa JT, Campochiaro PA, Nguyen QD. Vascular disrupting agent for neovascular age related macular degeneration: a pilot study of the safety and efficacy of intravenous combretastatin A‐4 phosphate. BMC Pharmacol Toxicol. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wan X, Dennis AT, Obejero‐Paz C, Overholt JL, Heredia‐Moya J, Kirk KL, Ficker E. Oxidative inactivation of the lipid phosphatase phosphatase and tensin homolog on chromosome ten (PTEN) as a novel mechanism of acquired long QT syndrome. J Biol Chem. 2011;286:2843–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Napolitano C, Schwartz PJ, Brown AM, Ronchetti E, Bianchi L, Pinnavaia A, Acquaro G, Priori SG. Evidence for a cardiac ion channel mutation underlying drug‐induced QT prolongation and life‐threatening arrhythmias. J Cardiovasc Electrophysiol. 2000;11:691–696. [DOI] [PubMed] [Google Scholar]
- 180. Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–948. [DOI] [PubMed] [Google Scholar]
- 181. Naing A, Veasey‐Rodrigues H, Hong DS, Fu S, Falchook GS, Wheler JJ, Tsimberidou AM, Wen S, Fessahaye SN, Golden EC, Aaron J, Ewer MS, Kurzrock R. Electrocardiograms (ECGs) in phase I anticancer drug development: the MD Anderson Cancer Center experience with 8518 ECGs. Ann Oncol. 2012;23:2960–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Borad MJ, Soman AD, Benjamin M, Casa D, Tembe WD, Piper BF, Ramanathan R, Tibes R, Jameson G, Ansaldo K, Von Hoff DD. Effect of selection of QTc formula on eligibility of cancer patients for phase I clinical trials. Invest New Drugs. 2013;31:1056–1065. [DOI] [PubMed] [Google Scholar]
- 183. Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol. 1992;70:797–801. [DOI] [PubMed] [Google Scholar]
- 184. Hodges M, Salerno D, Erlien D. Bazett QT correction reviewed‐evidence that a linear QT correction for heart‐rate is better. J Am Coll Cardiol. 1983;1:694. [Google Scholar]
- 185. Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(supplement):81–90. [DOI] [PubMed] [Google Scholar]
- 186. Bogossian H, Frommeyer G, Ninios I, Hasan F, Nguyen QS, Karosiene Z, Mijic D, Kloppe A, Suleiman H, Bandorski D. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm. 2014;11:2273–2277. [DOI] [PubMed] [Google Scholar]
- 187. Chiladakis J, Kalogeropoulos A, Zagkli F, Koutsogiannis N, Chouchoulis K, Alexopoulos D. Predicting torsade de pointes in acquired long QT syndrome: optimal identification of critical QT interval prolongation. Cardiology. 2012;122:3–11. [DOI] [PubMed] [Google Scholar]
- 188. Woosley RL, Heise CW, Romero KA. Qtdrugs list. Retrieved from: https://www.crediblemeds.org/. Accessed October 10, 2017.
- 189. Weissler‐Snir A, Gollob MH, Chauhan V, Care M, Spears DA. Evaluation of prolonged QT interval: structural heart disease mimicking long QT syndrome. Pacing Clin Electrophysiol. 2017;40:417–424. [DOI] [PubMed] [Google Scholar]
- 190. Tisdale JE. Drug‐induced QT interval prolongation and torsades de pointes: role of the pharmacist in risk assessment, prevention and management. Can Pharm J (Ott). 2016;149:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and the American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution‐wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–325. [DOI] [PubMed] [Google Scholar]
- 193. Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. [DOI] [PubMed] [Google Scholar]
- 194. Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, Benhorin J, Locati EH, Towbin JA, Keating MT, Lehmann MH, Hall WJ. Influence of genotype on the clinical course of the long‐QT syndrome. International Long‐QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. [DOI] [PubMed] [Google Scholar]
- 195. Roden DM. Drug‐induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 196. Roden DM. Predicting drug‐induced QT prolongation and torsades de pointes. J Physiol. 2016;594:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Keren A, Tzivoni D, Gavish D, Levi J, Gottlieb S, Benhorin J, Stern S. Etiology, warning signs and therapy of torsade de pointes. A study of 10 patients. Circulation. 1981;64:1167–1174. [DOI] [PubMed] [Google Scholar]
- 198. Khan IA. Long QT syndrome: diagnosis and management. Am Heart J. 2002;143:7–14. [DOI] [PubMed] [Google Scholar]
- 199. Chorin E, Hochstadt A, Viskin S, Rozovski U, Havakuk O, Baranchuk A, Enriquez A, Strasberg B, Guevara‐Valdivia ME, Márquez MF, González‐Pacheco H, Hasdemir C, Rosso R. Female gender as independent risk factor of torsades de pointes during acquired atrioventricular block. Heart Rhythm. 2017;14:90–95. [DOI] [PubMed] [Google Scholar]


