Abstract
The development of imaging agents was initially driven following the discovery of X-ray technologies, but quickly evolved and expanded to include radiolabeling of cells and tissues to assist disease diagnosis and progression. The first imaging agents preceded the Great War but the field did not gain momentum until the 1950s. The approval rate for imaging NMEs continued at a high level for the remainder of the 20th century, but substantially decreased thereafter. This decline in approval rates corresponds with industry consolidation. Such losses have stabilized, but could have important implications for a field that has conveyed direct benefits to medicine and that could ensure the future of the wider biopharmaceutical industry.
Keywords: FDA, NME, imaging agents
Introduction
It is generally understood that early intervention against disease improves patient outcomes. Before the comparatively recent advent of modern medical imaging, the first signs of disease often reflected a gross manifestation of pathology. The advent of new means to generate and detect electromagnetic radiation presaged improvements that facilitated the identification and monitoring of disease, giving rise to the modern science of radiology. Whereas the earliest applications of ‘Roentgen rays’ were restricted to somewhat passive applications, such as the detection of broken bones, more sophisticated applications and higher resolutions of X-ray and other forms of electromagnetic radiation were made possible by contrast agents and advances in the selective targeting of diseased cells, such as scintigraphy. Together, these advancements revolutionized disease diagnosis and treatment.
As a brief review of the history of early medical imaging, the chase was on to apply this new technology to human medicine immediately following upon the late 19th-century discovery by Wilhelm Conrad Roentgen of what are now known as ‘X-rays’. As was vividly demonstrated by Roentgen in an early experiment, X-rays were used to image his wife Bertha’s hand and the radiographic images distinguished high-density bones (which absorb or attenuate the energy) from the lower-density soft tissues (which attenuate less energy and thus allow more energy to expose the photographic plate) [1]. Indeed, the high-density metals within Bertha’s wedding band (presumably gold or silver) provided an unplanned internal control demonstrating further how the density of an object could be distinguished by the high-energy Roentgen rays.
The potential use of X-rays for medical imaging was quickly embraced by a group of European urologists seeking new ways of imaging the ureter and bladder. Starting with Paris-based Theodore Tuffier, investigators sought to develop optically dense media, known as ‘contrast agents’, to distinguish the ureter from the bladder [2]. Among the attempts included the use of metal-containing catheters as well as the filling of the urinary bladder and other tissues with air, bismuth, and other dense metals [3]. Indeed, 1906 witnessed the first widely used contrast agents for X-rays in the form of colloidal silver (trade name ‘Collargol’), following reports by Fritz Voelcker and Alexander von Lichtenberg from Frederick University demonstrating its successful use for X-ray pyelography.
Like many pioneering products, Collargol was severely limited by observed toxicities and, although great effort was invested into addressing these issues, colloidal silver was eventually replaced by newer and safer contrast agents [4]. Thorium nitrate was another early contrast agent, introduced in 1915 by J. Edwards Burns of Johns Hopkins University [5]. However, the use of thorium nitrate was discontinued within a few years upon recognition of its astringent properties [3]. Thorium would reappear during the late 1920s in a colloidal form and, while the new product lacked most of its astringent properties, it would later be rendered obsolete by improved products. Tragically, in the window in which colloidal thorium (thorium dioxide) was widely used (administered to as many as 50 000 people, primarily in Europe), it is now understood that exposure to this intrinsically radioactive and thereby carcinogenic contrast agent contributed to countless cases of bladder, hepatic, and renal cancers [6]. Interestingly, the radioactive danger of thorium was first recognized in the USA, which limited its adoption in that country.
Another early product resulted from the discovery of Lipiodol by Marcel Guerbet in 1901 and its demonstrated use as a contrast agent for imaging uterine and fallopian tube patency in 1918. The entrepreneurial French chemist founded the first company based on imaging agents in 1926 (and which remains active in the field), thereby establishing an industry that would thrive for the rest of the 20th century.
A major breakthrough in early urographic imaging was achieved by Moses Swick, a young American urologist studying under Professor Leopold Lichtwitz in Hamburg, Germany [7]. Working with a team of chemical scientists led by Arthur Binz, Swick identified Selectan as a promising contrast agent and soon thereafter, a collaboration with additional German scientists, including Alexander von Lichtenberg, resulted in the 1929 discovery of a variant of Selectan, known as Uroselectan (and later marketed by Schering as Iopax). The use of iodinated products increased the hydrosolubility for arterial and venous injection and thereby ushered in a revolution in contrast agents that supported the rise of the emerging field of radiology [3].
The Center for Research Innovation in Biotechnology at Washington University in St Louis has been evaluating the sources of innovation for biomedical improvements. A series of publications in Drug Discovery Today identified therapeutic new molecular entities (NMEs) approved for use in the USA, including many put into practice before the creation of the modern US Food and Drug Administration (FDA) [8]. These publications sought to identify trends in the scientific, regulatory, and business practices guiding drug development. In doing so, we unexpectedly revealed troubling questions about the future of the drug development enterprise, with particular concern about the sustainability of early-stage research and development activities. Our prior work focused upon therapeutic NMES and did not capture imaging agents, an oversight that we address here.
The studies herein identified 122 novel NMEs that had been approved for use as imaging agents as of the end of 2015. Our work suggests that the rate of approvals accelerated during the third quarter of the 20th century and peaked during the final quarter of the century. Whereas the approval of imaging agents was largely centered around improving the quality and applicability of the new X-ray technology in the early years of the 20th century, the use of radiolabeled tracers, also known as scintigraphy, has since become the primary source of NMEs. Finally, the private sector organizations responsible for the approval of imaging agents have undergone waves of growth and consolidation that are similar yet different from those seen with their pharmaceutical counterparts.
Methods
Data sources and compilation
The analyses herein sought to identify all medical-imaging NMEs utilized in the USA and used many of the same criteria we invoked to analyze therapeutics [8]. Most imaging products were introduced following the creation of the FDA and, for many of the products, information was accessed from the FDA website (www.fda.gov). Some imaging agents were introduced before the creation of the FDA or might have been absent from published FDA records if they were no longer accessible because of obsolescence, toxicity, or commercial reasons that motivated withdrawal from the market. This subset of imaging agents was identified through meticulous searches of public databases, including those published by the American Medical Association, which archived the introduction of new therapeutic options via a series of regular reports from 1909 through to the mid-1940s in the Journal of the American Medical Association (JAMA) titled ‘New and Nonofficial Remedies’ and later in an annual book form titled New and Nonofficial Drugs.
Given the complexity and inconsistent nature of compiling early information, all data were independently verified by searches of public databases, including, but not necessarily limited to, published literature (www.ncbi.nlm.nih.gov/pubmed/) and searches of patent and trademarks (www.uspto.gov).
The information collected included the generic and trade names of each product as well as the date of FDA approval (at least the year and, ideally, the month and day) for the organization receiving the first approval. To identify missing information, priority was placed upon peer-reviewed scientific journals (via PubMed searches) and historical reviews of the field. Lacking this, a general web-based search was conducted, with emphasis upon reliable sources (information from recognized public sector organizations and/or private sector filings with the FDA or Securities and Exchange Commission).
Identification of innovator organizations
The work herein emphasized sources of innovation. The source(s) of innovative products were generally identified using information provided in publically accessible FDA documents. For approvals in which the reviews were publically available from the FDA (generally products approved during or after the mid-1990s), the medical reviews generally conveyed the organizations involved in the regulatory life cycle, including those associated with the submission of the investigational new drug (IND) application or its equivalent, any changes in custody as a result of licensing or mergers and acquisitions, end of Phase 1 and/or 2 meetings, as well as correspondence associated with the approval of the product. For those products where FDA documentation was not readily available, the organization, receiving date, and date of the final approval were documented and literature-based searches of Federal sites identified objective evidence of those organizations that might have contributed to the clinical development of the specific product at any point from the initial IND submission through to the final approval.
For many products approved before or during the mid-1990s, the information provided by the FDA often identified the current distributor of the product (and not necessarily the innovator organization if the product had changed hands as a result of licensing or acquisitive activities). In light of extensive industry consolidation, it was necessary to work backwards by asking whether any predecessor organizations had originated or contributed to the product before FDA approval. This was accomplished, when possible, by assessing the prior regulatory interactions with the FDA as indicated in the supporting documents associated with each Biologics License Application (BLA) approval. As a result of inconsistencies in reporting, this work was often supplemented with press releases from the FDA or companies associated with the product, with emphasis upon announcements of clinical trials initiations or outcomes. Additional assistance in identifying contributors to the product development was provided by searches of the US Patent and Trademark Office (by searching both granted and published patent and trademark applications, including expired trademarks).
Data availability
All data analyzed herein have been made available to the scientific community and general public on the website of the Center for Research Innovation in Biotechnology (http://crib.wustl.edu). We actively encourage all interested parties to explore the data and identify any improvements or additions that might be of use for interested investigators.
Results
Trends in early development
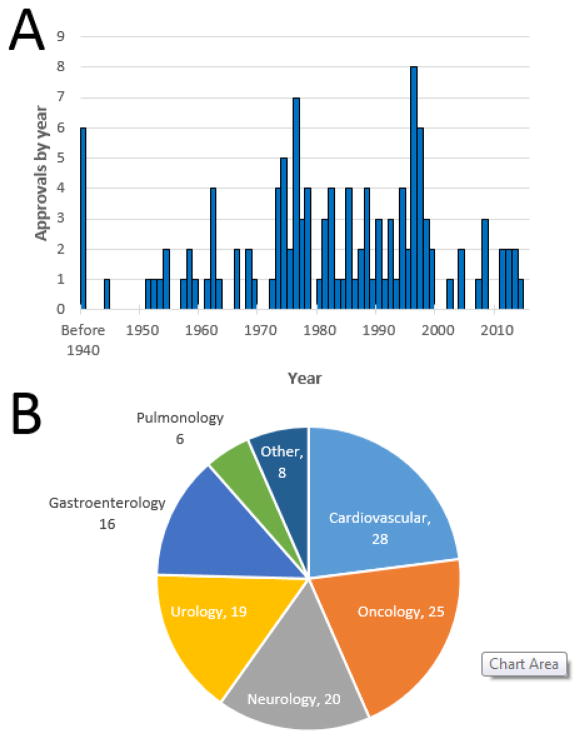
The introduction of Uroselectan/Iopax ushered in a new era of medical imaging. Early propagation of these initial successes was hampered by the onset of the Second World War, but the 1950s began an era of unprecedented advancements in radiology (Figure 1a). Nine NMEs were introduced during that decade and an impressive 11 NMEs followed during the 1960s. The progress continued and the number of NMEs for imaging more than doubled to 27 during the 1970s. The rate of new product introductions remained high, with 24 NMEs approved during the 1980s and 29 during the 1990s. With the advent of the new millennium, the rate slowed, with seven NMEs approved during 2001–2010 and seven additional NMEs approved during the first half of the ongoing decade.
Figure 1.
Overview of imaging new molecular entities (NMEs). (a) The approval rate of NMEs for imaging agents is shown on an annual basis over time. Please note that six NMEs were introduced in the USA from 1906 to 1940. (b) The 122 imaging NMEs were broadly grouped based on the indication for which they were first approved.
As indicated above, urology imaging was a major driver of early medical imaging and, overall, urology indications captured 20 of the 122 NMEs identified, including ten of the first 13 (77%) imaging agents introduced from 1906 through 1954 (Figure 1b). Other major clinical areas for which an imaging agent first gained FDA approval included cardiology (28 NMEs), oncology (25 NMEs), neurology (20 NMEs), and gastroenterology (16 NMEs). If one restricts the analyses to trends since the beginning of the new millennium, neurology has captured the most imaging NMEs (six of 14; 43%), followed by cardiology and oncology (three each).
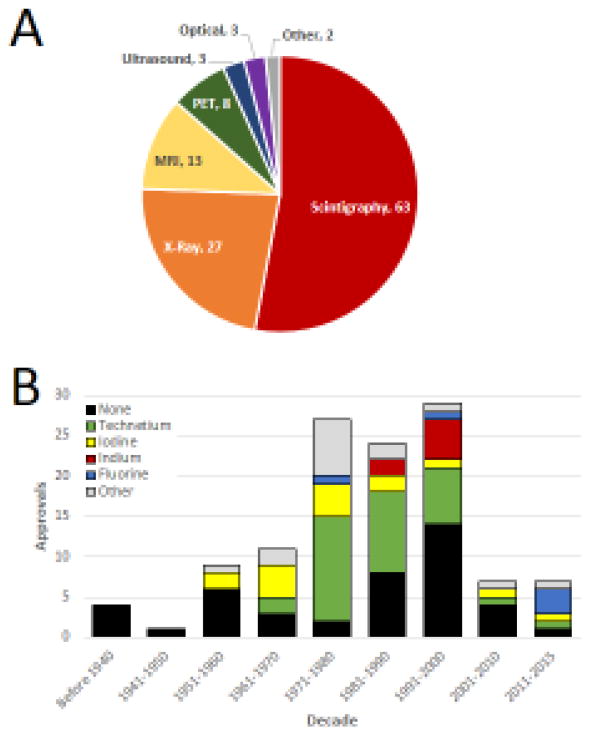
An alternative means to evaluate the application of radiology NMEs over time arose by linking each product to the technology for which it was originally approved (Figure 2a). Whereas X-ray technologies spurred much of the early years of the field (with emphasis on contrast agents), this was eclipsed by scintigraphy (defined herein as the use of a radio-labeled probes), which has captured more than half (63 of 122) of all imaging NMEs. Most of the remaining NMEs were developed for relatively newer technologies [magnetic resonance imaging (MRI), positron emission tomography (PET) and ultrasound].
Figure 2.
Uses and compositions of imaging agents. (a) All 122 imaging agents were broadly grouped into categories based on the imaging modality for which they were initially approved [or introduced if the new molecular entity (NME) pre-dated the formation of the modern US Food and Drug Administration (FDA). (b) The radionuclide incorporated into each imaging NME is indicated, with black bars signifying products that lacked a radiolabel.
Given the rise of scintigraphy and PET, we turned our attention to the radionuclides and asked whether and how these have changed over time (Figure 2b). Overall, just over one-third (46 of the 122) of the imaging agents identified were contrast agents or had other functions that did not encompass a radioactive tracer. Among the remaining radionuclide-containing NMEs, technetium was the most common tracer, accounting for 45% of all radioactive imaging products. When viewed over time, technetium-containing NMEs were first introduced during the late 1960s and the prominence of this radionuclide continued throughout the rest of the 20th century. Since then, the relative and absolute number of technetium-containing NMEs has shrunk, with only two approvals since 2001.
Similar findings were observed with iodine-labeled tracers, which first came to prominence during the 1950s, grew over the following two decades, and declining thereafter. Notably, a flurry of approvals for indium-labeled NMEs was observed during the late 1980s and 1990s, but no indium-containing NMEs have been approved since 1996. The first radioactive fluorine-labeled radiotracer, 18F-fluorodeoxyglucose (FDG), a glucose analog, was introduced in 1976. While this moved PET imaging forward into the clinical arena as a principal means of functional assessment of increased metabolic function [9], this particular radionuclide (18F) overall remained relatively rare until the current decade (2011–2015), during which three separate 18F PET radiotracers were approved.
Sources of innovation
Having evaluated major trends in the products themselves, we asked about the organizations responsible for the research and development of imaging-based NMEs. For products approved over the past three decades, much of the information about their research and development was available from the FDA directly in the form of the medical reviews for each product. For each imaging agent, we sought to identify the product sponsor and dates of key activities (IND submission, end of Phase 2 meetings, etc.). If the public FDA records were insufficient, historical data were evaluated in the form of patent and trademark holdings as well as public information obtained from the National Library of Medicine and archived corporate literature (e.g., annual reports and Securities and Exchange Commission filings).
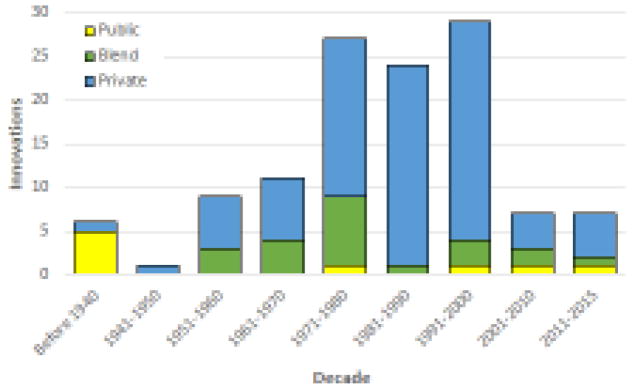
Public sector organizations (universities and government laboratories) were the source of early (five of the first six) products introduced in the USA (Figure 3). By contrast, the private sector was the primary or only driver of innovation for 98 of 100 products approved since the second half of the 20th century. Within this same period, public and/or public–private partnerships contributed to only 17 (17%) product approvals. However, the dominance of the private sector might be waning, because public and/or public–private sector partnerships contributed to five of the 14 (36%) NMEs approved so far since the start of the current century.
Figure 3.
The organizations that contributed to the research or development of each new molecular entity (NME). Many products were the result of collaboration among public and private sector organizations, which is indicated as a ‘blend’ and is marked with a green bar.
Forty different private sector organizations contributed to the approval of at least one imaging NME. The largest overall contributor to imaging agents was Mallinckrodt, which was responsible for the approval of 20 products (16% of all NMEs) (Table 1). Other major sources of NME innovation included Squibb, Roche (Medi-Physics), DuPont, and Schering AG, which together contributed to the approval of at least 40 additional products. Viewed another way, these five organizations account for almost half of all imaging agents.
Table 1.
The five organizations that contributed to the research or development of the largest number of imaging NMEsa
| Company | First | Total | Now |
|---|---|---|---|
| Mallinckrodt | 1962 | 20 | Acquired and relaunched |
| Squibb | 1989 | 13 | Bristol-Myers Squibb |
| Roche (Medi-Physics) | 1974 | 11 | GE Healthcare |
| DuPont | 1978 | 10 | Lantheus |
| Schering AG | 1952 | 6 | Bayer |
The table also gives the date of their first approval, the total number of NMEs introduced by the organization, and their current statues. Please note that the organizations shown in red are still active in imaging NME research and development based on publically available information, whereas there is no evidence for continued NME research for those companies shown in bold
Not all major sources of innovation continue to contribute to the research for the development of NMEs for imaging. For example, Mallinckrodt’s R&D efforts in the field appeared to wane in the years following its acquisition by Tyco International and subsequent changes in ownership and independence. Likewise, there is no evidence that research and development continues for novel imaging by Bristol-Myers Squibb (the current parent company of Squibb). Indeed, the other three major sources of innovation have themselves been subject to industry consolidation, with each being integrated into a larger entity (although we did find evidence for continued medical imaging R&D at GE Healthcare, Lantheus, and Bayer).
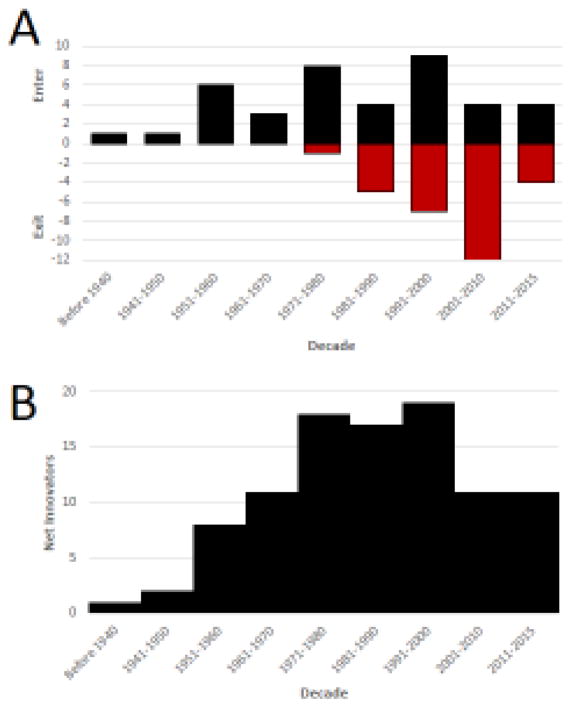
Such findings led us to assess the net number of private sector organizations that still contribute to the research and development of NMEs for medical imaging (Figure 4a). When viewed over time, the number of active and independent innovator organizations grew through to the end of the 20th century, peaking at 19 different companies in 2000 (Figure 4b). By the first decade of the new millennium, the net number of active and independent companies involved in imaging had declined to 11 and has remained stable thereafter. Four of the 29 companies that had existed since the 1970s were lost because of corporate dissolution or bankruptcy and the remaining 25 were subsumed or disbanded as a result of industry consolidation. Consequently, just over a quarter (11 of 40) companies that have ever contributed to the research or development of a novel imaging agent remain active and independent in imaging research today.
Figure 4.
Impact of industry consolidation. (a) The number of private sector organizations that have gained an approval for at least one imaging new molecular entity (NME) and which are still active in new NME research and development is shown on a decade-by-decade basis. Please note that the red bars on the negative axis reflect the impact of industry consolidation or bankruptcies that led to the loss of an organization involved in research and development. (b). The net number of private sector organizations with active NME research and development programs is shown over time.
Discussion
The major finding of our present study is that the introduction of FDA-approved medical imaging agents began in earnest during the 1950s and the rate of approvals peaked in the closing years of the 20th century. Whereas the science of urology drove the early years of medical imaging, cardiovascular, oncology, and neurology applications now dominate the field. Likewise, the foundations of medical imaging were initially based around the development of contrast agents to assist in radiological anatomic imaging. In total, 33 innovative products were approved with applications for anatomic imaging (see supplementary data online). However, scintigraphic and PET radiotracers designed to pursue metabolic and functional diagnoses have become the dominant technology for which NMEs have been developed over the past half century, which is reflected by the fact that 67 different products have been approved based on functional or molecular-based imaging of disease. These radiotracers provide information on tumor metabolism, demonstrating which tumors are metabolically active and malignant or indicating which tumors have reoccurred. The most basic 18F-FDG is a glucose analog, which is highly sensitive, but less specific and identifies any region of increased metabolic function. Others, such as the newly FDA-approved 11C-choline, designed to be used to assess for prostate cancer reoccurrence, are taken up only by tumors with upregulated choline transport and phosphorylation [10].
Imaging technological advances have often been the impetus for new imaging agent development. The introduction of CT during the mid 1970s ushered in additional iodinated contrast agents that could be used for both X-ray angiography and CT: ionic agents, and then non-ionic and low- and iso-osmolar agents, each providing added patient safety in terms of contrast reactions and nephrotoxicity. In total, 74 products were approved that utilized ionizing radiation, while 22 non-ionizing imaging agents have been introduced. Most (20 of 22) non-ionic products have been introduced since the late 1980s and reflect the growth of MRI. Developed during the 1980s, MRI required its own set of imaging agents, with the first MRI agent, gadopentetic acid, entering the market in 1987 [11]. Likewise ultrasound development initiated an influx of imaging agents, including gelatin and later albumin-shell microbubbles, to better depict anatomy, and sulfur hexafluoride microbubbles to characterize liver lesions [12,13]. Targeted microbubbles, currently experimental, provide the possibility of functional assessment using ultrasound. The latest imaging modality, optical imaging, not yet in widespread clinical use, provides the possibility to use infrared dyes to assess tumor margins intraoperatively.
However, it should be emphasized that not all technological advances require new imaging agents; for instance, low- and iso-osmolar CT contrast agents have translated well into use with newer dual-source, faster, higher temporal resolution CT scanners, which benefit the patient by allowing for less contrast agent use and radiation dose-lowering techniques.
The private sector has historically been responsible for the research and development of medical imaging agents, but in recent years, public sector organizations have increasingly contributed to imaging agent research and development. Such changes might be the result of industry consolidation, which has claimed almost three quarters of all private sector organizations that had been contributed to the approval of at least one imaging NME.
Similar to many fields of medicine, imaging has been disrupted by waves of scientific and technological advancements. The years following the identification of X-rays spurred the development of agents to increase the resolution and opacity of vital physiological structures, such as the ureter and bladder. Over time, the products introduced evolved from passive agents (e.g., contrast agents that enhanced the resolution of external beam radiation) to active agents. For example, 2D scintigraphy with radioactive tracers that seek out the desired cells or tissues facilitated the active search for diseased tissues as understanding of disease markers increased. These technologies in turn provided the framework for 3D and 4D analyses that allowed investigators to go beyond simply finding a particular tissue or organ to measuring its function using technologies such as PET scanning [14].
Despite profound advances in our understanding of disease in recent years, the number of NMEs has seemingly begun to decline during the 21st century. The trends observed over the past 15 years might simply reflect a nonrepresentative and short-term trend. Given the relatively short time since these changes have begun, it is too early to gain a true understanding of such findings. Nonetheless, the relative decline in new approvals coincides with a comparable decline in the number of private sector organizations involved in imaging research and development.
Were such a trend to remain durable, then new thinking might be required to ensure continued imaging advancements and NMEs in the future. One possibility, which might already be reflected in the findings presented herein, would be to emphasize a blend of private and public sector collaboration. The increasing sophistication and availability of both patients and medical imaging equipment to major academic centers could increase the emphasis upon public–private sector partnerships and, thus, ensure the continuity of novel NME development.
There are similarities and differences when comparing our results herein with imaging agents against prior analyses of therapeutic development. The similarities include the rise of the enterprise during the second half of the 20th century and the profound impact of consolidation in recent decades. A notable difference can be seen with the decline in the early years of the 21st century. In the case of biopharmaceuticals, the rate of decline, a result almost entirely of industry consolidation, has been considerably steeper and shows no signs of abating. By contrast, the number of private sector organizations involved in the research and development of imaging NMEs, while a fraction of the size of the biopharmaceutical industry in absolute terms, appears to have stabilized over the past two decades.
The need to conserve and expand our capabilities in medical imaging might be particularly relevant and vital for the larger therapeutic development enterprise. Many indicators suggest that future opportunities could incentivize a renewal in medical imaging and, thus, it is important to maintain the continuity as well as the breadth and depth of organizations involved. As demonstrated in studies by our group and others, declining efficiency and increasing costs caused many investors and organizations to partially or completely withdraw from the field [15–18]. Given the small number of ‘successful’ companies involved in imaging, such trends could easily disrupt the ability to continue delivering advancements in medical imaging, at a time when imaging itself could help promote a turnaround in the larger biopharmaceutical enterprise.
Despite these considerable opportunities, increasing regulatory constraints, combined with new challenges associated with the patenting of ‘natural products’, has undermined interest in the development of new imaging products. One hurdle arose as a result of the passage of HIPAA legislation, which required that information that could identify the patient be protected (delinked or decorrelated) by image acquisition and reading methodologies [19]. Compounding this, the FDA and other regulatory as well as reimbursement organizations are placing greater emphasis upon the need to definitively link new imaging markers with a demonstration of clinical benefit to ensure approval or reimbursement. All of these changes are also occurring within an industry that is facing newly realized pressures from generic competition as state-of-the-art technologies from decades before necessarily face patent expiries.
Recent advancements in the understanding and prosecution of oncology indications provides one means to understand how medical imaging could have an essential role in supporting the larger drug development enterprise. Targeted therapy is a prominent trend guiding the design of therapeutics targeting many cancers. Such strategies prosecute acquired mutations. Parallel advances in imaging technologies that identify and track such mutations throughout the body (as opposed to simply assessing levels in blood) could increase the effectiveness and efficiency of developing these new targeted therapies. Likewise, the ability to image immune-based clearance could guide the rapidly evolving field of immuno-oncology. As both imaging and targeted therapeutic technologies improve and are deployed more widely, it appears likely that many will be linked to image-based approaches that are used to identify patients for treatment and to monitor progress.
A forward-looking and highly promising opportunity is the potential use of new imaging agents to impact decision-making during the drug development process by addressing particularly challenging and historically intractable indications. A prominent example is the potential use of medical imaging to expedite or prioritize the development of agents meant to treat Alzheimer’s disease and other neurodegenerative conditions [20]. The development of new medicines to treat diseases of the brain and nervous system has generally fared poorly in recent years [21]. Many prominent failures have been attributed to a relative lack of reliable preclinical animal-based models for diseases in humans [22,23]. One can conceive that the use of targeted imaging could provide opportunities to decrease some of the risk that has plagued the development of therapies for neurological diseases. For example, low-dose imaging studies can provide opportunities for Phase 0 studies to assess whether, how, and when a targeted agent can be delivered to the desired site in the body and whether the intended therapy can act upon its intended targets [24]. Nonetheless, the challenges associated with predicting medical success have largely remained elusive and the regulatory challenges (including scientific challenges and increasing costs) needed to achieve this goal are not to be underestimated [25,26]. Given these potential opportunities, it appears essential that we retain our capabilities in the development of new medical imaging breakthroughs within both the public and private sectors.
Supplementary Material
Highlights.
122 new molecular entities (NMEs) for imaging have been approved for use in the United States as of the end of 2015.
Whereas the first products emphasized conventional x-rays, scintigraphy has dominated the past half century of approvals
Consolidation has reduced the number of active and independent companies by almost half since 2000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker GF, et al. Röntgen Rays: Memoirs. Harper & Brothers; 1899. [Google Scholar]
- 2.Tuffier T. Title. Br Med J. 1929;2:879. [Google Scholar]
- 3.Pollack HM. History of lodinated contrast media. In: Thomsen HS, et al., editors. Trends in Contrast Media. Springer; 1999. pp. 3–19. [Google Scholar]
- 4.Muller GL. Experimental bone marrow reactions: I. Anemia produced by collargol. J Exp Med. 1926;43:533–553. doi: 10.1084/jem.43.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns JE. Thorium: a new agent for pyelography: preliminary report. JAMA. 1915;64:2126–2127. [Google Scholar]
- 6.Casper J. The introduction in 1928–29 of thorium dioxide in diagnostic radiology. Ann NY Acad Sci. 1967;145:527–529. doi: 10.1111/j.1749-6632.1967.tb50255.x. [DOI] [PubMed] [Google Scholar]
- 7.Swick M. Intravenous urography by means of the sodium salt of 5-iodo2-pyridon-N-acetic acid. JAMA. 1930;95:1403–1409. [PubMed] [Google Scholar]
- 8.Kinch MS, et al. An overview of FDA-approved new molecular entities: 1827–2013. Drug Discov Today. 2014;19:1033–1039. doi: 10.1016/j.drudis.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med. 2008;49:17N–21N. [PubMed] [Google Scholar]
- 10.Parker WP, et al. Patterns of recurrence after postprostatectomy fossa radiation therapy identified by C-11 choline positron emission tomography/computed tomography. Int J Radiation Oncol Biol Physics. 2017;97:526–535. doi: 10.1016/j.ijrobp.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corot C, Warlin D. Superparamagnetic iron oxide nanoparticles for MRI: contrast media pharmaceutical company R&D perspective. Wiley Interdiscipl Rev Nanomed Nanobiotechnol. 2013;5:411–422. doi: 10.1002/wnan.1225. [DOI] [PubMed] [Google Scholar]
- 12.Carroll BA, et al. Ultrasonic contrast enhancement of tissue by encapsulated microbubbles. Radiology. 1982;143:747–750. doi: 10.1148/radiology.143.3.7079504. [DOI] [PubMed] [Google Scholar]
- 13.Bokor D, et al. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol. 2001;36:104–109. doi: 10.1097/00004424-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Singhal T. The history of cerebral PET scanning: From physiology to cutting-edge technology. Neurology. 2013;81:1275. doi: 10.1212/01.wnl.0000435802.68308.a1. [DOI] [PubMed] [Google Scholar]
- 15.Kinch MS, Moore R. Innovator organizations in new drug development: assessing the sustainability of the biopharmaceutical industry. Cell Chem Biol. 2016;23:644–653. doi: 10.1016/j.chembiol.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Scannell JW, et al. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 17.Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- 18.Pammolli F, et al. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10:428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- 19.Murad FM, et al. Image management systems. Gastrointestinal Endoscopy. 2014;79:15–22. doi: 10.1016/j.gie.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 20.Reardon S. Alzheimer antibody drugs show questionable potentia. Nat Rev Drug Discov. 2015;14:591–592. doi: 10.1038/nrd4709. [DOI] [PubMed] [Google Scholar]
- 21.Ringel M, et al. Does size matter in R&D productivity? If not, what does? Nat Rev Drug Discov. 2013;12:901–902. doi: 10.1038/nrd4164. [DOI] [PubMed] [Google Scholar]
- 22.Webster SJ, et al. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saraceno C, et al. Modeling Alzheimer’s disease: from past to future. Front Pharmacol. 2013;4:77. doi: 10.3389/fphar.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinders R, et al. Phase 0 clinical trials in cancer drug development: from FDA guidance to clinical practice. Mol Intervent. 2007;7:325. doi: 10.1124/mi.7.6.9. [DOI] [PubMed] [Google Scholar]
- 25.Wang YX, et al. Scientific and industrial challenges of developing nanoparticle-based theranostics and multiple-modality contrast agents for clinical application. Nanoscale. 2015;7:16146–16150. doi: 10.1039/c5nr03887a. [DOI] [PubMed] [Google Scholar]
- 26.Nunn AD. The cost of developing imaging agents for routine clinical use. Invest Radiol. 2006;41:206–212. doi: 10.1097/01.rli.0000191370.52737.75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed herein have been made available to the scientific community and general public on the website of the Center for Research Innovation in Biotechnology (http://crib.wustl.edu). We actively encourage all interested parties to explore the data and identify any improvements or additions that might be of use for interested investigators.