Abstract
Long-acting glucagon-like peptide 1 receptor agonists are increasingly used to treat type 2 diabetes. An increase of heart rate (HR) has been observed with their use. To elucidate the role of the cardiac sympatho-vagal balance as a possible mediator of the reported increase in HR, we performed power spectral analysis of HR variability (HRV) in patients receiving exenatide extended-release (ER). Twenty-eight ambulatory patients with type 2 diabetes underwent evaluation at initiation of exenatide-ER and thereafter at 3 and at 6 months. To obtain spectral analyses of HRV, a computerized acquisition of 10 minutes of RR electrocardiogram intervals (mean values of ~700 RR intervals) were recorded both in lying and in standing positions. All patients showed a substantial increase of HR both in lying and in standing positions. Systolic blood pressure, body weight, and glycated hemoglobin A1c significantly decreased both at 3 and 6 months compared with basal levels. The low-frequency/high-frequency ratio varied from 3.05 ± 0.4 to 1.64 ± 0.2 (P < 0.001) after 3 months and to 1.57 ± 0.3 (P < 0.001) after 6 months in a lying position and from 4.56 ± 0.8 to 2.24 ± 0.3 (P < 0.001) after 3 months and to 2.38 ± 0.4 (P < 0.001) after 6 months in a standing position compared with basal values, respectively. HR variations, induced by exenatide-ER treatment, do not appear to be related to sympathetic autonomic tone. Of note, we observed a relative increase of vagal influence on the heart.
Keywords: autonomic nervous system, GLP-1 receptor agonist, power spectral analysis
Sympatho-vagal balance was evaluated on diabetics after treatment with long-acting GLP-1 agonist. The major finding is a “shifting” of sympatho-vagal balance with a reduced LF/HF ratio.
Type 2 diabetes is a complex metabolic disorder characterized by hyperglycemia and associated with a high risk of cardiovascular complications. Glucagon-like peptide 1 receptor (GLP-1R) agonists represent a relatively new class of antihyperglycemic agents that address most of the pathophysiological mechanisms involved in the development of type 2 diabetes [1]. The recent development of longer-acting GLP-1R agonists, including the once-weekly form of exenatide extended-release (ER), represents an even more practical and acceptable therapeutic modality for the treatment of patients affected by type 2 diabetes.
A specific effect of these agents, i.e., a reduction of body weight, is a welcome contrast to the effects of other treatment options, e.g., sulfonylureas, glitazones, and insulin, all of which tend to induce an increase in body weight. Moreover, the favorable effects on body weight and metabolic profile, together with a reduction in blood pressure, could theoretically reduce cardiovascular risk [1], but only recent clinical trials have indicated a beneficial role of GLP-1R agonists on cardiovascular disease [2, 3].
In most GLP-1 trials, a small but substantial increase in heart rate (HR) has been reported [4]. This effect on HR may constitute a reason for caution, as it can potentially lead to adverse outcomes. In fact, some observations seem to suggest that an increase in HR, particularly as a consequence of β-adrenergic stimulation and/or reduced vagal tonic stimulation, may predict cardiac morbidity and potential mortality, and it is therefore undesirable [5].
A meta-analysis of data available from randomized controlled trials on GLP-1 analogs showed that the increase in HR was more evident for liraglutide than exenatide administered twice daily [4]. However, the underlying mechanisms associated with the increase of HR are still under investigation. The most important mechanism involved in HR regulation is the sympathetic/parasympathetic balance. However, both autonomic nervous system-dependent and independent mechanisms have been suggested as possible explanations of the rise in HR [6, 7].
Power spectral analysis (PSA) of HR variability (HRV) is a noninvasive, practical, and reproducible tool, able to define the relative balance of the autonomic nervous system at the cardiac level [8]. The present real-world clinical study was applied specifically to estimate, during long-acting GLP-1 agonist treatment, the sympatho-vagal balance variations, also considering that cardiovascular diseases are the major complications of type 2 diabetes [9].
1. Research Design and Methods
A. Patients
This clinical study was devoted to evaluate sympatho-vagal balance during long-acting GLP-1R agonist treatment. It was not intended to be an interventional study but rather, a preliminary study conducted in an ambulatory setting.
Twenty-eight consecutive outpatients with type 2 diabetes, who initiated treatment with a long-acting GLP-1 agonist (exenatide-ER administered subcutaneously, once weekly; 2 mg/dose) for clinical reasons, were included. In our institution (Verona), almost all patients with type 2 diabetes are periodically evaluated by PSA in an institutional-screening program. The identified patients were further examined after beginning the treatment, at 3 and 6 months, to study the trend of both HR and the sympatho-vagal balance. All patients were already being treated with hypoglycemic agents (see Table 1), but none of them were being treated with dipeptidyl-peptidase 4 inhibitors, sodium-glucose cotransporter 2 inhibitors, acarbose, or insulin. All hypertensive patients were treated with angiotensin-converting enzyme or angiotensin II receptor blocker inhibitors, and five of them were taken β-blockers. During the study, there was no variation either in the type or dose of medications or in the therapy applying to patients who were already taking antihypertensive drugs. The study was conducted in accordance with the Declaration of Helsinki and its amendments. The study was approved by the local Ethics Committee. All patients gave their informed consent to participate in the study.
Table 1.
Baseline Characteristics of Patients and Therapies During the Study (n = 28)
| Baseline | 3 Month | 6 Month | |
|---|---|---|---|
| Age, y | 62.9 ± 9.6 | ||
| Sex, men/women | 13/15 | ||
| Ethnicity: caucasic (%) | 28 (100) | ||
| Cardioaspirin (%) | 11 (39.3) | 11 (39.3) | 11 (39.3) |
| Hypoglycemic agents | |||
| Sulfonylureas (%) | 16 (57.1) | 16 (57.1) | 16 (57.1) |
| Metformin (%) | 19 (67.9) | 19 (67.9) | 19 (67.9) |
| Pioglitazone (%) | 4 (14.3) | 4 (14.3) | 4 (14.3) |
| Repaglinide (%) | 2 (7.1) | 2 (7.1) | 2 (7.1) |
| Alpha glucosidase inhibitor (%) | 1 (3.6) | 1 (3.6) | 1 (3.6) |
| Antihypertensive therapy | |||
| β-Blockers (%) | 5 (17.9) | 5 (17.9) | 5 (17.9) |
| ACE inhibitors (%) | 9 (32.1) | 9 (32.1) | 9 (32.1) |
| Sartanics (%) | 8 (28.6) | 8 (28.6) | 8 (28.6) |
| Calcium antagonists (%) | 5 (17.86) | 5 (17.86) | 5 (17.86) |
| Diuretics (%) | 3 (10.71) | 3 (10.71) | 3 (10.71) |
| Hypertension (%) | 17 (60.71) |
Data are presented as means ± standard deviation or absolute numbers and percentages.
Abbreviation: ACE, angiotensin-converting enzyme.
B. Clinical and Laboratory Data
Venous blood was drawn from all patients in the morning after an overnight fast. Hemoglobin A1c (HbA1c) was measured (according to the standard operating procedures of the International Federation of Clinical Chemistry Reference) using an automated high-performance liquid chromatography analyzer (Diamat; Bio-Rad Laboratories, Milan, Italy). The upper limit of normal for our laboratory was 5.8% (40 mmol/mol).
C. PSA
PSA of resting HRV was carried out on all patients in lying and standing positions (LS) to emphasize parasympathetic and sympathetic predominance, respectively.
A one-channel electrocardiogram (ECG) recorder was used to monitor the ECG trace and respiratory pattern (impedance based). This was in addition to a sampling of the RR interval, which was instantaneously calculated in milliseconds in sequence with the relative beat-to-beat blood pressure value, provided by a finger photopletismograph (Finometer; Finapres Medical Systems, Enschede, The Netherlands). The RR intervals were recorded over a relatively long, nearly artifact-free period, in this case for precisely 10 minutes in LS conditions. Mean values of all RR intervals (~600 to 800), recorded in milliseconds in this period, were calculated (in numbers of ~600 to 800), and HR, as beats per minute (bpm), was obtained.
Signals of RR values (in tachogram form) and the relative beat-to-beat systolic/diastolic values (in a linear pattern) were instantaneously transmitted and configured for calculation by means of an analogic/digital transducer, as previously described [10].
For the software, a mathematical autoregressive model was used for the PSA of HRV, which was carried out, as previously described, while the subjects were quietly lying down and resting [10]. The power of each component was measured in normalized units (NUs), as these give a better estimate of the balance between the various components. NUs are obtained by dividing the frequency band of interest by total power [total HRV minus very low frequency (LF); see later]. The RR interval series in normal subjects can be broken down, in terms of cyclic variations around the mean, into three major spectral components that characteristically fall into three principal frequency bands. The LF band, between 0.03 and 0.17 Hz, normally has a mean central frequency ~0.12 Hz and is thought to represent mainly sympathetic activity and to a much lesser extent, parasympathetic activity, as well as probably other physiological reflex mechanisms, such as baroreceptorial activity and peripheral vasomotor tone. It is partially inhibited by β-blockers and enhanced in many situations that stimulate sympathetic activity (standing, exercise, and mental stress) [11]. The high-frequency (HF) band, between 0.17 and 0.36 Hz, is synchronized with breathing phases and closely associated with vagal activity; it is enhanced by vagomimetic drugs and is totally abolished by atropine [12–14]. Finally, the very LF band (<0.02 Hz) is not under autonomic control but probably under the influence of the renin–angiotensin system or other possible hormonal mechanisms [14]. This component was not taken into account in this study, because our analysis focused on efferent vagal-sympathetic components. PSA of HRV was carried out in LS to highlight the influence of parasympathetic and sympathetic pathways, respectively [13]. In normal subjects, passive tilt or more simply, standing up is accompanied by an increase in the LF component and a decrease in the HF component of RR variability [11, 13, 14]. The power of each component was measured in NUs, as these give a better estimation of the balance among the various components. These were obtained by dividing the absolute power of each spectral component by the total variance of the RR intervals, from which the power of the continuous component (here, defined as the power density with a frequency between 0 and 0.02 Hz) was removed [11].
D. Cardiovascular Autonomic Assessment
The cardiovascular autonomic assessment was performed following Ewing and Clarke’s criteria [15] involving the measurement of the HRV in LS, during deep breathing (DB), and using the Valsalva ratio (VR). Blood pressure, taken both with the patient lying down and after 2 minutes of standing [15], was measured manually by the same operator using the same instrument (Gamma; Heine Optotechnik, Herrsching, Germany) at the same time each morning after fasting. The mean value of three successive measurements of blood pressure was calculated. Patients were requested to avoid strenuous physical exercise in the 24 hours preceding the cardiovascular tests. We advised them not to consume beverages containing caffeine or alcohol and to avoid smoking before the cardiovascular autonomic tests. No agreement exists on the number of abnormal cardiovascular tests required to reach a diagnosis of confirmed cardiac autonomic neuropathy (CAN) [16]. In light of the evidence available, experts have proposed that at least two abnormal HR tests (below the fifth percentile) are required, and only one abnormal test or two borderline tests (between the fifth and 10th percentiles) indicate a condition of “early or uncertain” CAN. A severe form of autonomic neuropathy is diagnosed when confirmed CAN is associated with orthostatic hypotension. DB, LS, and VR are considered as indexes of mainly “cardiovagal” function, with similar high sensitivity and specificity, whereas the orthostatic hypotension test is considered to be a test of sympathetic function [17] (more detailed methodological information is described elsewhere [18]). The patients in the current study were also evaluated for the presence or the absence of autonomic dysfunction.
E. Statistical Analysis
The differences intra patients with type 2 diabetes before and after 3 and 6 months of exenatide-ER treatment were analyzed using the Wilcoxon test for paired samples; the Mann-Whitney U test and the linear correlation test were used for all other analyses. P < 0.05 or less was considered to indicate statistical significance. Data are expressed as the means ± standard error (SE).
2. Results
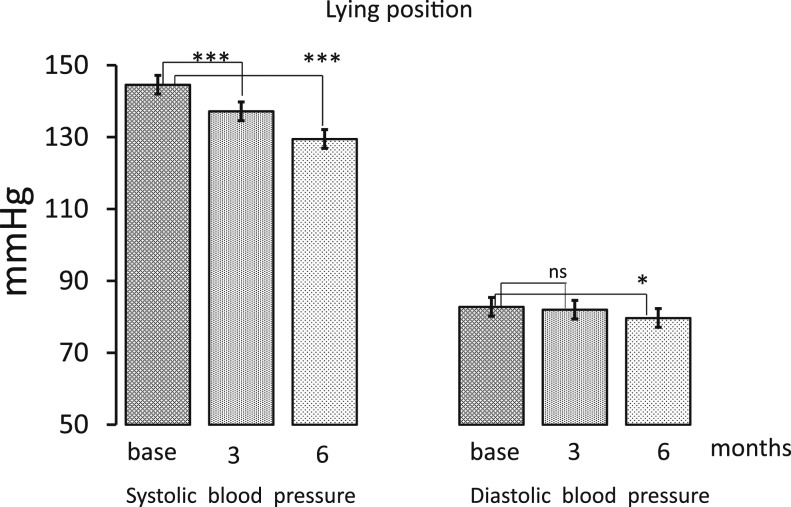
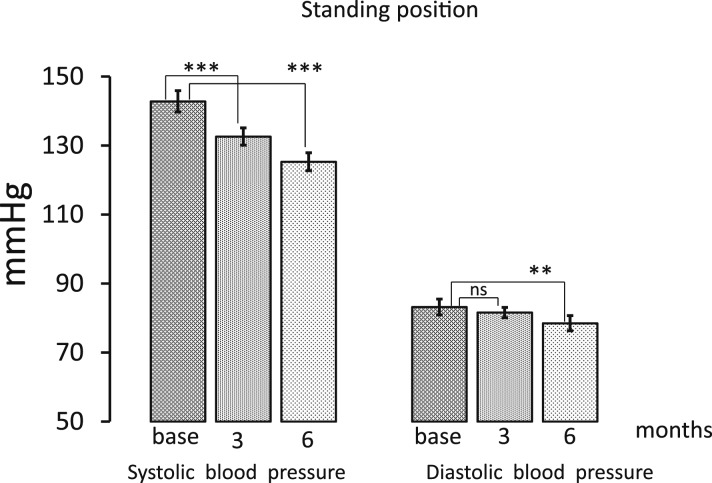
Baseline clinical characteristics of the patients are reported in Table 1. The mean age of participants was 62.7 ± 10.0, 53.6% were women, and none had a previous cardiovascular event. All subjects were caucasic. Aspirin was taken by 39.3% of subjects, all patients were on renin–angiotensin system inhibitor treatment (16 on angiotensin-converting enzyme and 12 on angiotensin receptor inhibitors), and 10.7% were taking diuretics. Approximately 82% of subjects were affected by hypertension. As shown in Table 1, medications were not changed during the study period. All patients completed the 6-month period of the study, and no adverse effects were reported. In all patients, treatment with exenatide-ER, given once weekly subcutaneously (Table 2), was associated with a significant increase in HR, both in lying position, from 75.7 ± 2.1 to 79.1 ± 2.1 bpm at 3 months (P < 0.001 vs basal value) and to 77.7 ± 2.4 at 6 months (not significant vs basal value), and in standing position, from 83.6 ± 2.2 to 86.0 ± 2.4 bpm after 3 months (P < 0.05 vs basal value) and to 86.7 ± 2.6 after 6 months (P < 0.05 vs basal value). During the treatment period, systolic blood pressure in lying position significantly decreased from 144.6 ± 2.6 to 137.2 ± 2.8 mmHg after 3 months (P < 0.001 vs basal value) and to 129.5 ± 2.5 after 6 months (P < 0.001 vs basal value), respectively, whereas diastolic blood pressure decreased from 82.8 ± 1.9 to 82.0 ± 1.5 mmHg (P = not significant) after 3 months and to 79.7 ± 1.9 mmHg (P < 0.05 vs basal value) after 6 months (Fig. 1, Table 2). In standing position, systolic blood pressure changed from 142.8 ± 3.1 to 132.6 ± 2.5 mmHg after 3 months (P < 0.001 vs basal value) and to 125.3 ± 2.3 after 6 months (P < 0.001 vs basal value), and diastolic blood pressure decreased from 83.2 ± 2.3 to 81.6 ± 1.5 mmHg after 3 months (not significant) and to 78.5 ± 2.2 mmHg after 6 months (P < 0.001 vs basal value; Fig. 2, Table 2). Mean HbA1c value before treatment was 8.4 ± 0.1% and decreased to 7.1 ± 0.1% (P < 0.001) after 3 months and to 6.8 ± 0.1% after 6 months (P < 0.001 vs basal value; Table 2). Mean body weight from 88.5 ± 3.7 decreased to 86.0 ± 3.6 kg (P < 0.001) after 3 months and to 85.8 ± 3.7 (P < 0.001) after 6 months (Table 2).
Table 2.
Different Variables Considered Before Treatment, After 3 and 6 Months of Therapy Both in Clinostatism and Orthostatism (n = 28)
| Weight, kg | HbA1c, % | LS | DB | VR | ||||
|---|---|---|---|---|---|---|---|---|
| Base | 88.5 ± 3.7 | 8.4 ± 0.10 | 1.12 ± 0.02 | 16.4 ± 1.4 | 1.41 ± 0.04 | |||
| 3 mo | 86.0 ± 3.6a | 7.1 ± 0.15a | 1.11 ± 0.02 | 17.3 ± 1.4 | 1.34 ± 0.04b | |||
| 6 mo | 85.8 ± 3.7a | 6.8 ± 0.19a | 1.10 ± 0.02 | 19.4 ± 2.0b | 1.39 ± 0.05 | |||
| HR, bpm | Systolic BP, mmHg | Diastolic BP, mmHg | HF, NU | LF, NU | PSA Total Milliseconds2 | LF/HF | Pressure Rate Product, mmHg × min | |
| Lying Position | ||||||||
| Base | 75.7 ± 2.1 | 144.6 ± 2.6 | 82.8 ± 1.9 | 72.1 ± 12 | 131.7 ± 19 | 641.3 ± 90 | 3.05 ± 0.4 | 11,012 ± 345 |
| 3 mo | 79.1 ± 2.1a | 137.2 ± 2.8a | 82.0 ± 1.5 | 75.6 ± 15 | 82.5 ± 20c | 581.0 ± 97 | 1.64 ± 0.2a | 10,861 ± 313 |
| 6 mo | 77.7 ± 2.4 | 129.5 ± 2.5a | 79.7 ± 1.9b | 72.9 ± 19 | 88.1 ± 25b | 745.7 ± 138 | 1.57 ± 0.3a | 10,115 ± 352b |
| Standing Position | ||||||||
| Base | 83.6 ± 2.2 | 142.8 ± 3.1 | 83.2 ± 2.3 | 71.2 ± 19 | 138.3 ± 22 | 684 ± 94 | 4.56 ± 0.8 | 11,962 ± 411 |
| 3 mo | 86.0 ± 2.4b | 132.6 ± 2.5a | 81.6 ± 1.5 | 45.9 ± 13 | 68.3 ± 10c | 574 ± 82 | 2.24 ± 0.3a | 11,380 ± 360b |
| 6 mo | 86.7 ± 2.6b | 125.3 ± 2.6a | 78.5 ± 2.2c | 68.7 ± 30 | 115.4 ± 42 | 620 ± 129 | 2.38 ± 0.4a | 10,917 ± 415c |
Classic cardiovascular autonomic tests are also reported. Data are expressed as means ± SE.
Abbreviation: BP, blood pressure.
P < 0.001 indicate the level of statistical significance; significance vs base.
P < 0.05 indicate the level of statistical significance; significance vs base.
P < 0.01 indicate the level of statistical significance; significance vs base.
Figure 1.
Systolic and diastolic blood pressure values before treatment and after 3 and 6 months of therapy in a lying position. Data are expressed as means ± SE. *P < 0.05 and ***P < 0.001 indicate the level of statistical significance (n = 28). ns, not significant.
Figure 2.
Systolic and diastolic blood pressure values before treatment and after 3 and 6 months of therapy, both in a standing position. Data are expressed as means ± SE. **P < 0.01 and ***P < 0.001 indicate the level of statistical significance (n = 28).
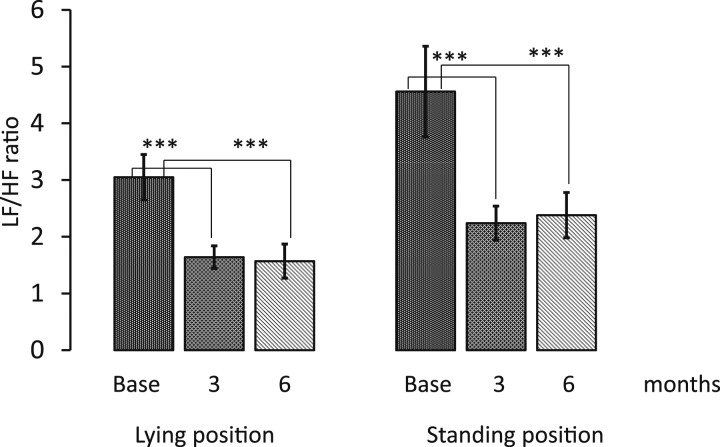
PSA of HRV showed that the LF/HF ratio in lying position decreased from 3.05 ± 0.4 to 1.64 ± 0.2 (P < 0.001) after 3 months and to 1.57 ± 0.3 (P < 0.001 vs basal value) after 6 months and in standing position from 4.56 ± 0.8 to 2.24 ± 0.3 (P < 0.001) after 3 months and 2.38 ± 0.4 (P < 0.001 vs basal value) after 6 months (Fig. 3, Table 2). The LF component in the lying position changed from 131.7 ± 19 to 82.5 ± 20 (P < 0.01) after 3 months and to 88.1 ± 25 (NU; P < 0.05) after 6 months, whereas in standing position, from 138.3 ± 22 to 68.3 ± 10 (P < 0.01) after 3 months and to 115.4 ± 42 (not significant) after 6 months (Table 2). The HF component in the lying position changed from 72.1 ± 12 to 75.6 ± 15 after 3 months and to 72.9 ± 19 (NU; not significant) after 6 months, whereas in standing position, from 71.2 ± 10.2 to 45.9 ± 13 (not significant) after 3 months and to 68.7 ± 30 (NU; not significant ) after 6 months. Finally, the total power density did not change substantially during the therapy period (Table 2). The classic autonomic tests (LS, DB, and VR) did not vary substantially during the study period, as shown in Table 2. The pressure rate product, an estimate of the cardiac oxygen consumption, was substantially reduced at 6 months in both in lying and in standing position and at 3 months in lying position (Table 2). According to the standardized criteria for the diagnosis of CAN (see methods section), two patients resulted with confirmed (although not severe) CAN, three with early CAN, and 23 without CAN.
Figure 3.
LF/HF ratio before treatment and after 3 and 6 months of therapy in LS. Data are expressed as means ± SE. ***P < 0.001 indicates the level of statistical significance (n = 28).
3. Discussion
Despite the important advances in antihyperglycemic therapies, macrovascular complications are still the most common cause of death in patients affected by type 2 diabetes [7, 19]. Clinical evidence is consistently growing that collectively demonstrates a beneficial effect of the GLP-1R agonists on cardiovascular risk [2, 3, 20]. At present, in addition to its antidiabetic effects, GLP-1R agonists have shown some cardioprotective actions, mainly related to the reduction in blood pressure levels [1–3]. It has been reported that a reduction of 5.6 mmHg resulted in an 18% decrease in the risk of death from cardiovascular diseases in patients with diabetes [21]. Furthermore, it has been suggested that GLP-1 may provide cardiac protection via an unknown second receptor or by means of metabolic protection [22, 23]. In contrast to this beneficial effect, there is a consistent evidence of an increase in HR [4, 24]. In our study, differently from the others, we measured HR in LS. Interestingly, we observed an increase of HR at 3 months and a trend toward basal at 6 months in the lying position, whereas in the standing position, in which the sympathetic predominance is emphasized, we observed a consistent increase in HR with no major changes in blood pressure. Furthermore, our study confirms an increase of HR during the GLP-1R agonist. However, our study is the evaluation of the sympatho-vagal balance using the PSA carried out on all patients, in LS, to emphasize parasympathetic and sympathetic predominance, respectively.
The principal physiological mechanism increasing HR is either an increase in the sympathetic drive or a decrease in the parasympathetic activity. However, until now, the precise extent to which GLP-1R agonists induce a modulation of the sympatho-vagal balance remains unclear, and to the best of our knowledge, only few studies have addressed this topic [25–27]. However, none of these studies involved exenatide-ER or evaluated PSA of resting HRV, both in lying and in standing positions under controlled conditions in a laboratory, to emphasize parasympathetic and sympathetic predominance, respectively. In other long acquisition data studies, obtained by Holter monitoring, more influences could likely occur by environmental and behavioral factors.
Of note, the main result of our study is a substantial reduced LF/HF ratio after therapy with the GLP-1R agonist, both in lying and standing positions; an unexpected “shifting” of sympatho-vagal balance, meaning reduced sympathetic influence and a relatively greater parasympathetic final influence on the heart. This finding can have clinical implications, as a shift toward an increased parasympathetic tone can substantiate a protective effect on the heart. Concordantly with the previous statement, we also observed a substantial reduction of the pressure rate product at 6 months, indicating decreased cardiac work and less oxygen consumption by the heart. Interestingly, the pressure-rate product was mainly reduced in a standing position when a predominant sympathetic tone is expected.
Hence, our findings do not seem to suggest a mediated autonomic effect of the GLP-1R agonist on HR, and probably other mechanisms are involved. For example, it might be an effect of the agonist on the heart, as also recently suggested [25, 26]. In line with this hypothesis, experimental studies of GLP-1 infusions in murine models have also reported dose-dependent inotropic and chronotropic effects that were not suppressed by propranolol, thereby suggesting a different mechanism of action [28]. In studies on animal models using exendin-(9-39) (with resulting antagonist effects that blocked the increases in both blood pressure and HR), the hypothesis of a receptor-mediated effect was confirmed when the pharmaceutical was coadministered with GLP-1-(7-36) [29]. In addition, further evidence suggests that the changes in autonomic function induced by the GLP-1 agonist on the autonomic tone are heterogeneous, and as the parasympathetic influence on the gut and pancreas is increased, the effect of GLP-1 on blood pressure and HR is conflicting and species specific [7].
Studies on animal models have demonstrated the presence of a GLP-1R on the heart, particularly on, but not limited to, the myocytes of the sinus atrial node and atria [30, 31]. Interestingly, signaling by means of GLP-1R directly increases the cardiomyocyte glucose uptake, independently of its insulinotropic actions [32], through the AKT pathway and the successive glucose transporter type 4 translocation [32], in addition to a cyclic adenosine monophosphate-induced phosphorylation of cardiac protein. The activation of the latter pathway also has been reported during the stimulation of cardiac β1 receptors, and opposite effects have also been described, as a result of activating the cholinergic muscarinic receptors [32].
Hence, as recently shown in animal models, GLP-1R agonists may increase HR by multiple mechanisms, including regulation of the autonomic nervous system [33]. More interestingly, it seems that cardiac GLP-1R circuits controlling HR require neural inputs and do not function in a heart-autonomous manner [33].
Finally, of note in our data is evidence that shows that patients with early and confirmed autonomic neuropathy also showed an improvement, at the end of the study, in the relative vagal influence on the heart in one traditional autonomic test, i.e., deep breathing, which it is considered to measure mainly vagal function. Consistently, this potentially beneficial therapeutic effect has already been reported in diabetic autonomic neuropathy [34].
This study has important limitations to take into account: it does not have a comparison group, and therefore, we cannot exclude that HR variations may change in a similar manner with initiation of other drugs, and the number of subjects is limited. Nevertheless, the study also has some strengths: (1) all subjects were followed and evaluated by a single operator; (2) a consolidated experimental approach was used to study the autonomic system; (3) PSA of resting HRV was carried out both in lying and in standing positions, thus emphasizing parasympathetic and sympathetic predominance, respectively; (4) all ECG acquisitions were carried out under controlled conditions in a laboratory; and (5) an effort was made not to change background medications during the study period.
In conclusion, we observed a relative increase in vagal influence on the heart; thus, an effect of exenatide on the heart frequency might theoretically be suggested. The reduced sympathetic vs parasympathetic balance might be the consequence of a compensatory mechanism, and in any case, a “more” relative vagal influence on the heart could reduce the risk of cardiovascular mortality.
Acknowledgments
Author Contributions: V.C. and G.Z. conceived of the study and wrote the manuscript. V.C. and R.R. performed the tests. I.P., M.T., and F.D.S. researched and input the data. L.S. and V.C. analyzed the data. G.T., K.T., and E.B. reviewed/edited the manuscript. F.B. and M.D. contributed to the discussion and reviewed/edited the manuscript. V.C. is the guarantor of the study.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bpm
- beats per minute
- CAN
- cardiac autonomic neuropathy
- DB
- deep breathing
- EC
- electrocardiogram
- ER
- extended-release
- GLP-1
- glucagon-like peptide 1
- GLP-1R
- glucagon-like peptide 1 receptor
- Hb
- hemoglobin
- HF
- high frequency
- HR
- heart rate
- HRV
- heart rate variability
- LF
- low frequency
- LS
- lying and standing position
- NU
- normalized unit
- PSA
- power spectral analysis
- SE
- standard error
- VR
- Valsalva ratio.
References and Notes
- 1.Saraiva FK, Sposito AC. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc Diabetol. 2014;13(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering CommitteeLEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 4.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aune D, Sen A, ó’Hartaigh B, Janszky I, Romundstad PR, Tonstad S, Vatten LJ. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017;27(6):504–517. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner SM, March JE, Kemp PA, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol. 2008;154(1):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffioen KJ, Wan R, Okun E, Wang X, Lovett-Barr MR, Li Y, Mughal MR, Mendelowitz D, Mattson MP. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011;89(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 9.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice . European Association for Cardiovascular Prevention and Rehabilitation. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012): The Fifth Joint Task force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representative of nine society and invited experts). Atherosclerosis. 2012;223(1):1–68. [DOI] [PubMed] [Google Scholar]
- 10.Bellavere F, Balzani I, De Masi G, Carraro M, Carenza P, Cobelli C, Thomaseth K. Power spectral analysis of heart-rate variations improves assessment of diabetic cardiac autonomic neuropathy. Diabetes. 1992;41(5):633–640. [DOI] [PubMed] [Google Scholar]
- 11.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84(2):482–492. [DOI] [PubMed] [Google Scholar]
- 12.Bernardi L, Ricordi L, Lazzari P, Soldà P, Calciati A, Ferrari MR, Vandea I, Finardi G, Fratino P. Impaired circadian modulation of sympatho-vagal activity in diabetes. Circulation. 1992;86:1443–1452. [DOI] [PubMed] [Google Scholar]
- 13.Ori Z, Monir G, Weiss J, Sayhouni X, Singer DH. Heart rate variability. Frequency domain analysis. Cardiol Clin. 1992;10(3):499–537. [PubMed] [Google Scholar]
- 14.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. [DOI] [PubMed] [Google Scholar]
- 15.Ewing DJ, Clarke BF. Autonomic neuropathy: its diagnosis and prognosis. Clin Endocrinol Metab. 1986;15(4):855–888. [DOI] [PubMed] [Google Scholar]
- 16.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P; Toronto Consensus Panel on Diabetic Neuropathy . Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639–653. [DOI] [PubMed] [Google Scholar]
- 17.The Consensus Committee of the American Autonomic Society and the American Academy of Neurology Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46(5):1470–1490. [DOI] [PubMed] [Google Scholar]
- 18.Zoppini G, Cacciatori V, Raimondo D, Gemma M, Trombetta M, Dauriz M, Brangani C, Pichiri I, Negri C, Stoico V, Bergamini C, Targher G, Santi L, Thomaseth K, Bellavere F, Bonadonna RC, Bonora E. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS). Diabetes Care. 2015;38(8):1487–1493. [DOI] [PubMed] [Google Scholar]
- 19.Simmons RK, Griffin SJ, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbæk A. Effect of population screening for type 2 diabetes and cardiovascular risk factors on mortality rate and cardiovascular events: a controlled trial among 1,912,392 Danish adults. Diabetologia. 2017;60(11):2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathanson D, Ullman B, Löfström U, Hedman A, Frick M, Sjöholm A, Nyström T. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55(4):926–935. [DOI] [PubMed] [Google Scholar]
- 21.Chalmers J, Joshi R, Kengne AP, Ninomiya T, Bi Y, Bompoint S, Billot L, Patel A; ADVANCE Collaborative Group . Efficacy and safety of fixed combination of perindopril and indapamide in type 2 diabetes: results from ADVANCE in context of available evidence. J Hypertens Suppl. 2008;26(3):S21–S27. [PubMed] [Google Scholar]
- 22.Clarke SJ, McCormick LM, Dutka DP. Optimising cardioprotection during myocardial ischaemia: targeting potential intracellular pathways with glucagon-like peptide-1. Cardiovasc Diabetol. 2014;13(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying Y, Zhu H, Liang Z, Ma X, Li S. GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J Mol Endocrinol. 2015;55(3):245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits MM, Tonneijck L, Muskiet MH, Hoekstra T, Kramer MH, Diamant M, van Raalte DH. Heart rate acceleration with GLP-1 receptor agonists in type 2 diabetes patients: an acute and 12-week randomised, double-blind, placebo-controlled trial. Eur J Endocrinol. 2017;176(1):77–86. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani Y, Kawabe A, Matsumura M, Aso Y, Yasu T, Banba N, Nakamoto T. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using Holter electrocardiography and power spectral analysis of heart rate variability. Diabetes Care. 2016;39(2):e22–e23. [DOI] [PubMed] [Google Scholar]
- 26.Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, Nielsen OW, Haugaard SB, Sajadieh A. Effects of liraglutide on heart rate and heart rate variability: a randomized, double-blind, placebo-controlled crossover study. Diabetes Care. 2017;40(1):117–124. [DOI] [PubMed] [Google Scholar]
- 27.Smits MM, Muskiet MH, Tonneijck L, Hoekstra T, Kramer MH, Diamant M, van Raalte DH. Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol. 2016;81(4):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barragán JM, Rodríguez RE, Blázquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36) amide in rats. Am J Physiol. 1994;266(3 Pt 1):E459–E466. [DOI] [PubMed] [Google Scholar]
- 29.Barragán JM, Rodríguez RE, Eng J, Blázquez E. Interactions of exendin-(9-39) with the effects of glucagon-like peptide-1-(7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67(1):63–68. [DOI] [PubMed] [Google Scholar]
- 30.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. [DOI] [PubMed] [Google Scholar]
- 31.Ussher JR, Baggio LL, Campbell JE, Mulvihill EE, Kim M, Kabir MG, Cao X, Baranek BM, Stoffers DA, Seeley RJ, Drucker DJ. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab. 2014;3(5):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110(8):955–961. [DOI] [PubMed] [Google Scholar]
- 33.Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, Mighiu AS, Zhang H, Ludwig A, Seeley RJ, Heximer SP, Drucker DJ. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol Metab. 2017;6(11):1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8(7):405–416. [DOI] [PubMed] [Google Scholar]