Abstract
We investigated factors underlying the varying effects of a high dietary iodide intake on serum T4 levels in a wide spectrum of mouse strains, including thyroiditis-susceptible NOD.H2h4, NOD.H2k, and NOD mice, as well as other strains (BALB/c, C57BL/6, NOD.Lc7, and B10.A4R) not previously investigated. Mice were maintained for up to 8 months on control or iodide-supplemented water (NaI 0.05%). On iodized water, serum T4 was reduced in BALB/c (males and females) in association with colloid goiters but was not significantly changed in mice that developed thyroiditis, namely NOD.H2h4 (males and females) or male NOD.H2k mice. Neither goiters nor decreased T4 developed in C57BL/6, NOD, NOD.Lc7, or B10.A4R female mice. In further studies, we focused on males in the BALB/c and NOD.H2h4 strains that demonstrated a large divergence in the T4 response to excess iodide. Excess iodide ingestion increased serum TSH levels to the same extent in both strains, yet thyroidal sodium iodide symporter (NIS) messenger RNA (mRNA) levels (quantitative polymerase chain reaction) revealed greatly divergent responses. NOD.H2h4 mice that remained euthyroid displayed a physiological NIS iodine autoregulatory response, whereas NIS mRNA was inappropriately elevated in BALB/c mice that became hypothyroid. Thus, autoimmune thyroiditis-prone NOD.H2h4 mice adapted normally to a high iodide intake, presumably by escape from the Wolff-Chaikoff block. In contrast, BALB/c mice that did not spontaneously develop thyroiditis failed to escape from this block and became hypothyroid. These data in mice may provide insight into the mechanism by which iodide-induced hypothyroidism occurs in some humans without an underlying thyroid disorder.
Keywords: hypothyroidism, iodine autoregulation, iodide-symporter, TSH
Iodide-induced hypothyroidism occurred in mice without autoimmune thyroiditis, associated with an abnormal autoregulatory NaI symporter response. Conversely, thyroiditis-prone mice remained euthyroid.
It is not generally appreciated that, in addition to the TSH negative feedback mechanism, thyroid hormone homeostasis in an environment of variable (inorganic) iodide intake is maintained by (organic) iodine autoregulation [1]. In this process, independent of the TSH level, intrathyroidal iodine influences many aspects of thyroid function, including iodide transport into the thyroid and thyroid hormone synthesis and secretion, as well as other enzymes and membrane transporters critical to thyroid growth and function [reviewed in 1]. In times of excessive iodide ingestion, the short-term inhibition of thyroid hormone synthesis (Wolff-Chaikoff “block”) [2] is normally followed by “escape” from this block by limitation of iodide transport into the thyroid [3, 4]. In humans, failure to adapt to iodide excess can lead to hypo- or hyperthyroidism, most clearly occurring in individuals with underlying subclinical thyroid dysfunction such as in autoimmune thyroid disease or nontoxic goiter [reviewed in 5]. However, for unclear reasons, iodide-induced thyroid dysfunction can also occur idiosyncratically in individuals with apparently normal thyroids.
In mice, increased iodide ingestion causes divergent outcomes in two strains not spontaneously susceptible to thyroid autoimmunity (Table 1). SJL mice develop colloid goiter and hypothyroidism, whereas CBA mice remain euthyroid with normal thyroid histology [6]. Hypothyroidism and TSH-induced goiter in the SJL mice are caused by their inability to downregulate thyroid sodium iodide symporter (NIS) expression [6] and consequent failure to escape from the Wolff-Chaikoff block [7]. Very limited, or contradictory, information on thyroid function is available for strains that spontaneously develop thyroid autoimmunity, namely NOD.H2h4 and NOD.H2k mice [8, 9], as well as the parent NOD strain that primarily develops type I diabetes and, to a lesser extent, thyroid autoimmunity [10–12]. For example, excess iodide administration to diabetes-prone NOD mice modestly decreased serum T4 levels [13], whereas in the NOD.H2h4 strain, iodide had either no effect [10] or consistently reduced serum T4 levels [14]. Unlike in the SJL and CBA strains, there are no reports on potential mechanisms underlying iodide-induced thyroid dysfunction in these thyroiditis-susceptible mouse strains.
Table 1.
Summary of the Effects of Increased Dietary Iodide on Thyroid Function and Lymphocytic Infiltration in Mouse Strains in the Present and Previous Studies
| Strain | I-A | M or F | Lymphocytic Infiltration | Goiter (Colloid) | T4 | Reference |
|---|---|---|---|---|---|---|
| BALB/c | I-Ad | M, F | No | Yes | Decreased | Present study |
| NOD.H2h4 | I-Ak | M, F | Moderate | No | No change | [10–12, 15–17] |
| M, F | Moderate | No | No change | [18], present study | ||
| NOD | I-Ag7 | M | Small | No | Not done | [12, 61, 62] |
| M | Small | No | Decreased | Present study | ||
| NOD.Lc7 | I-Ak | M | Small | No | No change | [18], present study |
| NOD.H2k | I-Ak | M | Moderate | No | No change | [18], present study |
| B10.A.4R | I-Ak | M, F | No | No | Decreased (M) | Present study |
| C57BL/6 | I-Ab | F | No | Yes | Decreased | Present study |
| CBA | I-Ak | F | None | No | None | [6, 11] |
| SJL | I-As | F | Small | Yes | Decreased | [6] |
Sex indicated by male or female.
Abbreviations: F, female; I-A, mouse major histocompatibility class II antigens; M, male.
Because of this strain-dependent variability, we investigated the serum T4 and thyroid histologic responses to excess dietary iodide in a wide spectrum of mouse strains, including thyroiditis-susceptible NOD.H2h4, NOD.H2k, and NOD mice, as well as other strains (BALB/c, C57BL/6, NOD.Lc7, and B10.A4R) for which the effect of increased dietary iodide on thyroid function has not been investigated. To examine the expression of genes related to thyroid function, namely thyroglobulin (Tg), thyroid peroxidase (TPO), the TSH receptor (TSHR), NIS, Duox1, Duox2, and pendrin (Pds), we focused on two mouse strains that do (NOD.H2h4), or do not (BALB/c), spontaneously develop thyroid autoimmunity. We confirmed that excess iodide ingestion had no effect on serum T4 levels in NOD.H2h4 mice [10–12, 15–17]. Further, hypothyroidism and goiter did develop in nonautoimmune-prone BALB/c mice because of an increase (rather than the anticipated decrease) in NIS on exposure to excess iodide.
1. Methods
A. Mice
The following mouse strains were investigated:
-
a.
Male and female BALB/c, male and female NOD.H2h4 (NOD.Cg_H2h4/DilTacUmmJ), and female B10.A4R [B10.A_H2h4/(4R) SgDvEgJ] mice, originally from The Jackson Laboratory (Bar Harbor, ME), were bred at Cedars-Sinai Medical Center (Los Angeles, CA).
-
b.
Female C57BL/6 mice were purchased from The Jackson Laboratory and housed at Cedars-Sinai Medical Center.
-
c.
Sera and thyroid tissue were available from previous studies of thyroid autoimmunity in male mice of the following strains: NOD.H2h4, NOD.H2k, NOD, B10.A4R, and NOD.Lc7 mice [18]. NOD.Lc7 mice are congenic for a segment on the distal end of mouse Chr 7, which coincides with the B10.A4R interval retained in the generation of NOD.H2h4 mice [18]. NOD mice had been tested for urinary glucose using Diastix Reagent Strips for Urinalysis (Glucose Bayer Health Care LLC, Mishawaka, IN) to exclude data from mice that developed diabetes.
All mouse studies were performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center and performed with the highest standards of care.
B. Exposure to Iodide-Supplemented Water
From the age of 8 weeks (2 months), when both males and females are sexually mature, mice were maintained on regular water or water supplemented with 0.05% sodium iodide (iodized water) [19] for 16 weeks in all mouse strains and for 32 weeks in NOD.H2h4, NOD.H2k, and BALB/c. Blood was drawn before treatment and after 8 or 16 weeks on regular water or NaI. At the 16-week time point, some mice (including all NOD and NOD.Lc7) were euthanized to harvest blood and thyroid tissue.
C. T4, TSH, and Thyroid Histology
Total serum thyroxine (T4) was measured in undiluted mouse serum (25 μL) using a radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA). T4 values were computed from kit standards and expressed as μg/dL. Serum TSH was measured (50 μL) in a radioimmunoassay by Dr. Samuel Refetoff (University of Chicago; fee for service). T4 values for male and female NOD.H2h4 mice after 16 weeks on NaI were included in a previous study [20].
Thyroid glands were preserved as previously described [18], and paraffin-embedded and serial sections were stained with hematoxylin and eosin (Research Animal Diagnostic Laboratory, University of Missouri, Columbia, MO). Thyroid histology was examined in Los Angeles. The extent of thyroid lymphocytic infiltration was previously reported for male NOD.H2h4, NOD.H2k, NOD, NOD.Lc7, and B10.A4R mice [18]. Histological data have not previously been reported for BALB/c, C57BL/6, or female B10.A4R mice.
D. Intrathyroidal mRNA Expression Measured by RT-PCR
Thyroids from mice exposed for 16 weeks to regular water (NOD.H2h4, n = 2; BALB/c, n = 4) or NaI water (NOD.H2h4, n = 4; BALB/c, n = 4) were stored in RNAlater (LifeTechnologies, Carlsbad, CA). Quantitative real-time (RT) polymerase chain reaction (PCR) was performed essentially as previously described [21]. Tissue was homogenized with QIAshredder columns (QIAGEN, Valencia, CA). Total RNA was prepared using RNeasy Plus Mini kit and treated with TURBO DNase (LifeTechnologies, Carlsbad, CA) to remove genomic DNA. Reverse transcription was performed with the AffinityScript QPCR cDNA Synthesis Kit (Agilent Technologies, Cedar Creek, TX) using oligo(dT) and random primers. Quantitative RT-PCR was performed using the FastStart SYBR Green Master Mix (Roche, Basel, Switzerland) with 2.5% of complementary DNA (20 μL final volume). Reactions were run on an iCycler Thermal Cycler with an iQ5 Real Time PCR Detection System module (Bio-Rad Laboratories, Hercules, CA). An initial denaturation step at 95°C (10 minutes) was followed by denaturation at 95°C (30 seconds) and annealing and extension at 55°C (30 seconds) for 40 cycles. Relative gene expression levels were calculated using the comparative Ct method (Delta delta Ct), according to the Pfaffl model [22] using Bio_Rad iQ5 2.0 software. Samples were tested in triplicate; parallel controls lacked reverse transcription. The genes studied included: NIS, Pds, dual oxidase 1 (Duox1) and Duox2, and the housekeeping genes β-actin and glutaraldehyde-3-phosphate dehydrogenase (GAPDH). Primers were obtained from QIAGEN, except for Pds primers, which were obtained from Bio-Rad. Data were normalized to mouse β-actin and GAPDH; results are shown as mean + standard deviation (SD).
E. Statistical Analyses
Significant differences between responses in different groups were determined by Mann-Whitney rank sum test or, when normally distributed, by Student t test. Multiple comparisons were made using analysis of variance. Tests were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA).
2. Results
A. Serum T4 in Mice on Iodized vs Regular Water
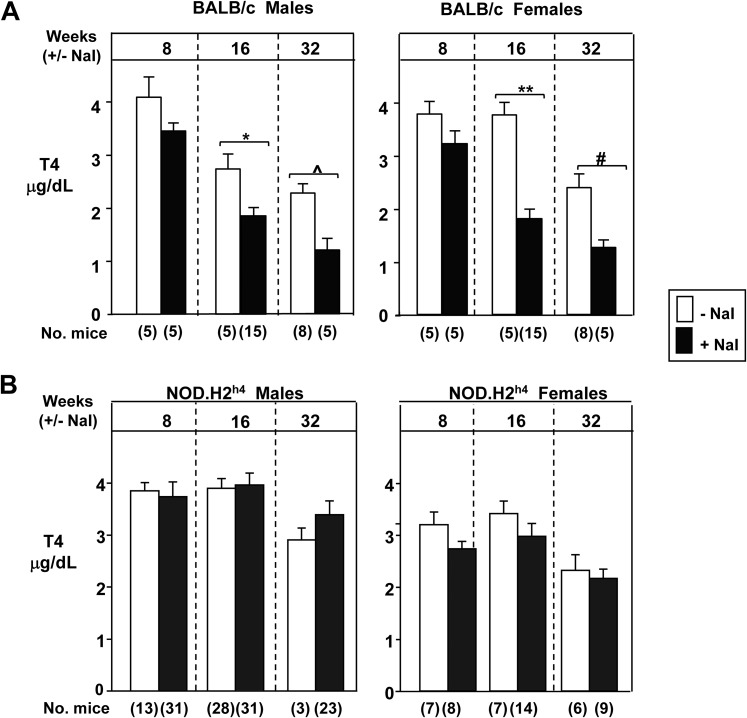
We initially compared the response to iodide supplementation of water in two mouse strains that do (NOD.H2h4), or do not (BALB/c), spontaneously develop thyroiditis when maintained on regular water. In the former, excess iodide ingestion exacerbates the thyroiditis. After 16 and 32 weeks on iodized drinking water, serum T4 levels were significantly reduced in BALB/c mice of both sexes (Fig. 1A). However, excess iodide ingestion in thyroid autoimmunity-prone NOD.H2h4 mice (male and female) did not alter serum T4 levels (Fig. 1B).
Figure 1.
After long-term exposure (up to 32 weeks, starting at the age of 2 months) to iodide (NaI 0.05%) in drinking water, serum T4 levels decrease in (A) BALB/c mice but are unchanged in (B) NOD.H2h4 mice. The numbers of mice studied are in parentheses. Statistically significant differences: panel A males, *P = 0.011, ^P = 0.035; females, #P < 0.001, **P = 0.005 (t tests).
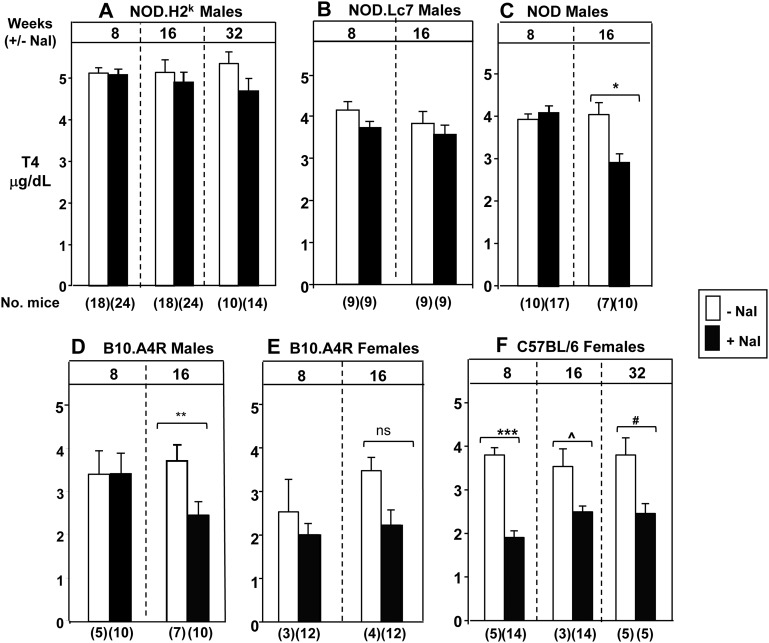
In addition to the two foregoing mouse strains, we studied the serum T4 responses to excess iodide ingestion in five other strains with variable susceptibility to autoimmune thyroiditis, and for whom such data have not previously been reported (Fig. 2). Like NOD.H2h4 (Fig. 1), NOD.H2k and NOD.Lc7 are derived from the original NOD strain. In a long-term study with NOD mice, development of overt diabetes requiring insulin therapy is a confounding factor in assessing thyroid function, and mice becoming hyperglycemic were excluded from analysis. Further, because diabetes develops earlier in females, we studied NOD males and, for comparison, males in the other NOD-derived strains (NOD.H2k and NOD.Lc7). Excess iodide ingestion only decreased serum T4 levels in the original NOD founders (Fig. 2C), not in the two NOD variants (Fig. 2A and B).
Figure 2.
Exposure to iodized water (up to 32 weeks, starting at the age of 2 months) does not induce significant changes in T4 levels in (A) NOD.H2k, (B) NOD.Lc7, or (E) B10.A4R female mice. Significantly reduced T4 levels on iodized water in (C) NOD, (D) B10.A4R males, and (F) C57BL/6. T4 values are in μd/dL (mean + standard error of the mean ). The numbers of mice studied are in parentheses. Statistically significant differences: panel C, *P = 0.004; panel D, **P < 0.001, ***P < 0.001, ^P = 0.008, #P = 0.018 (all t tests). Ns, not significantly different.
Thyroiditis-prone NOD.H2h4 mice (the focus of studies described later) are derived by crossing regular NOD with B10.A4R mice. In the latter strain, 16-week exposure to excess dietary iodide significantly decreased serum T4 levels in males (Fig. 2D), but the suggestive decline in females did not attain statistical significance (Fig. 2E). Finally, we studied C57BL/6 females because they differ markedly from BALB/c females in failing to develop hyperthyroidism in an induced model of Graves disease [23]. Despite this difference, ingestion of a high iodide diet by C57BL/6 mice, like BALB/c mice (Fig. 1A), clearly decreased serum T4 levels, a phenomenon even observable after 8 weeks, the earliest time point studied (Fig. 2F).
B. Thyroid Histology in Relation to Iodide Intake
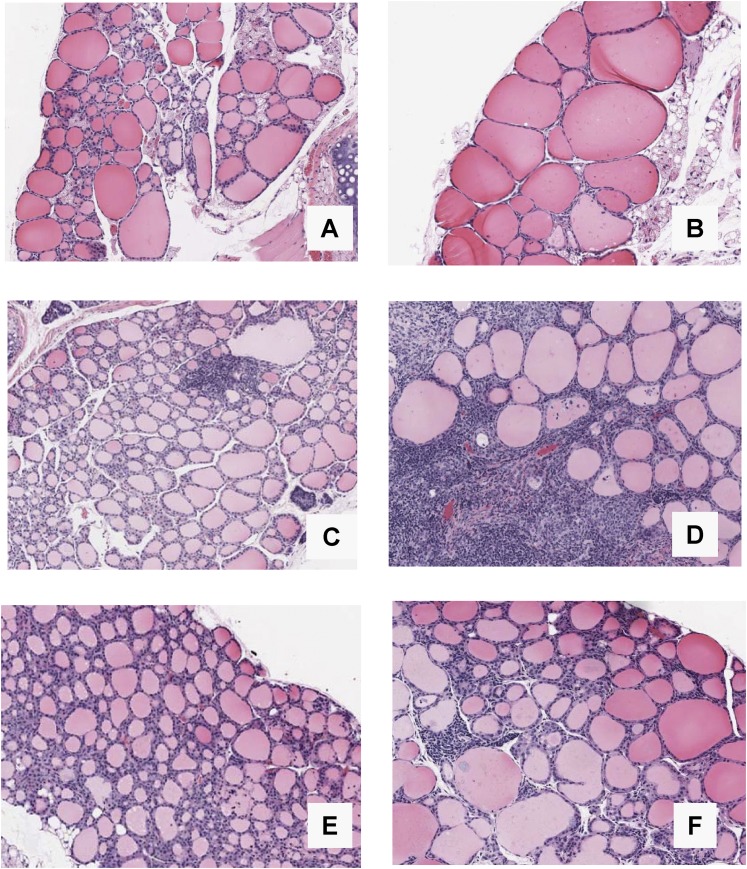
The histological changes induced by a high iodide diet were strikingly different in BALB/c and NOD.H2h4 mice of both sexes. BALB/c mice developed large colloid goiters in association with the decline in serum T4. In contrast, thyroids of NOD.H2h4 mice (whose T4 levels did not decrease) were not enlarged but had moderate thyroid lymphocytic infiltrates. Representative examples after 32 weeks on control or iodized water are shown for female BALB/c (Fig. 3A and 3B) and female NOD.H2h4 (Fig. 3C and 3D) mice. Moderate thyroiditis was previously reported for male NOD.H2h4 and NOD.H2k mice [18] and NOD.H2h4 females [20] after iodide exposure for 16 weeks. Thyroid tissue from male NOD mice on control vs iodized water revealed small lymphocytic infiltration (Fig. 3E and 3F) as reported for NOD.Lc7 [18]. After exposure to iodized water, C57BL/6 mice developed colloid goiters (although less striking than in the BALB/c strain), and thyroid histology was unchanged in B10.A4R mice (data not shown).
Figure 3.
BALB/c mice develop colloid goiters and NOD.H2h4 mice develop moderate thyroiditis after exposure to iodized drinking water for 32 weeks. Some NOD mice develop small lymphocytic infiltrates on iodized water (16 weeks). Thyroid histology in representative (A, B) female BALB/c and (C, D) NOD.H2h4 mice and (E, F) male NOD mice on regular water (left panels) and iodized water (right panels). Magnification: ×10. Number of thyroids examined in each group: A, 5; B, 5; C, 5; D, 5; E, 9; F, 12.
C. Serum TSH in Mice on Iodized Water
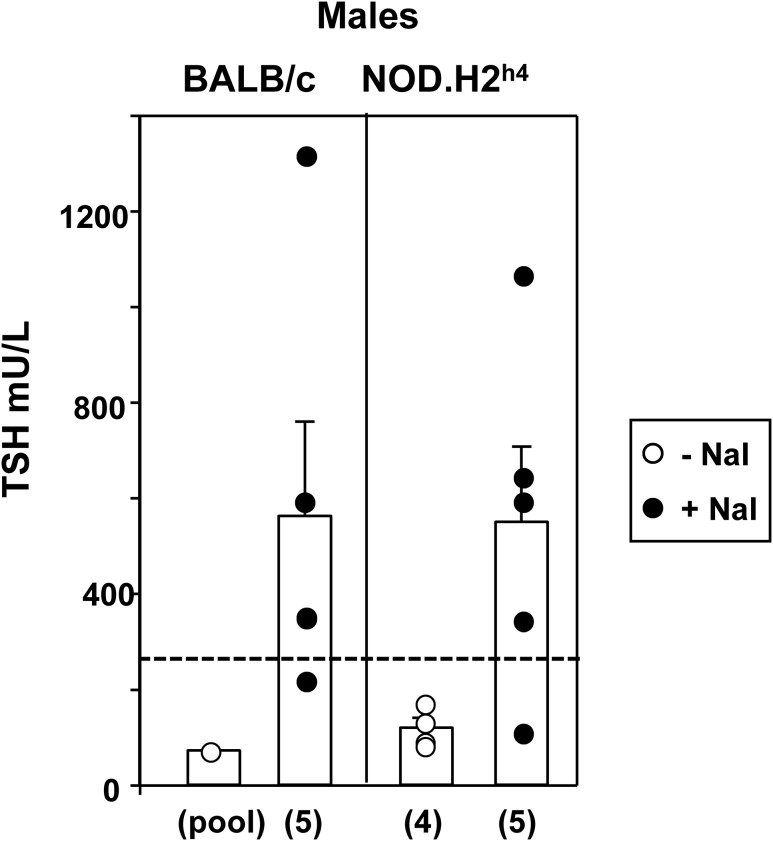
We measured TSH levels in BALB/c and NOD.H2h4 mice, the two strains showing the greatest divergence in response to iodized water, namely hypothyroid goiters in BALB/c and euthyroidism in association with moderate lymphocytic infiltration in NOD.H2h4 mice. Sera were from mice after 16 weeks on iodide, the first time point at which T4 levels were significantly reduced in the BALB/c strain. Because of limited serum available from venepuncture (serum used for multiple assays), and with TSH requiring a large volume, data from only male mice could be subject to statistical analysis. Despite the difference between the BALB/c and NOD.H2h4 mice in their T4 responses to excess dietary iodide (Fig. 1), the extent of the iodide-induced increases in serum TSH were comparable in males of these two strains (Fig. 4). A similar tendency was observed in female mice (data not shown), but the numbers were too small for statistical analysis.
Figure 4.
Serum TSH in male BALB/c and NOD.H2h4 mice exposed to iodized water for 16 weeks. TSH was measured by radioimmunoassay [24] and is expressed as mU/L; the number of sera tested is given in parentheses. The hatched lines provide a “yardstick” for TSH values in untreated recombinant inbred male mice strains (CXB, BXH, and AXB/BXA); mean + 2 SD (n = 51, pooled data from [25]). TSH levels in male BALB/c and NOD.H2h4 mice on iodide supplementation are not significantly (ns) different, but are higher than in recombinant inbred males (analysis of variance, P < 0.05). “Pool” comprises small aliquots of sera from five male BALB/c mice to provide sufficient volume (50 uL) for assay. Note this value is shown for interest and is not used for statistical analysis. T4 values for male NOD.H2h4 mice after 16 weeks on NaI were included in a previous study [20].
D. Expression of Genes Related to Thyroid Function
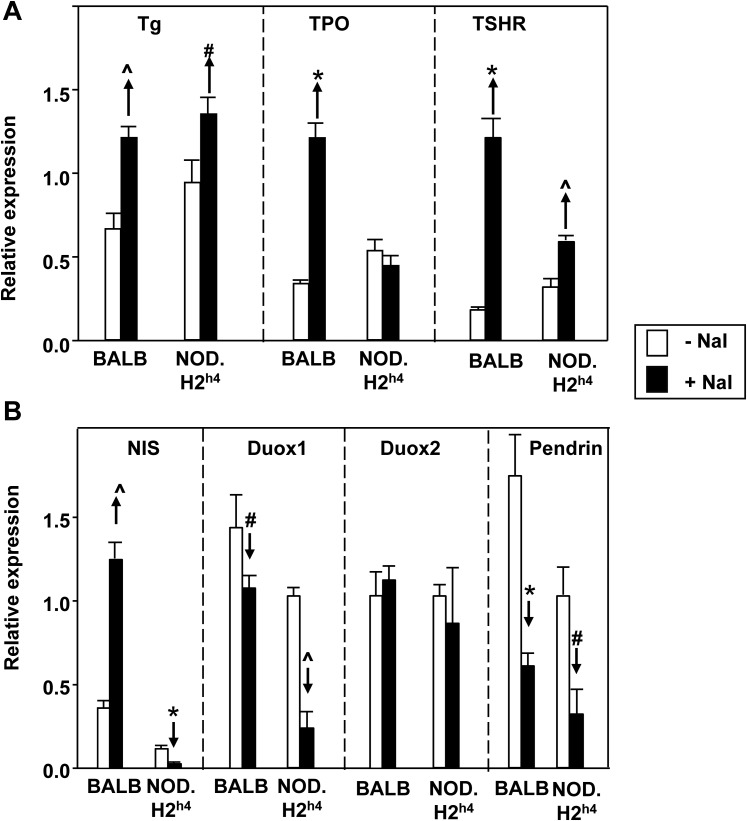
To examine factors underlying the marked difference between BALB/c and NOD.H2h4 mice in their response to excess dietary iodide intake, we assessed the messenger RNA (mRNA) expression level of thyroid genes related to regulation of thyroid hormone synthesis by quantitative PCR. Although we did not quantitate protein expression levels for these thyroid genes, protein concentrations correlate with their respective mRNA when measured under the same conditions [26]. Consistent with the comparable elevation of serum TSH in both mouse strains, expression of Tg, TPO, and the TSHR mRNA coding for the major thyroid-specific genes were all significantly increased on a high iodide intake vs control chow with one exception, namely TPO in NOD.H2h4 mice (Fig. 5A). The expression levels of Duox1 and Pds were reduced in both BALB/c and NOD.H2h4 mice on a high iodide diet (Fig. 5B). Duox2 expression was unchanged.
Figure 5.
Intrathyroidal expression in male BALB/c and NOD.H2h4 mice of mRNA coding for molecules involved in thyroid hormone expression. Mice were, or were not, maintained on iodized water for 16 weeks. Panel A: Tg, TPO, and the TSHR; panel B: NIS, Duox1, Duox 2, and Pds. The data (mean + SD, normalized to mouse β-actin and GAPDH) are from one of two similar experiments. Significant differences: panel A: ^P < 0.002, #P < 0.05, *P < 0.001 (t tests); panel B: ^P < 0.001, *P < 0.002, #P < 0.05 (t tests).
The major, and highly surprising, difference between the two mouse strains was in the expression of NIS, the iodide transporter normally downregulated in the escape from the Wolff-Chaikoff block (Fig. 5B). Increased iodide intake reduced NIS expression in NOD.H2h4 mice that remained euthyroid. In contrast, NIS expression was increased in the BALB/c mice that became hypothyroid. Of note, this difference in NIS expression between BALB/c and NOD.H2h4 was unrelated to TSH stimulation, with TSH levels being increased by a similar extent in both strains (Fig. 4).
3. Discussion
Deficiency of dietary iodide, an essential element for thyroid hormone synthesis, leads to hypothyroidism with severe metabolic consequences. Paradoxically, it has been recognized for more than 60 years [27] that ingestion of excess iodide, for which there are numerous sources [5], can, in some individuals, also cause hypothyroidism. Most susceptible to “iodide myxedema” are euthyroid individuals with pre-existing, subclinical thyroid disorders, particularly Hashimoto thyroiditis [28, 29] and Graves disease following radioiodine therapy or subtotal thyroidectomy [30] and after partial thyroidectomy for benign thyroid nodules [31]. Hemithyroidectomy of thyroid autoimmune-prone BbioBreeding Wistar (BB/W) rats also predisposes to iodide-induced hypothyroidism [32]. On the other hand, iodide-induced hypothyroidism may be idiosyncratic, occurring in normal individuals [33], or may not develop in some patients with Hashimoto thyroiditis with features identical to affected individuals [28]. The underlying mechanism for iodide-induced hypothyroidism in humans is generally accepted to be failure to escape from the Wolff-Chaikoff block [2, 3] because of the inability to reduce iodide transport into the thyroid [4], although it should be appreciated that this phenomenon can only be directly observed ex vivo in experimental animals after removal of their thyroids.
The current study, taken together with previous reports, provides insight into the mechanism of “iodide myxedema,” and it is useful to categorize the contributing factors, or lack thereof, as follows:
-
1.
Thyroid lymphocytic infiltration is not a factor in iodide-induced hypothyroidism in rodents. Consistent with previous reports [10, 16], excess iodide ingestion increases the extent of thyroiditis (lymphocytic infiltration) in thyroid autoimmunity-prone mouse strains, particularly NOD.H2h4 and NOD.H2k, without suppressing serum T4 levels. Conversely, increased dietary iodide did suppress serum T4 levels in BALB/c and C57BL/6 mice that do not develop thyroid lymphocytic infiltration. These data are consistent with BB/W rats in whom thyroiditis was enhanced by dietary iodide excess without alteration in serum T4 levels [34]. As in mice, most humans with thyroid autoantibodies (a reflection of thyroid lymphocytic infiltration) have normal thyroid function [35, 36]. Of course, thyroid lymphocytic infiltration so severe as to eliminate most thyroid follicles can cause hypothyroidism in a number of mouse models, even without increased iodide ingestion [37, 38].
-
2.
NaI symporter autoregulation. The inorganic iodide transporter at the thyrocyte basal surface, subsequently identified as NIS [39], is the focal point of organic iodine autoregulation [1, 4]. Excess dietary iodide intake reduced NIS mRNA expression in thyroiditis-prone NOD.H2h4 mice that remained euthyroid on this dietary regimen, consistent with a normal iodine autoregulatory response. These data confirm the previous finding in Sprague-Dawley rats that iodide administration reduced both NIS mRNA and protein expression in the thyroid [7]. On the other hand, the most remarkable observation in the current study is that hypothyroidism induced by excess dietary iodide in BALB/c mice was associated with an unphysiological, marked increase in NIS mRNA expression (Fig. 5B). Consequently, it is likely that iodide-induced hypothyroidism in BALB/c, but not NOD.H2h4, mice can be ascribed to the inability of the former strain to escape from the Wolff-Chaikoff block by decreasing NIS mRNA levels. We did not study a role for Mct8 or anoctamin_1 [40] in iodine autoregulation in these animals, a possible future investigation.
Li and Carayanniotis [6] observed that iodide-induced hypothyroidism in SJL mice was associated with failure to suppress NIS mRNA (which remained unchanged), whereas in CBA mice that remained euthyroid on the same regimen, NIS mRNA levels were greatly reduced. Although not obtained using quantitative PCR, these data taken together with our quantitative data suggest that there is a graded inherent variability among different mouse strains in iodine autoregulation of NIS expression. Thus, excess dietary iodide increased NIS mRNA levels in BALB/c mice, had no effect in SJL mice, and suppressed NIS mRNA levels in NOD.H2h4 and CBA mice. Hypothyroidism ensues when autoregulation fails in BALB/c and SJL mice.
-
3.
TSH stimulation of NIS expression or functional activity. Iodide transporter functional activity is influenced in a yin-yang manner by opposing factors. Suppression by excess dietary iodide of NIS activity is counterbalanced by TSH stimulation of NIS mRNA expression and translation in rat thyroid cells [41] as well as in human thyroid tissue in vivo [42]. Therefore, conditions in which TSH levels are elevated could counter the physiological iodine autoregulatory suppression of NIS activity. Indeed, subclinical thyroid disorders that are susceptible to iodide myxedema such as Hashimoto thyroiditis [29] and treated Graves disease [30] may have slightly elevated TSH levels. Depression of serum T4 would further increase TSH secretion in a vicious cycle with ensuing overt hypothyroidism. Consistent with a role for TSH in failure to escape from the Wolff-Chaikoff block is the observation of an increased TSH level in SJL, but not CBA, female mice, only the former becoming hypothyroid on iodized water [6].
At face value, the current study provides evidence against the foregoing concept that a TSH-driven increase in NIS expression is an important factor determining whether excess iodide ingestion leads to hypothyroidism. Iodide administration to BALB/c and NOD.H2h4 mice led to comparable increases in serum TSH levels (Fig. 4), yet hypothyroidism only developed in the former strain (Fig. 1). However, as discussed later, the likely reason for this apparent inconsistency is that, because of the iodine autoregulation mechanism, the TSH level in serum does not reflect the potency of the TSH stimulus at the level of adenylyl cyclase activation.
-
4.
Iodine autoregulation and TSH functional activity. A corollary of iodine autoregulation is that this phenomenon modulates TSH functional activity at the level of the thyrocyte. When the serum TSH level is maintained constant, excess iodide diminishes the potency of TSH action on a number of metabolic responses in experimental animals in vivo (TSH injection posthypophysectomy), as well as in isolated thyroids in vitro [reviewed in 1]. Conversely, iodide depletion amplifies TSH action, for example increasing its goitrogenic effect [43]. Inhibition of iodide organification by methimazole or propylthiouracil reverses this effect, as well as having effects on multiple thyrocyte functions [reviewed in 1], implicating an, as yet unidentified, autoregulatory organic iodine intermediary (termed “X-I”) [44], perhaps an iodolactone or iodoaldehyde [reviewed in 45].
An interesting finding in our study is the divergence among molecules involved in thyroid hormone synthesis in their iodine autoregulatory responses, suggesting variability in the yin-yang balance between TSH (positive) and the putative X-I (negative) influences on molecules involved in thyroid hormone synthesis. Thus, elevated serum TSH levels in BALB/c and NOD.H2h4 mice were associated with increases in Tg, TPO, and TSHR mRNA (with the exception of unchanged TPO mRNA in NOD.H2h4 mice). These data, consistent with previous reports of TSH stimulation of these thyroid-specific proteins [for example, 46–52] suggest that Tg, TPO, and the TSHR are not involved in iodine autoregulation. That is, TSH action on these molecules is not significantly opposed by the putative X-I. On the other hand, iodide-induced suppression of Pds mRNA levels in the face of an elevated TSH level is consistent with the greater influence of X-I. Despite an increase in serum TSH, excess dietary iodide either had no effect (Duox2) or suppressed (Duox1) mRNA levels, consistent with transcriptional control independent of the TSH/X-I balance (Duox2) or a balance toward X-I (Duox1).
Most important, in terms of understanding the underlying mechanism for idiosyncratic susceptibility to iodide-induced hypothyroidism in rodents (and possibly in humans), our data suggest an imbalance in the TSH/X-I ratio between BALB/c and NOD.H2h4 mice. Despite similar elevations in serum TSH in both strains, reduced or absent inhibition of NIS by X-I in BALB/c mice [whether directly or indirectly via cyclic adenosine monophosphate (cAMP), discussed later] could lead to unrestrained TSH stimulation of NIS with consequent failure to escape from the Wolff-Chaikoff block. On the other hand, in NOD.H2h4 mice, the balance in the TSH/X-I ratio favors the latter. Consequently, escape from the Wolff-Chaikoff block and euthyroidism is maintained by a combination of NIS suppression and a compensatory increase in TSH, blunted, at least in part, by an autoregulatory reduction in TSH activation of adenylyl cyclase (discussed later).
-
5.
Adenylyl cyclase activity. As mentioned previously, despite a constant TSH level in serum, iodine autoregulation modulates TSH functional activity. TSH action on the thyroid is largely effected by activation of adenylyl cyclase. Cyclic AMP, the product of adenylyl cyclase, is the major positive regulator for expression of NIS [41], TPO [53], and Tg [54], and also stimulates thyrocyte proliferation [55] in synergy with IGF-1 [56]. Control by cAMP of other thyroid-specific proteins is more complex. The functional activity of Pds (one of the apical transporters involved in iodide efflux from the thyrocyte) is increased by adenylyl cyclase activation [57], although its mRNA expression is not stimulated by TSH [42]. Duox2, and to a lesser extent Duox1, generate H2O2 necessary for iodide organification at the thyrocyte follicular lumen and are activated by TSH in a nontranscriptional mechanism [58]. The functional activity of only Duox1 is increased by forskolin via cAMP and protein kinase A [58].
Adenylyl cyclase is itself a pivotal target for iodine autoregulation in vivo [19] and in vitro [44, 59]. The unidentified organic iodine intermediary (X-I) directly suppresses the adenylyl cyclase catalytic unit [60]. Because of its central role in modulating the expression or function of different molecules involved in thyroid hormone synthesis, adenylyl cyclase could be the proximal target of X-I and be the mechanism by which iodine autoregulation modulates TSH activation of more downstream molecules. Alternatively, X-I could be targeting numerous other proteins, at a transcriptional or posttranscriptional level.
In conclusion, the idiosyncratic development of iodide myxedema in some humans, even in the absence of thyroid autoimmunity [33] as well as in diverse mouse strains, suggests a genetic contribution to this susceptibility. As mentioned previously, there is a graded inherent variability among different mouse strains in their susceptibility to iodine autoregulation of NIS expression. Future identification of genes contributing to this variability will be more readily accomplished in genetically identical mouse strains than in genetically diverse humans.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health Grants DK 54684 (to S.M.M.) and 19289 (to B.R.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cAMP
- cyclic adenosine monophosphate
- Duox1
- dual oxidase 1
- Duox2
- dual oxidase 2
- GAPDH
- glutaraldehyde-3-phosphate dehydrogenase
- mRNA
- messenger RNA
- NIS
- sodium iodide symporter
- PCR
- polymerase chain reaction
- Pds
- pendrin
- RT
- real-time
- SD
- standard deviation
- T4
- thyroxine
- Tg
- thyroglobulin
- TPO
- thyroid peroxidase
- TSHR
- TSH receptor.
References and Notes
- 1.Ingbar SH. Autoregulation of the thyroid. Response to iodide excess and depletion. Mayo Clin Proc. 1972;47(11):814–823. [PubMed] [Google Scholar]
- 2.Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174(2):555–564. [PubMed] [Google Scholar]
- 3.Wolff J, Chaikoff IL, Goldberg RC, Meier JR. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45(5):504–513, illust. [DOI] [PubMed] [Google Scholar]
- 4.Braverman LE, Ingbar SH. Changes in thyroidal function during adaptation to large doses of iodide. J Clin Invest. 1963;42(8):1216–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li HS, Carayanniotis G. Induction of goitrous hypothyroidism by dietary iodide in SJL mice. Endocrinology. 2007;148(6):2747–2752. [DOI] [PubMed] [Google Scholar]
- 7.Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, Chin WW, Braverman LE. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140(8):3404–3410. [DOI] [PubMed] [Google Scholar]
- 8.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. I-E+ nonobese diabetic mice develop insulitis and diabetes. J Exp Med. 1993;178(3):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guimont-Desrochers F, Cappello ZJ, Chagnon M, McDuffie M, Lesage S. Cutting edge: genetic characterization of IFN-producing killer dendritic cells. J Immunol. 2009;182(9):5193–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81(3):287–292. [DOI] [PubMed] [Google Scholar]
- 11.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12(3):157–165. [DOI] [PubMed] [Google Scholar]
- 12.Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A. Both CD4(+) T cells and CD8(+) T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol. 1999;192(2):113–121. [DOI] [PubMed] [Google Scholar]
- 13.Tani J, Mori K, Hoshikawa S, Nakazawa T, Satoh J, Nakagawa Y, Ito S, Yoshida K. Prevention of lymphocytic thyroiditis in iodide-treated non-obese diabetic mice lacking interferon regulatory factor-1. Eur J Endocrinol. 2002;147(6):809–814. [DOI] [PubMed] [Google Scholar]
- 14.McLachlan SM, Braley-Mullen H, Chen CR, Aliesky H, Pichurin PN, Rapoport B. Dissociation between iodide-induced thyroiditis and antibody-mediated hyperthyroidism in NOD.H-2h4 mice. Endocrinology. 2005;146(1):294–300. [DOI] [PubMed] [Google Scholar]
- 15.Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun. 2007;29(2-3):195–202. [DOI] [PubMed] [Google Scholar]
- 16.Teng X, Shan Z, Teng W, Fan C, Wang H, Guo R. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin Exp Med. 2009;9(1):51–59. [DOI] [PubMed] [Google Scholar]
- 17.Oppenheim Y, Kim G, Ban Y, Unger P, Concepcion E, Ando T, Tomer Y. The effects of alpha interferon on the development of autoimmune thyroiditis in the NOD H2h4 mouse. Clin Dev Immunol. 2003;10(2-4):161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier AN, Aliesky HA, Banuelos B, Chabot-Roy G, Rapoport B, Lesage S, McLachlan SM. Evidence that MHC I-E dampens thyroid autoantibodies and prevents spreading to a second thyroid autoantigen in I-A(k) NOD mice. Genes Immun. 2015;16(4):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapoport B, West MN, Ingbar SH. Inhibitory effect of dietary iodine on the thyroid adenylate cyclase response to thyrotropin in the hypophysectomized rat. J Clin Invest. 1975;56(2):516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapoport B, Aliesky HA, Banuelos B, Chen CR, McLachlan SM. A unique mouse strain that develops spontaneous, iodine-accelerated, pathogenic antibodies to the human thyrotrophin receptor. J Immunol. 2015;194(9):4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misharin AV, Rapoport B, McLachlan SM. Thyroid antigens, not central tolerance, control responses to immunization in BALB/c versus C57BL/6 mice. Thyroid. 2009;19(5):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CR, Aliesky H, Pichurin PN, Nagayama Y, McLachlan SM, Rapoport B. Susceptibility rather than resistance to hyperthyroidism is dominant in a thyrotropin receptor adenovirus-induced animal model of Graves’ disease as revealed by BALB/c-C57BL/6 hybrid mice. Endocrinology. 2004;145(11):4927–4933. [DOI] [PubMed] [Google Scholar]
- 24.Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9(12):1265–1271. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan SM, Hamidi S, Aliesky H, Williams RW, Rapoport B. Sex, genetics, and the control of thyroxine and thyrotropin in mice. Thyroid. 2014;24(7):1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165(3):535–550. [DOI] [PubMed] [Google Scholar]
- 27.Oliner L, Rubinstein HM. Myxedema induced by prolonged iodide administration. N Engl J Med. 1957;256(2):47–52. [DOI] [PubMed] [Google Scholar]
- 28.Braverman LE, Ingbar SH, Vagenakis AG, Adams L, Maloof F. Enhanced susceptibility to iodide myxedema in patients with Hashimoto’s disease. J Clin Endocrinol Metab. 1971;32(4):515–521. [DOI] [PubMed] [Google Scholar]
- 29.Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. [DOI] [PubMed] [Google Scholar]
- 30.Braverman LE, Woeber KA, Ingbar SH. Induction of myxedema by iodide in patients euthyroid after radioiodin or surgical treatment of diffuse toxic goiter. N Engl J Med. 1969;281(15):816–821. [DOI] [PubMed] [Google Scholar]
- 31.Clark OH, Cavalieri RR, Moser C, Ingbar SH. Iodide-induced hypothyroidism in patients after thyroid resection. Eur J Clin Invest. 1990;20(6):573–580. [DOI] [PubMed] [Google Scholar]
- 32.Allen EM, Appel MC, Braverman LE. Iodine-induced thyroiditis and hypothyroidism in the hemithyroidectomized BB/W rat. Endocrinology. 1987;121(2):481–485. [DOI] [PubMed] [Google Scholar]
- 33.Konno N, Yuri K, Taguchi H, Miura K, Taguchi S, Hagiwara K, Murakami S. Screening for thyroid diseases in an iodine sufficient area with sensitive thyrotrophin assays, and serum thyroid autoantibody and urinary iodide determinations. Clin Endocrinol (Oxf). 1993;38(3):273–281. [DOI] [PubMed] [Google Scholar]
- 34.Allen EM, Appel MC, Braverman LE. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986;118(5):1977–1981. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Amino N, Yagawa K, Uemura K, Satoh M, Miyai K, Kumahara Y. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46(6):859–862. [DOI] [PubMed] [Google Scholar]
- 36.Acar T, Ozbek SS, Erdogan M, Ozgen AG, Demirel SO. US findings in euthyroid patients with positive antithyroid autoantibody tests compared to normal and hypothyroid cases. Diagn Interv Radiol. 2013;19(4):265–270. [DOI] [PubMed] [Google Scholar]
- 37.Martin AP, Marinkovic T, Canasto-Chibuque C, Latif R, Unkeless JC, Davies TF, Takahama Y, Furtado GC, Lira SA. CCR7 deficiency in NOD mice leads to thyroiditis and primary hypothyroidism. J Immunol. 2009;183(5):3073–3080. [DOI] [PubMed] [Google Scholar]
- 38.McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148(12):5724–5733. [DOI] [PubMed] [Google Scholar]
- 39.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379(6564):458–460. [DOI] [PubMed] [Google Scholar]
- 40.Silveira JC, Kopp PA. Pendrin and anoctamin as mediators of apical iodide efflux in thyroid cells. Curr Opin Endocrinol Diabetes Obes. 2015;22(5):374–380. [DOI] [PubMed] [Google Scholar]
- 41.Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138(6):2227–2232. [DOI] [PubMed] [Google Scholar]
- 42.Bruno R, Feretti E, Tosi E, Arturi F, Giannasio P, Mattei P, Scipioni A, Presta I, Morisi R, Gulino A, Filetti S, Russo D. Modulation of thyroid-specific gene expression in normal and nodular human thyroid tissue from adults: an in vivo effect of thyrotropin. J Clin Endo Metab. 2017;90(10):5692–5697. [DOI] [PubMed] [Google Scholar]
- 43.Bray GA. Increased sensitivity of the thyroid in iodine-depleted rats to the goitrogenic effects of thyrotropin. J Clin Invest. 1968;47(7):1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Sande J, Grenier G, Willems C, Dumont JE. Inhibition by iodide of the activation of the thyroid cyclic 3′,5′-AMP system. Endocrinology. 1975;96(3):781–786. [DOI] [PubMed] [Google Scholar]
- 45.Boeynaems JM, Van Sande J, Dumont JE. Which iodolipids are involved in thyroid autoregulation: iodolactones or iodoaldehydes? Eur J Endocrinol. 1995;132(6):733–734. [DOI] [PubMed] [Google Scholar]
- 46.Chazenbalk G, Magnusson RP, Rapoport B. TSH stimulation of cultured thyroid cells increases steady state levels of the messenger RNA for thyroid peroxidase. Mol Endocrinol. 1987;1:913–917. [DOI] [PubMed] [Google Scholar]
- 47.Chiovato L, Vitti P, Lombardi A, Ceccarelli P, Cucchi P, Marcocci C, Carayon P, Pinchera A. Studies on the mechanism responsible for thyrotropin-induced expression of microsomal/peroxidase antigen in FRTL-5 cells. Endocrinology. 1988;123(2):1140–1146. [DOI] [PubMed] [Google Scholar]
- 48.Isozaki O, Kohn LD, Kozak CA, Kimura S. Thyroid peroxidase: rat cDNA sequence, chromosomal localization in mouse, and regulation of gene expression by comparison to thyroglobulin in rat FRTL-5 cells. Mol Endocrinol. 1989;3(11):1681–1692. [DOI] [PubMed] [Google Scholar]
- 49.Nagayama Y, Yamashita S, Hirayu H, Izumi M, Uga T, Ishikawa N, Ito K, Nagataki S. Regulation of thyroid peroxidase and thyroglobulin gene expression by thyrotropin in cultured human thyroid cells. J Clin Endocrinol Metab. 1989;68(6):1155–1159. [DOI] [PubMed] [Google Scholar]
- 50.Gerard CM, Lefort A, Libert F, Christophe D, Dumont JE, Vassart G. Transcriptional regulation of the thyroperoxydase gene by thyrotropin and forskolin. Mol Cell Endocrinol. 1988;60(2-3):239–242. [DOI] [PubMed] [Google Scholar]
- 51.Huber GK, Concepcion ES, Graves PN, Davies TF. Positive regulation of human thyrotropin receptor mRNA by thyrotropin. J Clin Endocrinol Metab. 1991;72(6):1394–1396. [DOI] [PubMed] [Google Scholar]
- 52.Maenhaut C, Brabant G, Vassart G, Dumont JE. In vitro and in vivo regulation of thyrotropin receptor mRNA levels in dog and human thyroid cells. J Biol Chem. 1992;267(5):3000–3007. [PubMed] [Google Scholar]
- 53.Magnusson RP, Rapoport B. Modulation of differentiated function in cultured thyroid cells: thyrotropin control of thyroid peroxidase activity. Endocrinology. 1985;116(4):1493–1500. [DOI] [PubMed] [Google Scholar]
- 54.Van Heuverswyn B, Leriche A, Van Sande J, Dumont JE, Vassart G. Transcriptional control of thyroglobulin gene expression by cyclic AMP. FEBS Lett. 1985;188(2):192–196. [DOI] [PubMed] [Google Scholar]
- 55.Dere WH, Rapoport B. Control of growth in cultured rat thyroid cells. Mol Cell Endocrinol. 1986;44(3):195–199. [DOI] [PubMed] [Google Scholar]
- 56.Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119(2):940–942. [DOI] [PubMed] [Google Scholar]
- 57.Pesce L, Bizhanova A, Caraballo JC, Westphal W, Butti ML, Comellas A, Kopp P. TSH regulates pendrin membrane abundance and enhances iodide efflux in thyroid cells. Endocrinology. 2012;153(1):512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem. 2009;284(11):6725–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapoport B, West MN, Ingbar SH. On the mechanism of inhibition by iodine of the thyroid adenylate cyclase response to TSH. Endocrinology. 1976;99:11–22. [DOI] [PubMed] [Google Scholar]
- 60.Filetti S, Rapoport B. Evidence that organic iodine attenuates the adenosine 3′,5′-monophosphate response to thyrotropin stimulation in thyroid tissue by an action at or near the adenylate cyclase catalytic unit. Endocrinology. 1983;113(5):1608–1615. [DOI] [PubMed] [Google Scholar]
- 61.Weatherall D, Sarvetnick N, Shizuru JA. Genetic control of diabetes mellitus. Diabetologia. 1992; 35(S2, Suppl 2):S1–S7. [DOI] [PubMed] [Google Scholar]
- 62.Bernard NF, Ertug F, Margolese H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes. 1992;41(1):40–46. [DOI] [PubMed] [Google Scholar]