Abstract
Background and Objective:
Administration of growth hormone (GH) during ovarian stimulation has been shown to improve success rates of in vitro fertilization. GH beneficial effect on oocyte quality is shown in several studies, but GH effect on uterine receptivity is not clear. To assess it, we studied whether GH administration can improve the chance of pregnancy and birth in women who experienced repeated implantation failure (RIF) using donated oocyte programs.
Design and Study Population:
A total of 105 infertile women were enrolled in the randomized controlled trial: 70 women were with a history of RIF with donated oocytes, and 35 infertile women underwent the first oocyte donation attempt. Women receiving donated oocytes were treated with progressively increasing doses of oral estradiol, followed by intravaginal progesterone after previous pituitary desensitization with gonadotropin-releasing hormone agonist. Thirty-five RIF patients were treated with GH (GH patients), whereas the rest of the 35 RIF patients (non-GH patients) and 35 first-attempt patients (positive control group) were not.
Results:
RIF patients receiving GH showed significantly thicker endometrium and higher pregnancy and live birth rates as compared with RIF patients of non-GH study group, although these rates remained somewhat lower as compared with the non-RIF patients of the positive control group. No abnormality was detected in any of the babies born.
Conclusion:
Our data of improved implantation, pregnancy, and live birth rates among infertile RIF patients treated with GH indicate that GH improves uterine receptivity.
Keywords: endometrial receptivity, growth hormone, implantation failure, IVF, RIF
Growth hormone administration improves pregnancy and birth rates among infertile women experiencing repeated implantation failure after receiving donated oocytes.
Administration of growth hormone (GH) during ovarian stimulation has been shown to improve success rates of in vitro fertilization (IVF) treatment [1, 2], and especially in women with poor ovarian response [3–6]. It is widely assumed that this improvement is related to the beneficial effects of GH on oocyte quality, as suggested by the observations of a higher number of oocytes collected, higher fertilization rate, and a higher number of embryos reaching the transfer stage in GH-treated patients [3, 5–8]. Furthermore, ovarian costimulation with GH has been shown to increase pregnancy rate [2, 9, 10], implantation rate [1, 2, 11], and live birth rate [1, 4]. Additionally, a recent meta-analysis of GH costimulation in controlled ovarian stimulation demonstrates significant increase in clinical pregnancy and live birth rates among poor ovarian responders [5].
Growth hormone (GH) is a peptide hormone secreted by the anterior pituitary gland in pulsatile manner, and it has important roles in cell growth and metabolism throughout the body. GH receptor is expressed in human oocytes and cumulus cells [12, 13], and GH has been shown to promote in vitro nuclear maturation of denuded human oocytes [14]. In addition to its direct effect on the oocytes and/or cumulus cells, GH may also influence oocyte quality indirectly, through activation of insulin-like growth factor-I synthesis or promotion of follicle-stimulating hormone–induced ovarian steroidogenesis (reviewed in [15]).
The data published on the beneficial effect of GH on assisted reproduction outcomes do not exclude the possibility that this effect is due, at least in part, to an action of GH on the uterus, enhancing the receptivity of endometrium for the implanting embryo. In fact, the uterus is a site of both GH production and GH action [15]. Indeed, GH has been shown not only to increase embryonic development in superovulated cows, but also to improve posttransfer pregnancy rates when given to embryo recipients [16]. In humans, the first study indicating GH beneficial effect on endometrium has been published recently, in which it was shown that simultaneous administration of GH with hormone-replacement therapy could improve clinical outcomes after frozen embryo transfer by increasing endometrial blood perfusion and expression of cytokines related to endometrial receptivity [17]. Further studies of GH effects on human uterine receptivity are clearly warranted before any clinical recommendations/adjustments in infertility treatment protocols could be done.
In the current study, we used a model of oocyte donation to evaluate the possible beneficial effects of GH on uterine receptivity. Oocyte donation usually enables very high success rates, but some patients can suffer repeated implantation failure (RIF) even with the use of this approach. In general, RIF is diagnosed when good-quality embryos repeatedly fail to implant. In this study, patients who had undergone two failed oocyte donation attempts at our center were considered as RIF patients. They were randomly assigned to two groups, as follows: patients in one group received GH treatment during endometrium preparation with estradiol, and those in the other group underwent a standard protocol without GH (non-GH group). The results of both RIF groups were compared with a group of supposedly good-prognosis patients undergoing their first oocyte donation attempt (positive controls).
1. Subjects and Methods
A. Study Design and Participants
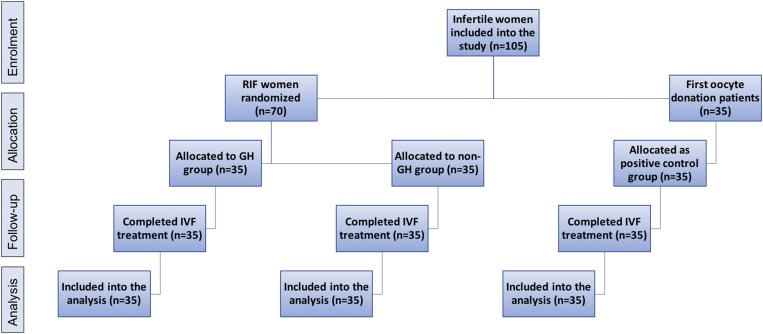
This is a randomized controlled trial, conducted between 2010 and 2017 at the MARGen Clinic. The allocation ratio of the three patient groups involved in this study (GH group as treatment group, non-GH group as negative control group, and women undergoing first donated oocyte treatment as positive control group) is 1:1:1 (Fig. 1). All subjects were fully informed about the procedure to be used, and a written consent was obtained. This study has been approved by the Ethics Committee of the University of Granada.
Figure 1.
Flow chart of the study design. All 105 infertile women underwent oocyte donation program, in which 70 women were with history of RIF and 35 women were the first oocyte donation patients with no history of RIF. No losses or exclusion occurred after randomization. The recruitment of patients started in 2010, and the follow-up ended in 2017, when the analysis of all pending outcomes was terminated.
The upper limit of oocyte recipient age and of oocyte donor age established at our clinic is 52 years and 25 years, respectively. These criteria of eligibility were applied from the beginning until the end of this study.
This study included in total 105 infertile couples treated by assisted reproduction with oocyte donation (Fig. 1). Seventy couples who experienced at least two previous failures with this approach at our clinic are referred to as RIF patients throughout this study. Thirty-five of these couples were included in the GH-treatment protocol [mean ± standard deviation (SD) implantation failures 3.17 ± 1.33, median implantation failure 3], whereas the remaining 35 couples were treated in a usual way (non-GH group) (mean ± SD implantation failures 3.34 ± 1.16, median implantation failure 3). The other 35 couples treated in the same period and undergoing their first oocyte donation attempt, were included as a positive control group. No changes to methods were done after trial commencement.
B. Allocation and Randomization
RIF patients were randomly allocated into two groups (GH and non-GH patients). All GH injections were performed by an independent nurse who was informed by the trial coordinator of each woman’s number and the treatment allocation. The positive control (non-RIF) group was created by allocating all consecutive couples undergoing their first oocyte donation attempt. This allocation was started immediately after the allocation of the first RIF couple and terminated as soon as 35 cases were enrolled.
C. Oocyte Donation and IVF/Intracytoplasmic Sperm Injection
According to the rules of our clinic, the maximum age of all oocyte donors was 25 years. The age of women receiving embryos from this program (oocyte recipients) was between 30 and 51 years. IVF was made by intracytoplasmic sperm injection in all cases included in this study because of the semen quality, as described before [18, 19].
Basically, ovarian stimulation of the oocyte donors, as well as the treatment of oocyte recipients, was carried out as described [20]. Briefly, oocyte donors were stimulated with the use of a long gonadotropin-releasing hormone agonist protocol and human recombinant follicle-stimulating hormone (Puregon or Gonal F). Human menopausal gonadotropin (Menopur) was added when blood luteinizing hormone concentration, which was repeatedly determined during ovarian stimulation, fell <1 IU/L. Final oocyte maturation was triggered by subcutaneous injection of 250 μg recombinant human chorionic gonadotropin (hCG; Ovitrelle), when at least five follicles measured 18 mm or more. Ovarian puncture for oocyte recovery was performed 36.5 hours after recombinant hCG injection.
Oocyte recipients were treated with progressively increasing doses of orally administered pure estradiol (Provames) or estradiol valerate (Progynova) after previous pituitary desensitization with a single injection of the long-acting preparation of gonadotropin-releasing hormone agonist triptorelin (3.75 mg Decapeptyl). The interval between triptorelin injection and the beginning of oral estradiol treatment ranged between 8 and 20 days. This interval was determined individually, in each case, in view of the optimal synchronization of the recipient’s endometrial development and the follicular growth of the corresponding donor.
These protocols made it possible to synchronize the ovarian stimulation of each donor with the development of the endometrium of the respective oocyte recipient. Consequently, all attempts of oocyte donation included in this study were carried out with fresh oocytes. Supernumerary embryos were cryopreserved; the results with cryopreserved embryos are not included in this study.
D. Outcome Measures
Pregnancy rate, live birth rate, and live born baby rate were the primary outcomes. Secondary outcomes included ongoing pregnancy rate, implantation rate, ongoing implantation rate, and miscarriage rate.
Pregnancy was defined as a positive β-hCG test, and the ongoing pregnancy was defined as the presence of at least one fetal heart pulsation on ultrasound beyond 20 weeks. Pregnancy rate was calculated as the number of patients with positive β-hCG test divided by the number of patients in whom embryos were transferred, whereas the ongoing pregnancy rate was the number of patients with the presence of fetal heart activity divided by the number of transfer procedures. Implantation rate was the number of embryonic sacs detected on ultrasound divided by the number of embryos transferred, and the ongoing implantation rate was calculated by dividing the number of living fetuses developing beyond 20 weeks divided by the number of embryos transferred. The miscarriage rate was defined as the number of miscarriages before 20 weeks divided by the number of women with a positive pregnancy test. Live birth rate was calculated as the number of births by the number of transfer procedures, and the live born baby rate was obtained by dividing the number of babies born with the number of embryos transferred. No changes were made to the trial outcome measures after the trial commenced.
E. Sample Size Calculation
Sample size calculation was performed using Stat version 13 (StataCorp, College Station, TX). The pregnancy rate of RIF patients in oocyte donation program in our clinic has been rather low (<20%) as compared with good-prognosis patients (>75%). Assuming that there is a 50% increase in pregnancy rate with GH administration (i.e., an increase from 20% to 30%), 31 subjects are required in each group to give a test of significance on 0.05, power of 90%, and SD of 0.6 (SD among our outcome variables is 0.5, but for stringent analysis we have chosen SD 0.6).
F. Statistical Analyses
Statistical analyses were performed in SPSS 20 for Macintosh (IBM, Chicago, IL). Most continuous variables (e.g., age of patients and total number of embryos) were normally distributed. Those continuous variables that were not normally distributed (e.g., endometrial thickness) were ln transformed. One-way analysis of variance was used was to explore the differences in the distribution of baseline and cycle characteristics across the groups. In case of significant group differences, Tukey post hoc test was used to explore the differences between subgroups. Logistic regression was performed for analyzing differences in outcome measures between GH patients and controls, in which age of the patient, age of the partner, and the number of embryos transferred were entered as confounders. Binary logistic regression was used to determine the odds ratios (ORs) and 95% confidence intervals (C.I.) of having a better outcome measures among GH patient group compared with non-GH group. These analyses were adjusted for patient age (model 1), and additionally for partner’s age and the number of embryos transferred (model 2). In all analyses, P value < 0.05 was considered statistically significant.
2. Results
A. Baseline and Cycle Characteristics
The three groups of infertile couples included in this study did not differ as to the female and male age. The women in the GH group had mean (±SD) age 42.2 ± 4.7 years, those in the non-GH group 42.4 ± 3.7 years, and those in the positive control group 43.8 ± 2.5 years. Husband ages in the study groups were 44.7 ± 7.4 years in GH group, 45.1 ± 5.4 years in non-GH group, and 47.1 ± 5.0 years in the positive control group. The three groups did not differ as to the duration and cause of infertility as well as to body mass index and the percentage of smokers among the female and the male partners. As the cycle characteristics of the three patient groups were similar, with the exception of the number of embryos transferred (Table 1), all of the following analyses were controlled with respect to the number of embryos transferred. For each of the three groups, all 35 randomly assigned participants received intended treatment and were analyzed for the primary outcomes. No losses or exclusions occurred after randomization.
Table 1.
Clinical Characteristics of the Three Study Groups (n = 35 in Each One) Treated by Oocyte Donation
| GH Patients | Non-GH Patients | Positive Controls | P Value | |
|---|---|---|---|---|
| Endometrial thickness (mm) mean (SD) | 9.3 (1.5) | 8.6 (1.0) | 9.4 (1.7) | 0.046 |
| No. of embryos obtained | 7.9 (2.2) | 8.2 (1.5) | 8.3 (1.3) | 0.643 |
| Embryos transferred | 0.036a | |||
| 1 | 2 (5.7%) | 0 (0%) | 5 (14.3%) | |
| 2 | 26 (74.3%) | 27 (77.1%) | 27 (77.1%) | |
| 3 (%) | 7 (20.0%) | 8 (22.9%) | 3 (8.6%) | |
| β-hCG (positive) | 19 (54.3%) | 6 (17.1%) | 26 (74.3%) | <0.001a,b |
| Heartbeat (positive) | 18 (51.4%) | 6 (17.1%) | 25 (71.4%) | <0.001a,b |
| No. of sacs | <0.001a,b | |||
| 1 | 13 (37.1%) | 4 (11.4%) | 17 (48.6) | |
| 2 | 6 (7.1%) | 2 (5.7%) | 9 (25.7) | |
| Delivery (yes) | 18 (51.4%) | 6 (17.1%) | 24 (68.6%) | <0.001a,b |
| Babies born | <0.001a,b | |||
| 1 | 14 (40.0%) | 5 (14.3%) | 16 (45.7%) | |
| 2 | 4 (11.4%) | 1 (2.9%) | 8 (22.9%) |
Significant differences between non-GH patients vs. positive controls in a multiple-comparison–adjusted post hoc test.
bSignificant differences between non-GH patients vs. GH patients in a multiple-comparison–adjusted post hoc test.
B. Treatment Outcomes
Endometrial thickness was positively increased by the GH administration in RIF patients (P = 0.046) (Table 1). In fact, RIF women in the GH group had similar endometrial thickness compared with the positive control women (9.3 ± 1.5 mm and 9.4 ± 1.7 mm, respectively), whereas RIF women in the non-GH group had a significantly thinner endometrium (8.6 ± 1.0 mm). There were significant differences in the pregnancy rates, live birth rates, and live born baby rates between the three study groups. RIF patients from the non-GH group demonstrated significantly lower treatment success rates as compared with each of the two other groups (Tables 1 and 2). The RIF patient group receiving GH administration demonstrated significantly higher treatment success rates when compared with the non-GH patient group (Table 2; Fig. 2). OR, the odds for having a positive β-hCG test, was very strong: 6.9 (95% C.I. 2.2 to 22.5), positive heart beat 6.4 (95% C.I. 2.0 to 20.9), and live birth 6.4 (95% C.I. 2.0 to 20.9) for the GH group compared with non-GH group (Table 3; Fig. 2). OR > 3.0 indicates a strong association, that there is no confounding or minimal confounding effects. In our study, the high OR indicates clearly that these significant improvements in IVF treatment outcomes must have resulted from the positive effects of GH on uterine receptivity, as only donated oocytes were used in our IVF program, which were devoid from the effects of GH. Nevertheless, as expected, the success rates in the group of RIF patients treated with GH were somewhat lower as compared with the positive controls who underwent their first treatment with donated oocytes (Tables 1, 2, and 3). No abnormality was detected in any of the babies born and during the follow-up until 1 year of age.
Table 2.
Summary of Treatment Success Rates in Studied Groups
| Non-GH Patients (n = 35) | GH Patients (n = 35) | Positive Controls (n = 35) | |
|---|---|---|---|
| Pregnancy rate (n° of women with positive hCG) | 17.1% [6] | 54.3% [19] | 74.3% [26] |
| Implantation rate (n° of embryo sacs) | 10.3% [8] | 33.3% [25] | 51.5% [35] |
| Ongoing pregnancy rate (n° of women with presence of fetal heart activity) | 17.1% [6] | 51.4% [18] | 71.4% [25] |
| Ongoing implantation rate (n° of living fetuses >20 weeks) | 7.7% | 24.0% [18] | 36.8% |
| Miscarriage rate (n° of miscarriages) | 0% (0) | 5.3% [1] | 3.8% [1] |
| Live birth rate (n° of women with live birth) | 17.1% [6] | 51.4% [18] | 68.6% [24] |
| Live born baby rate (n° of babies born) | 9.0% [7] | 29.3% [22] | 47.1% [32] |
Figure 2.
ORs of having a better treatment outcome after GH administration. Non-GH patients were set as reference group in the analyses and are graphically represented by the dashed line. RIF women cotreated with GH in donated oocyte program demonstrate >5 times higher chance of obtaining positive pregnancy test and live birth than women in the non-GH group.
Table 3.
Chances of Treatment Success Among Infertile Women Administrating GH During IVF Treatment vs. Women in the Non-GH Group
| P Value | Odds Ratio | Confidence Interval | |
|---|---|---|---|
| Positive β-hCG | 0.001 | 6.9 | 2.2 to 22.5 |
| Positive heart beat | 0.002 | 6.4 | 2.0 to 20.9 |
| Live birth | 0.002 | 6.4 | 2.0 to 20.9 |
Controlled/Adjusted for patient age, age of husband, and the number of embryos transferred.
3. Discussion
The results of our study show that the addition of GH to the treatment protocol in infertile women with a history of at least two previous implantation failures with donor oocytes improves significantly their chances of achieving pregnancy. Previous studies have shown that GH increases delivery and live birth rates in women aged >40 years [1], improves oocyte competence in women with multiple IVF failures [9], and increases pregnancy and live birth rates in poor ovarian responders [5]. In all of these studies, however, GH was used during ovarian stimulation, and the observed effects were interpreted as a result of GH effect on the oocytes. Our study design, in contrast, enabled us to evaluate the possible effects of GH exclusively on endometrial receptivity because only patients receiving donated oocytes (and not the respective oocyte donors) were treated with GH.
Little information is currently available regarding the effects of GH on endometrial receptivity. Global endometrial transcriptome studies demonstrate that women with RIF show dysregulations of several genes, gene networks, and signaling pathways when compared with healthy fertile women [21–25]. GH is a mitogen that triggers signal transduction pathways that influence gene expression regulation, including Janus kinase–signal transducer and activator of transcription pathway [26], which is highlighted as an important pathway in endometrial receptivity [27–29]. GH administration might positively stimulate genes and pathways that otherwise would be dysregulated in the endometrium of women with RIF, leading to an improvement of endometrial receptivity in this subgroup of infertile women. Indeed, a previous study on infertile women receiving hormone-replacement therapy with or without GH for frozen embryo transfers demonstrated that GH administration improved expression of cytokines related to endometrial receptivity [17].
GH-positive effect on the endometrial thickness was an important finding in the GH administration group, as adequate endometrial thickness is crucial for successful implantation [30, 31]. In line with this result, previous studies also have reported positive effect of GH on endometrial thickness in IVF programs [2, 6, 17]. These findings are further supported by a meta-analysis that concluded that adding GH during IVF in women with underdeveloped endometria (<6 mm thickness) significantly improved the morphology and thickness of the endometrium, resulting in a significantly higher clinical pregnancy rate [2]. GH is expressed in the glandular cells of endometrium from the late luteal phase and in the decidual tissue throughout pregnancy [32], and its possible effects as promoting proliferation and differentiation of the endometrium, ion exchange, and extracellular matrix synthesis have been proposed [33]. In addition, studies on mice have demonstrated that GH increases the expression of receptivity-related factors such as leukemia inhibitory factor, integrins, and matrix metalloproteinase 9 [2]. Further studies clearly are needed to understand the molecular mechanisms of GH on the endometrium and its functions.
In contrast, no significant impairment of uterine receptivity appears to occur with increasing female age, and oocyte donation leads to excellent success rates in older women [34]. The results of the current study must be interpreted in this context. We show in this work that >70% of couples undergoing their first oocyte donation attempt achieve clinical pregnancy with positive heartbeat, and this group of patients is thus unlikely to benefit from additional GH treatment. Nevertheless, additional analysis of GH administration among non-RIF patients undergoing oocyte donation would help to define better the target group benefiting from GH.
In spite of the high success rate of oocyte donation in the overall patient population, there does exist patients in whom the technique fails repeatedly. The results of our study demonstrate that this latter category of infertile women can benefit from GH treatment in an oocyte donation program. Additionally, women with RIF in other IVF programs (treated with their own oocytes) might also benefit from GH treatment. Of special interest are infertile patients with negative Clonidine test, who have low endogenous GH reserve and cannot increase its secretion after stimulation with Clonidine [35]. Indeed, previous studies have shown enhanced conception with GH cotreatment in Clonidine-negative and not in Clonidine-positive patients undergoing ovarian stimulation and IVF [36–38, 35]. Further research is warranted to refine the identification of patients who could truly benefit from getting GH treatment in assisted reproduction.
In conclusion, our study demonstrates that cotreatment with GH in RIF patients in donated oocyte cycles improves significantly embryo implantation, pregnancy, and live birth rates via beneficial actions on endometrial receptivity. A question arises as to how to identify the target patient population without the need to experience several previous treatment failures. The answer to this question is pending on the understanding of the mechanism through which GH improves endometrial receptivity. The evaluation of the effects of GH on endometrial transcriptome, subendometrial vascularization, and uterine artery blood flow are possible ways for future research.
Acknowledgments
Financial Support: S.A. was supported by Incorporación de Jovenes Doctores Program from University of Granada; Enterprise Estonia (Grant EU48695); and Estonian Ministry of Education and Research (Grant IUT34-16).
Clinical Trial Information: ClinicalTrials.gov no. NCT03237117 (registered 2 August 2017).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- GH
- growth hormone
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- OR
- odds ratio
- RIF
- repeated implantation failure
- SD
- standard deviation.
References and Notes
- 1.Tesarik J, Hazout A, Mendoza C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum Reprod. 2005;20(9):2536–2541. [DOI] [PubMed] [Google Scholar]
- 2.Du XF, Yang XH, Li J, Hao M, Guo YH. Growth hormone co-treatment within a GnRH agonist long protocol improves implantation and pregnancy rates in patients undergoing IVF-ET. Arch Gynecol Obstet. 2016;294(4):877–883. [DOI] [PubMed] [Google Scholar]
- 3.Kolibianakis EM, Venetis CA, Diedrich K, Tarlatzis BC, Griesinger G. Addition of growth hormone to gonadotrophins in ovarian stimulation of poor responders treated by in-vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(6):613–622. [DOI] [PubMed] [Google Scholar]
- 4.Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database Syst Rev. 2010;(1):CD000099. [DOI] [PMC free article] [PubMed]
- 5.Li X-L, Wang L, Lv F, Huang X-M, Wang L-P, Pan Y, Zhang X-M. The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(12):e6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassiouny YA, Dakhly DMR, Bayoumi YA, Hashish NM. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril. 2016;105(3):697–702. [DOI] [PubMed] [Google Scholar]
- 7.Kucuk T, Kozinoglu H, Kaba A. Growth hormone co-treatment within a GnRH agonist long protocol in patients with poor ovarian response: a prospective, randomized, clinical trial. J Assist Reprod Genet. 2008;25(4):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassiouny YA, Dakhly DMR, Bayoumi YA, Hashish NM. Does the addition of growth hormone to the in vitro fertilization/intracytoplasmic sperm injection antagonist protocol improve outcomes in poor responders? A randomized, controlled trial. Fertil Steril. 2016;105(3):697–702. [DOI] [PubMed] [Google Scholar]
- 9.Hazout A, Junca A, Ménézo Y, Demouzon J, Cohen-Bacrie P. Effect of growth hormone on oocyte competence in patients with multiple IVF failures. Reprod Biomed Online. 2009;18(5):664–670. [DOI] [PubMed] [Google Scholar]
- 10.Lattes K, Brassesco M, Gomez M, Checa MA. Low-dose growth hormone supplementation increases clinical pregnancy rate in poor responders undergoing in vitro fertilisation. Gynecol Endocrinol. 2015;31(7):565–568. [DOI] [PubMed] [Google Scholar]
- 11.Yovich JL, Stanger JD. Growth hormone supplementation improves implantation and pregnancy productivity rates for poor-prognosis patients undertaking IVF. Reprod Biomed Online. 2010;21(1):37–49. [DOI] [PubMed] [Google Scholar]
- 12.Ménézo YJR, el Mouatassim S, Chavrier M, Servy EJ, Nicolet B. Human oocytes and preimplantation embryos express mRNA for growth hormone receptor. Zygote. 2003;11(4):293–297. [DOI] [PubMed] [Google Scholar]
- 13.Abir R, Garor R, Felz C, Nitke S, Krissi H, Fisch B. Growth hormone and its receptor in human ovaries from fetuses and adults. Fertil Steril. 2008;90(4, Suppl):1333–1339. [DOI] [PubMed] [Google Scholar]
- 14.Menezo YJR, Nicollet B, Rollet J, Hazout A. Pregnancy and delivery after in vitro maturation of naked ICSI-GV oocytes with GH and transfer of a frozen thawed blastocyst: case report. J Assist Reprod Genet. 2006;23(1):47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull KL, Harvey S. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int J Endocrinol. 2014;2014:234014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira F, Badinga L, Burnley C, Thatcher WW. Bovine somatotropin increases embryonic development in superovulated cows and improves post-transfer pregnancy rates when given to lactating recipient cows. Theriogenology. 2002;57(4):1371–1387. [DOI] [PubMed] [Google Scholar]
- 17.Xue-Mei W, Hong J, Wen-Xiang Z, Yang L. The effects of growth hormone on clinical outcomes after frozen-thawed embryo transfer. Int J Gynaecol Obstet. 2016;133(3):347–350. [DOI] [PubMed] [Google Scholar]
- 18.Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17(1):184–189. [DOI] [PubMed] [Google Scholar]
- 19.Hazout A, Dumont-Hassan M, Junca A-M, Cohen Bacrie P, Tesarik J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod Biomed Online. 2006;12(1):19–25. [DOI] [PubMed] [Google Scholar]
- 20.Tesarik J, Mendoza C. Effects of exogenous LH administration during ovarian stimulation of pituitary down-regulated young oocyte donors on oocyte yield and developmental competence. Hum Reprod. 2002;17(12):3129–3137. [DOI] [PubMed] [Google Scholar]
- 21.Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod. 2009;24(10):2541–2548. [DOI] [PubMed] [Google Scholar]
- 22.Altmäe S, Martínez-Conejero JA, Salumets A, Simón C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16(3):178–187. [DOI] [PubMed] [Google Scholar]
- 23.Koot YE, van Hooff SR, Boomsma CM, van Leenen D, Groot Koerkamp MJ, Goddijn M, Eijkemans MJ, Fauser BC, Holstege FC, Macklon NS. An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep. 2016;6:19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lédée N, Munaut C, Aubert J, Sérazin V, Rahmati M, Chaouat G, Sandra O, Foidart JM. Specific and extensive endometrial deregulation is present before conception in IVF/ICSI repeated implantation failures (IF) or recurrent miscarriages. J Pathol. 2011;225(4):554–564. [DOI] [PubMed] [Google Scholar]
- 25.Altmäe S, Tamm-Rosenstein K, Esteban FJ, Simm J, Kolberg L, Peterson H, Metsis M, Haldre K, Horcajadas JA, Salumets A, Stavreus-Evers A. Endometrial transcriptome analysis indicates superiority of natural over artificial cycles in recurrent implantation failure patients undergoing frozen embryo transfer. Reprod Biomed Online. 2016:32(6):597–613. [DOI] [PubMed] [Google Scholar]
- 26.Binder G, Wittekindt N, Ranke MB. Noonan syndrome: genetics and responsiveness to growth hormone therapy. Horm Res Paediatr. 2007;67(Suppl 1):45–49. [Google Scholar]
- 27.Altmäe S, Reimand J, Hovatta O, Zhang P, Kere J, Laisk T, Saare M, Peters M, Vilo J, Stavreus-Evers A, Salumets A. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol. 2012;26(1):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aghajanova L, Altmäe S, Bjuresten K, Hovatta O, Landgren BM, Stavreus-Evers A. Disturbances in the LIF pathway in the endometrium among women with unexplained infertility. Fertil Steril. 2009;91(6):2602–2610. [DOI] [PubMed] [Google Scholar]
- 29.Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci USA. 2005;102(24):8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221–228. [DOI] [PubMed] [Google Scholar]
- 31.Momeni M, Rahbar MH, Kovanci E. A meta-analysis of the relationship between endometrial thickness and outcome of in vitro fertilization cycles. J Hum Reprod Sci. 2011;4(3):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sbracia M, Scarpellini F, Poverini R, Alò PL, Rossi G, Di Tondo U. Immunohistochemical localization of the growth hormone in human endometrium and decidua. Am J Reprod Immunol. 2004;51(2):112–116. [DOI] [PubMed] [Google Scholar]
- 33.Gunin AG. Influence of growth hormone on the uterine response to oestradiol in rats. J Reprod Fertil. 1997;110(2):299–306. [DOI] [PubMed] [Google Scholar]
- 34.Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–1377. [DOI] [PubMed] [Google Scholar]
- 35.Blumenfeld Z, Amit T. The role of growth hormone (GH), GH-receptor and GH-binding protein in reproduction and ovulation induction. J Pediatr Endocrinol Metab. 1996;9(2):145–162. [PubMed] [Google Scholar]
- 36.Blumenfeld Z, Barkey RJ, Youdim MB, Brandes JM, Amit T. Growth hormone (GH)-binding protein regulation by estrogen, progesterone, and gonadotropins in human: the effect of ovulation induction with menopausal gonadotropins, GH, and gestation. J Clin Endocrinol Metab. 1992;75(5):1242–1249. [DOI] [PubMed] [Google Scholar]
- 37.Blumenfeld Z, Amit T, Barkey RJ, Lunenfeld B, Brandes JM. Synergistic effect of growth hormone and gonadotropins in achieving conception in “clonidine-negative” patients with unexplained infertility. Ann N Y Acad Sci. 1991;626:250–265. [DOI] [PubMed] [Google Scholar]
- 38.Blumenfeld Z, Dirnfeld M, Gonen Y, Abramovici H. Growth hormone co-treatment for ovulation induction may enhance conception in the co-treatment and succeeding cycles, in clonidine negative but not clonidine positive patients. Hum Reprod. 1994;9(2):209–213. [DOI] [PubMed] [Google Scholar]