Abstract
In mouse ovaries, liver receptor homolog-1 [nuclear receptor subfamily 5, group A, member 2 (Nr5a2)] expression is restricted to granulosa cells. Mice with Nr5a2 depletion in this cell population fail to ovulate. To determine whether Nr5a2 is essential for granulosa cell proliferation during follicular maturation, we generated granulosa-specific conditional knockout mice (genotype Nr5a2 floxed Cre-recombinase driven by the anti-Müllerian type II receptor, hereafter cKO) with Nr5a2 depletion from primary follicles forward. Proliferation in cKO granulosa cells was substantially reduced relative to control (CON) counterparts, as assessed by bromodeoxyuridine incorporation, proliferative cell nuclear antigen expression, and fluorescent-activated cell sorting. Microarray analysis revealed >2000 differentially regulated transcripts between cKO and CON granulosa cells. Major gene ontology pathways disrupted were proliferation, steroid biosynthesis, female gamete formation, and ovulatory cycle. Transcripts for key cell-cycle genes, including Ccnd1, Ccnd2, Ccne1, Ccne2, E2f1, and E2f2, were in reduced abundance. Transcripts from other cell-cycle-related factors, including Cdh2, Plagl1, Cdkn1a, Prkar2b, Gstm1, Cdk7, and Pts, were overexpressed. Although the follicle-stimulating hormone and estrogen receptors were overexpressed in the cKO animals, in vivo treatment with estradiol-17β failed to rescue decreased proliferation. In vitro inactivation of Nr5a2 using the ML180 reverse agonist similarly decreased cell-cycle-related gene transcripts and downstream targets, as in cKO mice. Pharmacological inhibition of β-catenin, an Nr5a2 cofactor, decreased cyclin gene transcripts and downstream targets. Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling immunofluorescence and quantitative polymerase chain reaction of pro/antiapoptotic and autophagic markers showed no differences between cKO and CON granulosa cells. Thus, Nr5a2 is essential for granulosa cell proliferation, but its depletion does not alter the frequency of apoptosis nor autophagy.
Keywords: conditional knock-out mice, granulosa cells, Nr5a2, ovary, proliferation
The nuclear receptor, Nr5a2, regulates gonadotropin-induced proliferation in mouse granulosa cells by regulating the expression of cell-cycle kinases and their inhibitors.
In mammals, the process of follicle development serves to provide the structure and mechanisms to bring about maturation of the ovum and its subsequent expulsion during ovulation [1]. It begins before birth with the formation of the primordial follicles. In its earliest postnatal stage, the primordial follicle is surrounded by one dozen or so granulosa cells [2]. Its activation, by processes not yet completely understood, initiates a long developmental process in which most follicles that are activated are lost to atresia. In those that survive to the preovulatory stage, the granulosa cells undergo multiple rounds of replication with a consequent follicular population of 4.0 × 107 cells in the bovine model [2]. Associated with granulosa cell replication is the formation of the follicular antrum, beginning following ~10 rounds of proliferation [2]. Gene-deletion studies in mice have shown that regulation of proliferation is stage dependent, and in primordial and primary follicles, paracrine stimuli from the oocyte dominate, whereas growth factors are important to preantral development [3]. Antrum formation coincides with the acquisition of receptors for the follicle-stimulating hormone (FSHr), and the downstream synthesis of estrogens serves as a formidable, proliferative stimulus to granulosa cells in antral follicles [4, 5].
Recent studies have demonstrated that in addition to estrogens, signals from orphan nuclear receptors play a role in granulosa proliferation and thus, follicle growth. Those in the nuclear receptor subfamily 5, group A, member 2 (Nr5a) family—steroidogenic factor 1 (Nr5a1) [6] and liver receptor homolog 1 (Nr5a2) [7]—are expressed in the ovary. Nr5a2 and Nr5a1 are closely related, classic zinc finger transcription factors that are believed to interact with the same or similar DNA sequences [8, 9]. Whereas Nr5a1 is expressed in both follicular theca and granulosa cells, Nr5a2 is restricted to granulosa cells of primary and all subsequent follicles in the ovary [8]. Its expression in human ovarian follicles, as measured by transcript abundance, increases as follicles proceed through the developmental trajectory [10]. Conditional ablation studies of these orphan nuclear receptors have shown that both are essential for successful development of the mouse follicle and consequent ovulation [6, 7]. Both are known play important roles as regulators of cell proliferation in tumor tissues. Nr5a1 supports proliferation of prostate cancer cells indirectly, by promoting the steroid hormone synthesis that stimulates replication [11]. Nr5a2, on the other hand, is a mitogen, in that it has been shown to induce proliferation directly in cancer cells from the liver [12], mammary gland [13], colon [14], and bone [15]. Its effects on proliferation in vivo in granulosa cells, as well as the extent to which conditional depletion of Nr5a2 in these cells contributes to infertility in mouse models, have been little explored.
Nr5a2 is a constitutively active transcription factor reported to be a direct regulator of proliferation in intestinal crypt cells, by acting to promote transition from the G0/G1 phase to the S phase of the cell cycle [16]. In that study, it was shown that it directly promotes expression of cell-cycle genes, cyclin D1 and E1. It was further concluded that the intracellular signaling molecule, β-catenin, is a potent coactivator of Nr5a2 in the induction of proliferation [16]. The synergistic effect of these two factors has been extended to include several cancer cell lines [17], but there is no current evidence for this interaction in vivo or in ovarian cells.
The objectives of the current study were to explore the role of Nr5a2 in ovarian function, with focus on its contribution to granulosa cell proliferation. We used a mutant mouse model, where Nr5a2 has been depleted from the granulosa cells of follicles at all stages, from the primary follicle forward.
1. Materials and Methods
A. Animals and Colony Maintenance
Animal experiments were approved by the University of Montreal Animal Care Committee and were conducted according to the guidelines of the Canadian Council on Animal Care. All mutant and control (CON) mice were maintained on the C57BL/6 background, under a 14-hour light, 10-hour dark cycle and provided food and water ad libitum. Euthanasia was performed with isofluorane anesthesia, followed by cervical dislocation, as previously described [18]. Nr5a2 floxed (Nr5a2f/f) mice have been described previously [7, 18]. Granulosa-specific depletion of Nr5a2 from primary follicle forward was generated by crossing these animals with mice expressing Cre-recombinase driven by the anti-Müllerian type II receptor (Amhr2Cre/+) [7, 18] to produce conditional knockout (cKO) mice (genotype Nr5a2f/fAmhr2Cre/+). Following DNA extraction from tails, littermates were genotyped. CON mice in these trials were nonmutant, Nr5a2f/fAmhr2Cre/− females [19]. Wild-type mice were used as controls only in experiments where granulosa cells were isolated and treated with the Nr5a2 inverse agonist ML180 (Cayman Chemical, Ann Arbor, MI).
B. Superstimulation Protocol
Superstimulation [18] was achieved in 22- to 25-day-old mice by intraperitoneal injection of 5 IU equine chorionic gonadotropin (eCG; Folligon; Intervet, Kirkland, QC, Canada) to stimulate follicular development. Animals were euthanized 44 to 48 hours later. Ovaries were collected, weighed, fixed in paraformaldehyde or formalin (Sigma-Aldrich, Oakville, ON, Canada), and embedded in paraffin. In other trials, granulosa cells were isolated by ovarian puncture with 25 g needles in phosphate-buffered saline (PBS) 1× or culture medium and mechanically separated from the oocyte before filtration with a 40-μm BD nylon Falcon Cell Strainer (Becton Dickinson, Mississauga, ON, Canada). As it has previously been shown that Nr5a2 regulates the expression of cytochrome P450 19a1 (Cyp19a1; or aromatase), the rate-limiting enzyme in estrogen synthesis, we addressed the possibility that the effects of Nr5a2 depletion were solely a result of disruption of estrogen synthesis. This was achieved by treatment of CON and cKO mice with a single injection of estradiol-17β, 1 mg/animal, according to our previous protocol [5]. Ovaries were collected for analysis of proliferation (see later).
C. Bromodeoxyuridine (BrdU) Incorporation and Proliferating Cell Nuclear Antigen (PCNA) and Ki-67 Expression
Immature cKO and CON mice were superstimulated with eCG (Folligon; Intervet), as described previously, and injected with BrdU (Sigma-Aldrich), 30 mg/kg, 24 hours before euthanasia. Ovaries were fixed, processed, and sectioned. Slides were then rehydrated and incubated with trypsin (Invitrogen, Burlington, ON, Canada) for 20 minutes at 37°C and 10 minutes at room temperature. DNA was denatured by HCl 1N and 2N for 10 minutes on ice and 10 minutes at room temperature/20 minutes at 37°C, respectively, before being blocked for 1 hour with 5% normal rabbit serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS. Tissues were then incubated overnight with the first antibody sheep polyclonal against BrdU (Abcam, Toronto, ON, Canada), 1:100 in 5% normal rabbit serum overnight. The following day, slides were incubated with the second antibody rabbit polyclonal-to-sheep immunoglobulin G (IgG)-heavy and light chain (fluorescein isothiocyanate; Abcam) in PBS, 1× 1:400 for 1 hour, and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), 1:1000 in PBS 1× for 5 minutes. Slides were mounted with Permafluor (Thermo Fisher Scientific, Mississauga, ON, Canada). Ovarian distribution of BrdU was observed by Axio Imager M1 (Zeiss Microscopy, Toronto, ON, Canada), and dividing cells were counted using CellProfiler Software (Broad Institute, Cambridge, MA) [4]. Colored pictures were filtered to isolate the channel of interest (blue for DAPI and green for BrdU) and then converted to grayscale. A sample of five follicles per section was filtered individually to obtain an important average, and then the total number of cells in the selected follicles was counted based on shape recognition in the DAPI image, and the number of dividing cells that incorporated BrdU was determined based on intensity measurement in the BrdU image. The ratio of these values gave the percentage of proliferating cells. For PCNA immunofluorescence, the slides followed the same rehydration protocol before being boiled for 30 minutes in sodium citrate for antigen retrieval and blocked for 1 hour with 5% bovine serum albumin (BSA; Jackson ImmunoResearch Laboratories) in PBS at room temperature. We then treated with a rabbit polyclonal antibody against PCNA (SA194; Biomol, Hamburg, Germany): first antibody 1:200 in BSA 5%/PBS at 4°C overnight and with cyanine-3 (Cy3)-conjugated goat anti-rabbit IgG second antibody (111-165-144; Jackson ImmunoResearch Laboratories), diluted 1:400 in PBS 1× for 1 hour at room temperature. Finally, granulosa cells treated for Ki-67 immunofluorescence were fixed with platelet-activating factor 4% for 20 minutes and blocked with 5% BSA/PBS before being treated with the Ki-67 goat polyclonal-conjugated first antibody 1:500 (ab15580; Abcam) and Cy3-conjugated donkey anti-goat IgG second antibody (705-165-003; Jackson ImmunoResearch Laboratories ) 1:400, both for 1 hour at room temperature. In both cases, DAPI was used for counterstaining. A similar paradigm to that used for BrdU was used to measure the signal intensity following immunostaining for PCNA and Ki-67.
D. Global Analysis of Gene Expression by Microarray
Mice were superstimulated as described previously and ovaries collected at 40 hours after eCG treatment. Granulosa cells were isolated from large antral follicles by laser microdissection, as previously described [7] and RNA extracted with RNeasy kits (Qiagen, Toronto, ON, Canada). Samples comprising pooled granulosa cells of large antral follicles from each mouse RNA sample (10 μg; three per genotype) with RNA integrity number > 7 were chosen for microarray analysis. Microarray experiments were performed using the Affymetrix mouse 430-2 chips to profile the gene-expression levels with ∼40,000 unique probes. Each RNA sample was converted to complementary RNA and hybridized on an individual chip, according to the manufacturer’s instructions (Affymetrix, Thermo Fisher Scientific; and http://genomeast.igbmc.fr/). Raw microarray data (.CEL files) were normalized using the Gcrma R-pipeline. Background normalized data were then subjected to bioinformatics analysis using the web application, Network Analyst [20]. Differential expression analysis was performed on quantile-normalized data using the Limma algorithm on the Network Analyst platform. Probe densities (mean probe densities for genes with multiple probe sets) were compared between cKO and CON granulosa cells, and differentially regulated genes were identified with parameters: false discovery rate (FDR) <0.05 and fold change ≥2. The microarray data will be deposited in the National Center for Biotechnology Information Gene Expression Omnibus (http://ncbi.nlm.nih.gov/geo/). Subsequent gene ontology and pathway analyses were conducted using the Panther (http://pantherdb.org/) and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) databases.
E. RNA Extraction and Real-Time PCR
RNA from granulosa cells was extracted with a shredder, followed by an RNeasy Mini Kit (Qiagen), following the manufacturer’s instructions. Reverse transcription was performed using the SuperScript III reverse transcription (Invitrogen). Real-time quantitative polymerase chain reaction (qPCR) was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) with the CFX 96 Real-Time System, C1000 Touch Thermal Cycler (Bio-Rad Laboratories). The transcripts were amplified following the same cycling program: 30 seconds at 95°C and then 40 cycles of 15 seconds at 95°C and 30 seconds at 60°C, followed by 5-second steps of a 0.5°C increase between 65°C and 95°C. Primers used can be found in Supplemental Table 1 (256.7KB, pdf) .
F. Pharmacological Treatments
Granulosa cells from superstimulated immature CON mice were treated at 19 hours after plating with 3 µM ML180. Treatments were dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich), and in both cases, a DMSO-only control group was included. Treatment duration was 6 hours, after which, cells were harvested for analysis of cell-cycle genes by qPCR . To evaluate the effects of ML180 on granulosa cell proliferation, cells from CON mice were treated in vitro every 6 hours for 24 hours before quantifying proliferation by measuring incorporation of the cellular marker, Ki-67. In addition, cKO cells were treated with the inhibitor to β-catenin-responsive transcription (iCRT3; Sigma-Aldrich) at 20 µM, respectively, for 6 hours and compared with DMSO-treated controls.
G. Cleaved Caspase-3 Immunofluorescence
Slides of formalin-fixed, paraffin-embedded ovaries from superstimulated immature mice were treated, as previously described [18]; blocked 1 hour with 5% normal goat serum (Jackson ImmunoResearch Laboratories); incubated with cleaved caspase-3 antibody (Cell Signaling Technology, Danvers, MA) as the first antibody 1:150 in 5% normal goat serum overnight, with Cy3-conjugated goat anti-rabbit IgG second antibody (Jackson ImmunoResearch Laboratories), diluted 1:400 in PBS 1× for 1 hour at room temperature; and counterstained with DAPI, diluted 1:1000 in PBS 1× for 5 minutes before being mounted with Permafluor (Thermo Fisher Scientific). The same CellProfiler Software pipeline used for BrdU was used to count the number of atretic follicles and apoptotic cells marked with cleaved caspase-3 in ovaries of CON and cKO mice.
H. Terminal Deoxynucleotidyltransferase-Mediated Deoxyuridine Triphosphate Nick End Labeling Evaluation of Apoptosis
The in situ Apoptosis Detection Kit (ab206386; Abcam) that allows the recognition of apoptotic nuclei was used, according to the manufacturer’s instructions on slides from the same blocks as used for caspase-3 immunofluorescence. In summary, samples were rehydrated and permeabilized using Proteinase K for 23 minutes; endogenous peroxidases were inactivated with 3% H2O2 over 5 minutes; and samples were labeled with terminal deoxynucleotidyl transferase enzyme for 2 hours, blocked with blocking buffer for 10 minutes, incubated with conjugate for 35 minutes, incubated with diaminobenzidine solution for 20 minutes, and counterstained with Methyl Green for 1 minute, before being dehydrated and mounted with a coverslip.
I. Flow Cytometry
Fluorescence-activated cell sorting (FACS) was performed on granulosa cells isolated by follicle puncture and pooled from both ovaries of superstimulated immature CON and cKO mice using the BD Accuri C6 Cytometer (Becton Dickinson) according to the manufacturer’s instructions. After calibrating the cytometer using beads provided by the manufacturer, the samples were diluted in Krishan buffer to a concentration allowing the machine to count 30,000 events. Results were analyzed using ModFit LT Software (Verity Software House, Topsham, ME).
J. Statistical Analyses
All data were analyzed using JMP (version 9.0; SAS Institute, Cary, NC) statistical software. Differences between mutant and CON mice were determined by Student’s t test. All numerical data are represented as means ± standard error of the mean. A significant difference was recognized at P < 0.05.
2. Results
A. Conditional Depletion of Nr5a2 in Granulosa Cells Decreases Cell Proliferation
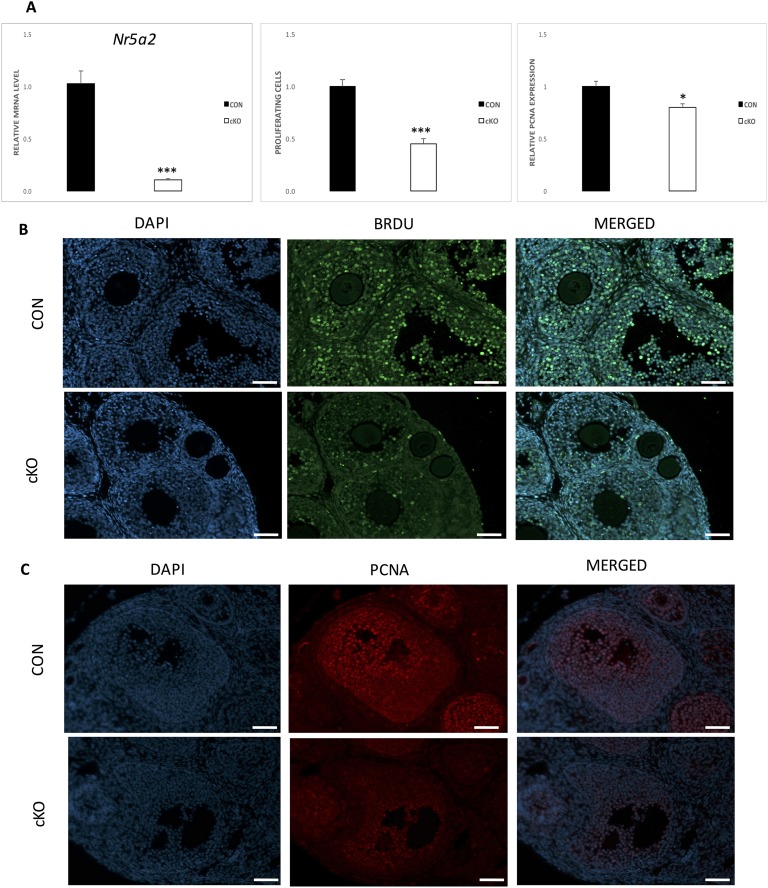
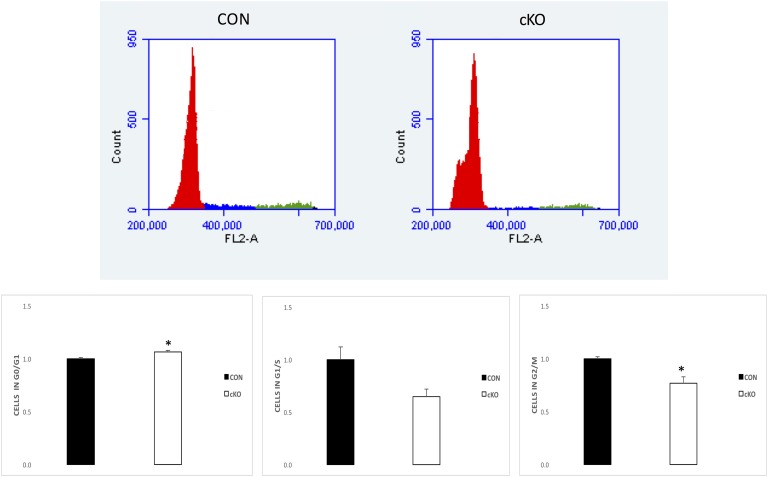
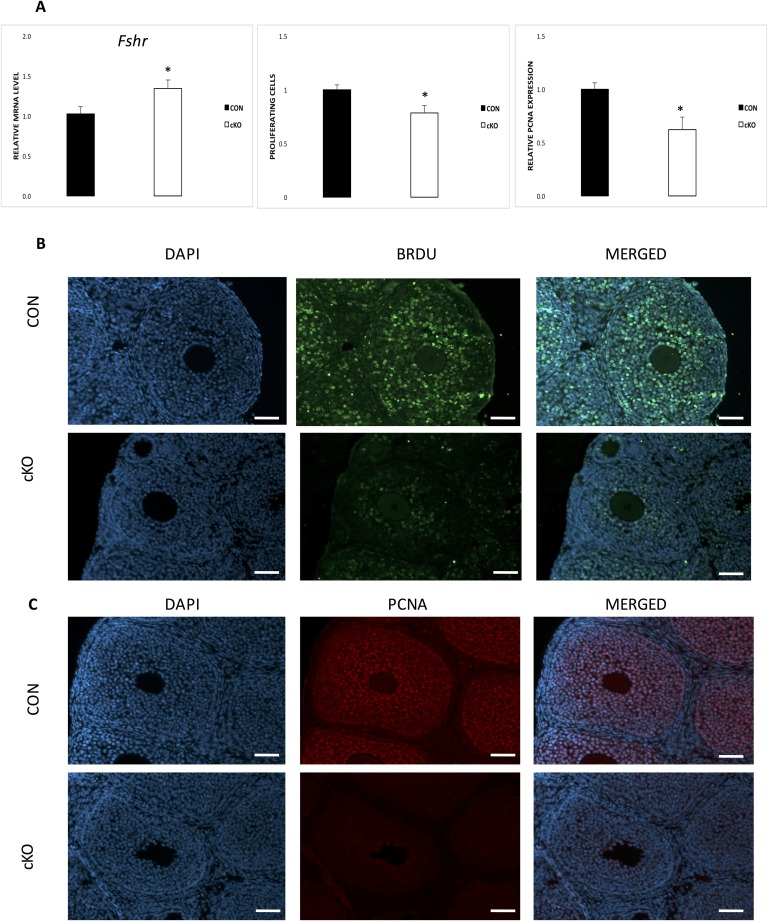
We have previously shown that the cKO mouse model, obtained by crossing Nr5a2f/f mice with Amhr2Cre/+ mice are infertile as a result of multiple factors, including failure to ovulate [7]. Analysis by qPCR of the Nr5a2 transcript from granulosa cells at 44 hours after eCG treatment confirmed depletion of the Nr5a2 gene by >90% in the cKO relative to the CON animals (Fig. 1A). At 24 hours after eCG treatment, there was no difference in mean ovarian mass (CON, 6.3 ± 0.6; cKO, 7.3 ± 1.1 µg), indicating that the cKO ovaries were not hypomorphic. Figure 1B depicts ovarian sections displaying nuclear incorporation of BrdU in cKO and CON mice and Fig. 1C, PCNA. The evident reductions in abundance of incorporation of BrdU and the intensity of the signal for PCNA were quantified (Fig. 1A). Both markers demonstrated that the number of proliferating granulosa cells in cKO mice relative to their CON counterparts was reduced by >50% in BrdU incorporation (Fig. 1A). FACS analysis (Fig. 2) of granulosa cells revealed that there were significantly more cells in the G0/G1 phase of the cell cycle in the cKO mice relative to the CON counterparts but fewer cells in the G1/S and G2/M phases of the cell cycle. Together, these results indicate that proliferation is substantially impaired in granulosa cells in cKO mice relative to their wild-type counterparts.
Figure 1.
Proliferation of mouse granulosa cells is compromised in Nr5a2-depleted follicles. (A, left) Amhr2Cre depletion of Nr5a2 transcripts in granulosa cells from mouse antral follicles (n = 5). (A, middle and right) BrdU and PCNA expression in CON and cKO granulosa cells at 44 h after gonadotropin treatment, as evaluated by quantitative image analysis. Data are means ± standard error (SE); *P < 0.05, ***P < 0.001. (B) Representative images showing the expression of BrdU in CON and cKO ovaries at 44 hours after gonadotropin stimulation in vivo (n = 10/n = 6). (C) Representative images for PCNA at 44 hours after gonadotropin stimulation (n = 4). In this and subsequent figures, CON signifies control animals, cKO the granulosa-specific mouse knockout line (genotype Nr5a2f/fAmhr2Cre/+). Original scale bars, 50 µM.
Figure 2.
FACS of granulosa cells from gonadotropin-stimulated CON and cKO mice. (Upper) Representative scan data. (Lower) Means ± SE of cell numbers in G0/G1 (red), G1/S (blue), and G2/M (green) phases of the cell cycle (n = 3). *P < 0.05. FL-2, fluorescence 2.
B. Substantial Modification of the Granulosa Cell Transcriptome Accompanies Nr5a2 Depletion
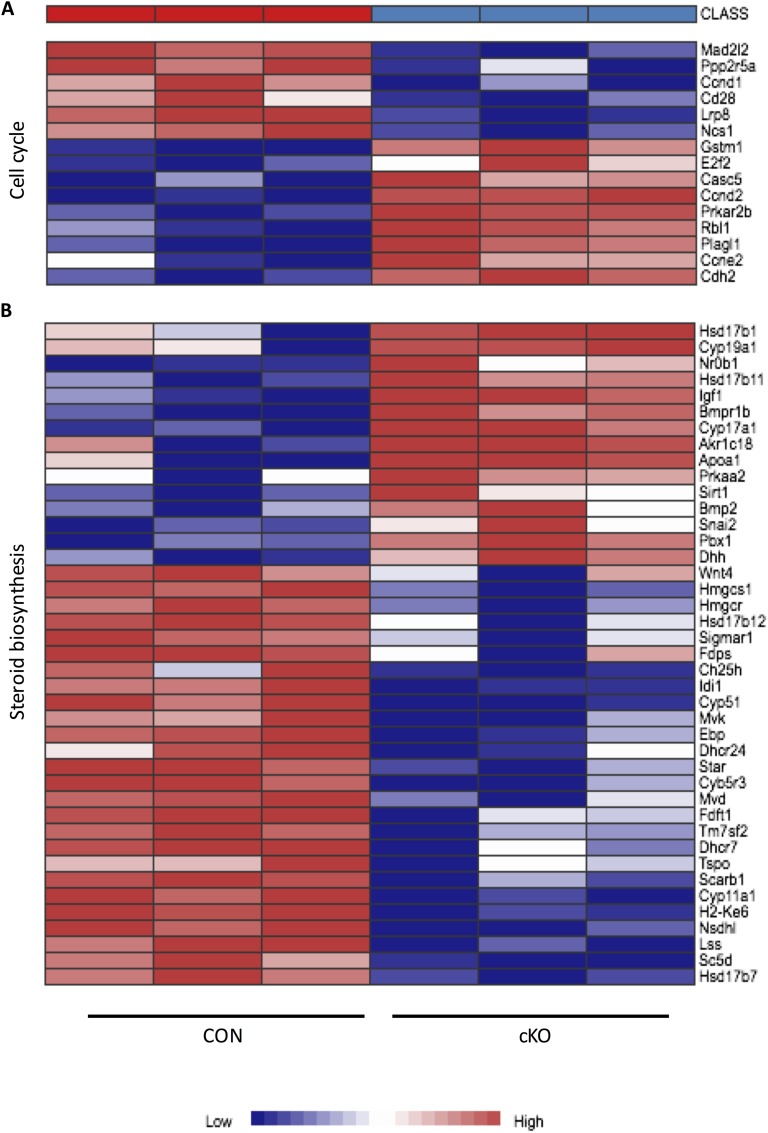
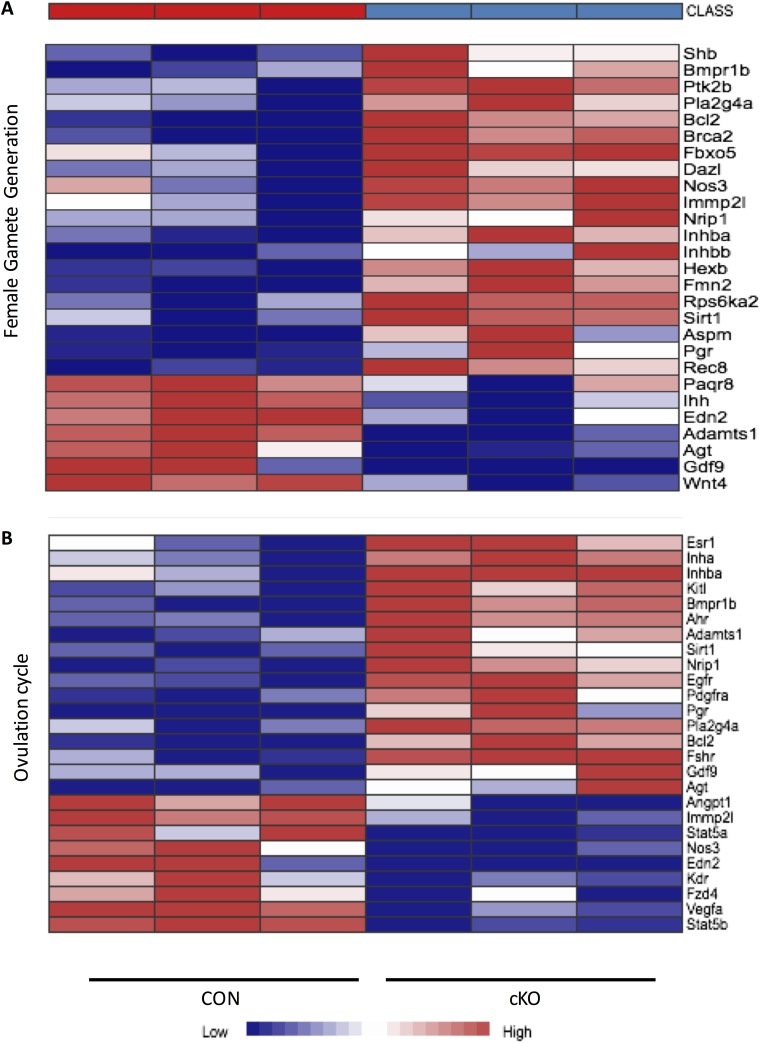
Microarray data analysis using the Network Analyst platform yielded 2136 differentially regulated genes at the statistical parameters of FDR = 0.05 and a twofold or greater change between cKO and CON mice at 48 hours post-eCG. Of these, 1089 genes were upregulated, and 1047 genes were downregulated. Lists of the 100 genes with the greatest definable variation relative to CON granulosa cells are presented in Supplemental Tables 2 (256.7KB, pdf) (upregulated genes) and 3 (256.7KB, pdf) (downregulated genes). Tables 1 and 2 represent gene ontology analysis by Panther and KEGG, respectively, demonstrating pathways and gene clusters that differed substantially between the cKO and CON samples. Pathway analysis revealed, as expected, that proliferation and mitotic cell-cycle processes were major cellular functions disrupted by Nr5a2 depletion, as shown in the heat map in Fig. 3A. We had previously demonstrated, using this [7] and other depletion paradigms [18, 21], that genes associated with steroidogenesis are, with few exceptions, reduced, as reflected in the heat map (Fig. 3B). The gene clusters associated with the ovulation cycle and female gamete generation, not surprisingly, showed inappropriate up- and downregulation of multiple genes (Fig. 4A and 4B).
Table 1.
Top Nine Gene Ontology Pathways (GO: Biological Processes)
| Pathway | Total | Expected | Hits | P Value | FDR |
|---|---|---|---|---|---|
| Cell division | 509 | 54.6 | 95 | 3.83E-08 | 3.14E-05 |
| Mitotic cell cycle | 683 | 73.2 | 118 | 7.90E-08 | 3.24E-05 |
| Regulation of lipid metabolic process | 232 | 24.9 | 52 | 1.67E-07 | 4.57E-05 |
| Steroid metabolic process | 342 | 36.7 | 68 | 3.13E-07 | 5.47E-05 |
| Steroid biosynthetic process | 164 | 17.6 | 40 | 4.39E-07 | 5.47E-05 |
| Mitosis | 366 | 39.2 | 71 | 4.60E-07 | 5.47E-05 |
| Lipid biosynthetic process | 587 | 62.9 | 102 | 4.67E-07 | 5.47E-05 |
| Female gamete generation | 94 | 10.1 | 27 | 1.13E-06 | 0.000108 |
| M phase of mitotic cell cycle | 382 | 40.9 | 72 | 1.18E-06 | 0.000108 |
Top nine gene ontology pathways enriched among all differentially regulated genes in the granulosa cells of mice with depletion of Nr5a2 in granulosa cells (cKO mice) relative to CON mice, as revealed by microarray and Panther bioinformatic analyses.
Table 2.
Top 10 KEGG Pathways
| Pathway | Total | Expected | Hits | P Value | FDR |
|---|---|---|---|---|---|
| Cell cycle | 127 | 14.1 | 33 | 1.81E-06 | 0.000386 |
| Prostate cancer | 87 | 9.68 | 24 | 1.59E-05 | 0.00141 |
| Pathways in cancer | 306 | 34.1 | 58 | 2.24E-05 | 0.00141 |
| Steroid biosynthesis | 17 | 1.89 | 9 | 2.64E-05 | 0.00141 |
| HTLV-I infection | 223 | 24.8 | 45 | 4.03E-05 | 0.00152 |
| Fat digestion and absorption | 8 | 0.891 | 6 | 4.27E-05 | 0.00152 |
| Pancreatic cancer | 70 | 7.79 | 19 | 0.000153 | 0.00467 |
| p53 signaling pathway | 69 | 7.68 | 18 | 0.000389 | 0.00981 |
| Progesterone-mediated oocyte maturation | 81 | 9.02 | 20 | 0.000415 | 0.00981 |
| Focal adhesion | 199 | 22.2 | 38 | 0.000516 | 0.011 |
Top 10 KEGG pathways enriched among all differentially regulated genes in the granulosa cells of mice with depletion of Nr5a2 in granulosa cells (cKO mice) relative to CON mice, as revealed by microarray and bioinformatic analysis, as enriched among all differentially regulated genes.
Abbreviation: HTLV-I, human T-lymphotropic virus type 1.
Figure 3.
Heat map derived from gene ontology analysis of microarray data from CON and cKO mice following gonadotropin stimulation in vivo. Represented are two of the top nine enriched pathways for (A) cell-cycle and (B) steroidogenesis-related genes, demonstrating twofold or greater variation in expression in granulosa cells. As noted in the color bar, genes in the cKO column are underexpressed relative to CON values, whereas genes in red are overexpressed. Probability cutoff was P < 0.05.
Figure 4.
Heat map derived from gene ontology analysis of microarray data from CON and cKO mice following gonadotropin stimulation in vivo. Represented are two of the top 10 enriched pathways, in this case for (A) female gamete generation and (B) ovulation cycle.
C. Differential Regulation of Cell-Cycle Genes and Downstream Targets in Granulosa Cells Depleted of Nr5a2
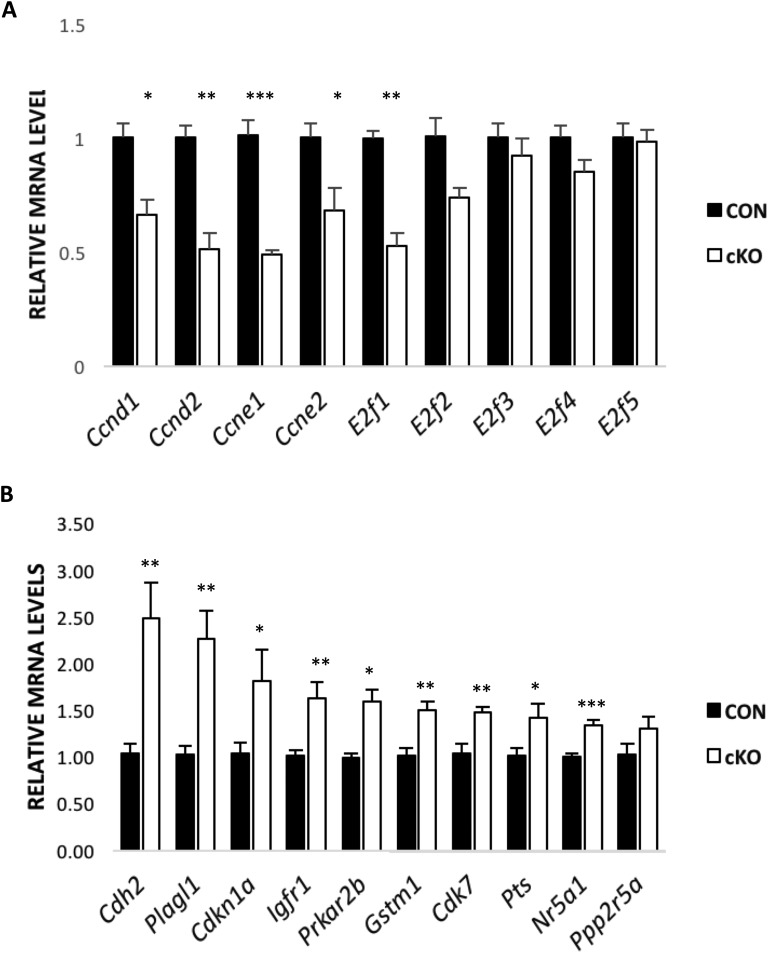
By real-time PCR, it was found that the abundance of transcripts of numerous genes involved in the cell cycle, including cyclins D1 and E1, is significantly decreased in granulosa cells depleted of Nr5a2 (Fig. 5A). The same effects were observed on their paralogs, cyclins D2 and E2. It is known that downstream targets of these cyclins play key roles in execution of the S phase by acting on the transcription of genes responsible for DNA replication [22]. These include the E2f activators, E2f1 and E2f2, which are also downregulated in granulosa cells from cKO mice (Fig. 5A). Cyclin-dependent kinases associated with cyclins D and E; Cdk4, Cdk6, and Cdk2, respectively; and transcripts for the E2f repressors, E2f4 and E2f5, did not show any substantial difference in their abundance between the cKO and CON animals. PCR validation of the global transcriptome also demonstrated that the number of genes associated with the cell cycle was upregulated in the granulosa cells of cKO animals relative to the CON counterparts, including Cdh2, Plagl1, Igfr1, Prkar2b, Gstm1, Cd7, Cdk7, and Pts (Fig. 5B). We further examined two cell-cycle inhibitors, Cdkn1a (p21) and Cdkn1b, and discovered the former to be substantially upregulated, whereas the latter showed no difference between CON and cKO granulosa cells following gonadotropin stimulation.
Figure 5.
Real-time PCR analysis of differentially expressed genes in mouse granulosa cells harvested following gonadotropin stimulation. Data are expressed as means ± SE for genes (A) underexpressed in cKO mice relative to CON and (B) overexpressed in cKO mice relative to CON mice (n = 5 to 9 animals/group). *P < 0.05, **P < 0.01, ***P < 0.001.
D. Nr5a2 Depletion Affects Gonadotropin Receptor Transcripts, and Estrogen Cannot Rescue Proliferation
Another transcript that was substantially upregulated in the microarray analysis was the FSHr, whereas the receptor for luteinizing hormone (Lhcgr) was less affected (Fig. 6A). In this model, in particular, there is overexpression of Cyp19a1 [7], a target of FSH and the rate-limiting enzyme in estrogen production. Furthermore, we have shown that estradiol-17β concentrations are more than double in the follicles of cKO mice following superstimulation [7]. Estrogens are potent mitogens for granulosa cells [5], and microarray analysis indicated that both estrogen receptors, Esr1 and Esr2, are overexpressed by approximately threefold in cKO granulosa cells. Therefore, we treated 24-day-old immature CON and cKO mice with estradiol-17β, followed by euthanasia, 24 hours later, according to our earlier protocol [5]. Ovarian sections were probed for BrdU and PCNA expression by quantification of immunofluorescence to establish whether the reduction in proliferation in cKO mouse granulosa cells could be reversed by estrogen treatment. The results (Fig. 6A and 6B) indicate that in spite of treatment with pharmacological doses of estrogen, the rate of proliferation remained well below that induced in the CON ovaries.
Figure 6.
FSHr is elevated in cKO granulosa cells, and exogenous estrogen cannot rescue proliferation relative it CON animals. (A, left) Abundance of FSHr transcripts in CON and cKO mice following gonadotropin stimulation (n = 5). (A, middle) The number of proliferating cells following 1 mg estrogen administration in vivo, as determined by quantitative imaging of BrdU expression. (A, right) The quantitative analysis of PCNA expression between granulosa cells from CON and cKO animals. Data are expressed as means ± SE of n = 4 animals/group. *P < 0.05. (B) Representative photomicrographs of BrdU incorporation in ovaries from estrogen-treated CON and cKO mice. (C) Representative photomicrographs showing PCNA expression in the ovaries of estrogen-treated CON and cKO mice. Original scale bars, 50 µM.
E. Pharmacological Inhibition of Nr5a2 and Its Cofactor β-Catenin Interferes With In Vitro Expression of Cell-Cycle Genes in Granulosa Cells of CON and cKO Mice
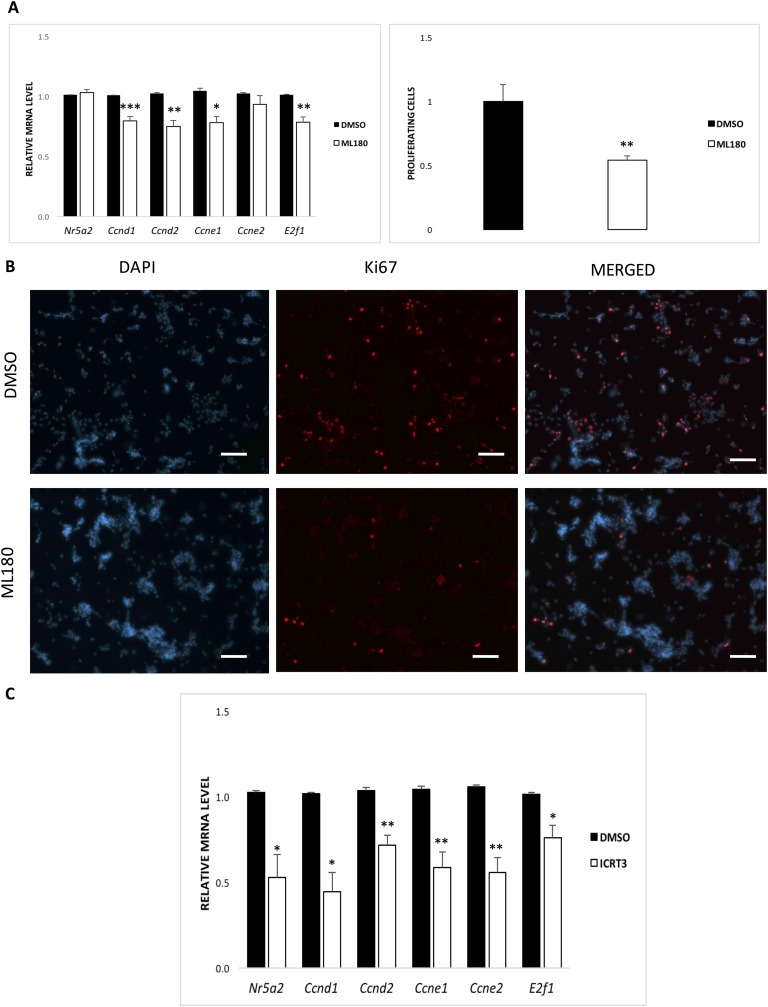
Incubation of CON granulosa cells with the inverse Nr5a2 agonist, ML180, for 6 hours caused no net difference in the abundance of Nr5a2 transcripts relative to DMSO controls. There was a demonstrable reduction in transcripts for Ccnd1, Ccnd2, Ccne1, and E2f1, further recapitulating the reduction in these genes in cKO granulosa cells at 6 hours of treatment (Fig. 7A). At 24 hours of treatment, proliferation was suppressed by one-half (Fig. 7A and 7B), consistent with in vivo results.
Figure 7.
(A) Effects of the Nr5a2 reverse agonist, ML180. (A, left) Effects on genes associated with cell proliferation. (A, right) Mean reduction in proliferation, as indicated by the reduced expression of the marker Ki-67 by quantitative image analysis. (B) Representative image demonstrating reduction in Ki-67 expression. Original scale bars, 100 μM. (C) Effects of the β-catenin activity inhibitor iCRT3 on cell-cycle genes in granulosa cells in vitro harvested from gonadotropin-stimulated cKO mice. Means ± SE from cultures of granulosa cells from five mice per group. *P < 0.05, **P < 0.01, ***P < 0.001.
Nr5a2 is a constitutively active transcription factor, and previous studies have shown that β-catenin functions as its coactivator [16]. To explore its potential role in the ovary, we treated cKO granulosa cell cultures with the oxazole compound iCRT3, which interferes with β-catenin action, by inhibition of its translocation to the nucleus and its interaction with the transcription factor, Tcf4 [23]. Tcf4, as measured by microarray, was twofold greater in cKO granulosa cells, indicating no impairment of this element of the β-catenin signaling mechanism (data not shown). Nonetheless, analysis at 6 hours indicated that there was depletion of Nr5a2; its targets, the cyclins D and E; and other downstream genes, such as the E2fs activator, E2f1 (Fig. 7C).
F. Nr5a2 Depletion Does Not Affect Apoptosis or Autophagy in Ovarian Granulosa Cells
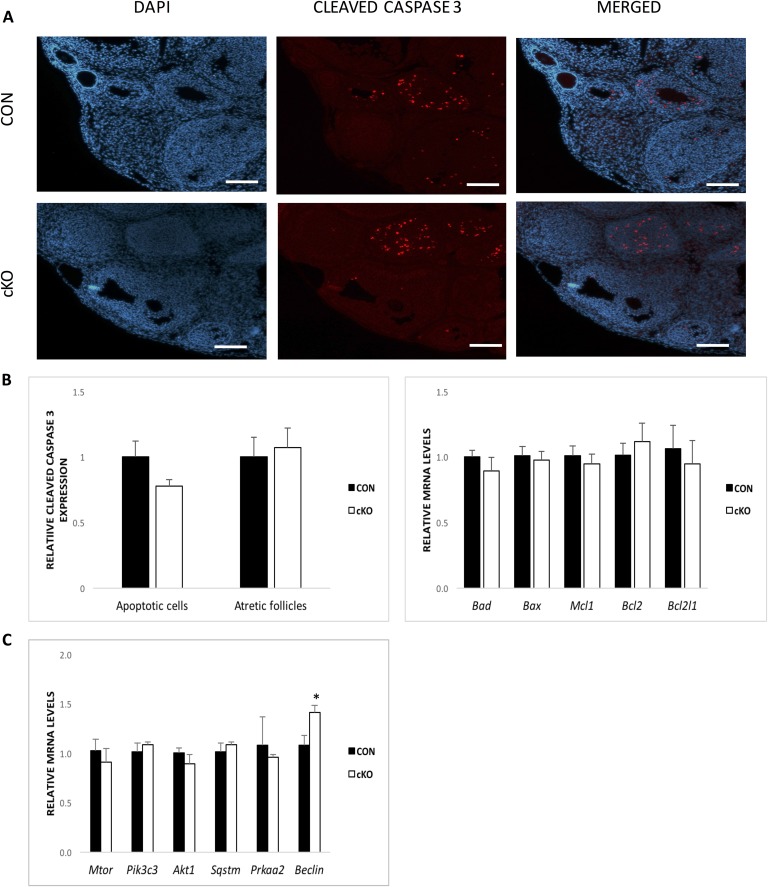
We next determined whether the reduced proliferation in cKO mouse granulosa cells in vivo could be attributed to programmed cell death or autophagy. In this context, cleaved caspase 3 immunofluorescence results were evaluated using CellProfiler software (Fig. 8A and 8B). There was no difference in the number of atretic follicles nor in the number of atretic cells in these follicles between the CON and the cKO mice (n = 5/genotype). Terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling analysis (Supplemental Fig. 1 (256.7KB, pdf) ) confirmed this observation. Evaluation of the pattern of gene expression of antiapoptotic Bcl2, Bcl2l1, and Mcl1 and proapoptotic Bax and Bad (n = 5/genotype) further indicated that there is no difference in patterns of programmed cell death genes (Fig. 8B). Although it is known that granulosa cell depletion can also be the result of autophagy [24], qPCR analysis indicated no difference in transcript abundance for a panel of genes associated with autophagy and signaling of autophagy beyond a moderate increase in the abundance of Beclin (Fig. 8C). The results indicate that Nr5a2 depletion does not increase the frequency of autophagy.
Figure 8.
Depletion of Nr5a2 does not increase apoptosis or autophagy in mouse granulosa cells. (A) Immunolocalization of cleaved caspase-3, a marker of apoptosis in the ovaries of gonadotropin-stimulated CON and cKO mice. Original scale bars, 200 µM. (B, left) Counts of the total number of follicles with apoptotic nuclei by cleaved caspase-3 immunofluorescence analysis from ovaries of CON and cKO mice. (B, right) Means ± SE of apoptotic marker genes. (C) Means ± SE of the effects of Nr5a2 depletion in vivo on expression of autophagy marker genes in gonadotropin-stimulated mice (n = 5/group). *P < 0.05.
3. Discussion
We have previously shown that depletion of Nr5a2 from mouse granulosa cells, either before [7, 18] or after the ovulatory gonadotropin signal [21], results in infertility. Short-term depletion of the Nr5a2 transcript by short hairpin RNA results in infertility with the same anovulatory phenotype [25]. Herein, we provide clear evidence that when depletion is initiated from the primary follicle forward, gonadotropin-induced proliferation of the granulosa cell population in antral follicles is compromised. The role of Nr5a2 in proliferation has been studied primarily in embryonic tissue and cancer cells, in vitro. Although germline deletion of Nr5a2 is lethal during early embryogenesis, proliferation of the embryo to the gastrulation stage occurs [26], indicating that proliferation can occur in the absence of this nuclear receptor. Likewise, suppression of Nr5a2 in colon cancer [27] or osteosarcoma cells [15] inhibited, but did not eliminate, proliferation, consistent with the present findings.
In concordance with studies of Nr5a2 depletion in cancer cell lines [13, 27], FACS analysis revealed that a greater proportion of the granulosa cells was in the G0/G1 phase, whereas there were fewer in both the S and G2/M phases. The decrease we report in granulosa cells following Nr5a2 depletion is of lower magnitude than that seen in cancer cells (7% vs 12% to 15%), whereas the abundance of the cell population in the G2/M phase was greater by 23% in cKO mice vs 10% to 18% in cancer cell lines [13, 27]. Thus, these in vivo results of disruption of the cell cycle by Nr5a2 depletion indicate the ubiquity of the role of Nr5a2 in proliferation.
We used microarray and gene abundance evaluation in search of the potential explanations for the reduced proliferation in granulosa cells. The number of dysregulated transcripts (>2000) far exceeded the number (637) observed in the ovary in a study using an 80% short hairpin RNA depletion in vivo in a similar model at 40 hours after eCG stimulation [25]. The present results further show that depletion of Nr5a2 reduced abundance of cell-cycle transcripts, such as Ccnd1 and Ccne1, both in vivo and in vitro, consistent with results of depletion studies in cancer cell lines [27]. Another remarkable similarity between our in vivo data and cancer cell line results is the increase in expression of Cdkn1a, or p21, the cell-cycle inhibitor that was identified by both microarray and PCR validation. Suppression of expression of this gene has previously been implicated as a major mechanism by which Nr5a2 influences proliferation in breast [13] and colon [14] cancer cell lines. In addition, Cdkn1a has been shown by chromatin immunoprecipitation assays to be a direct target of Nr5a2 in breast cancer cells [13]. Promoter assays revealed that Nr5a2 recruitment to the Cdkn1a promoter region in colon cancer cell lines activates transcription [14]. Male mice with germline deletions of Cdkn1a are fertile but displayed a 48% increase in the number of Sertoli cells, the male analog of the granulosa cell [28]. Global deletion of Cdkn1a appears to have little effect on mouse granulosa cell proliferation in the periovulatory follicle after the ovulatory signal, whereas double knockout of Cdkn1a and Cdkn1b dramatically increases proliferation in this context [29]. Based on our findings and the information in the literature, we propose that the failure of Cdkn1a downregulation in the absence of Nr5a2 is the principal mediator of the abrogation of gonadotropin-induced granulosa cell proliferation in the current study.
Follicle progression to the antral stages occurs in this cKO model [7], an event that could not occur without granulosa cell proliferation [2]. It is well known that the early phases of follicle development are gonadotropin independent, driven primarily by elements of the transforming growth factor-β family [3]. The experimental paradigm of the current study was the comparison of granulosa cell proliferation in cKO and CON mice following treatment with eCG, a potent stimulator of follicle development from the early antral stage forward. As noted previously, our data demonstrate that the gonadotropin induction of proliferation is substantially impaired by depletion of Nr5a2. Both the luteinizing hormone receptor and FSHr are present in the granulosa cells of the cKO mouse, and the latter is overexpressed.
Insulin-like growth factors (IGFs) act in synergy with FSH in induction of antral follicle formation, and deletion of Igfr1 from granulosa cells abrogates this process completely [30]. In the current study, the abundance of both FSHr and Igf1r transcripts was elevated in cKO granulosa cells relative to the CON model. In ruminant follicles, FSH induces the expression of Igfr1 [31, 32]. It is therefore possible that superstimulation by eCG, in the presence of elevated FSHr, is responsible for the observed increase Igfr1 transcript abundance.
One of the principal roles of FSH in the ovarian follicle is the induction of Cyp19a1 and consequent production of estradiol [33]. Neither of these events appears impaired in this study, indeed, in microarray data, and our previous reports [7] indicate that Cyp19a1 is overexpressed. Not only are both isoforms of the estrogen receptor (Esr1 and Esr2) overexpressed, but also, intrafollicular concentrations of estradiol are more than twofold greater in this cKO mouse model [7]. Furthermore, proliferation could not be rescued by injection of cKO mice with pharmacological doses of estradiol, a treatment that we previously showed to induce granulosa cell mitosis in vivo [5]. The estrogen signaling pathway appears intact, as microarray data show a robust expression of its iconic target mediator of proliferation, growth regulation by estrogen in breast cancer 1 [34]. We conclude that the mitogenic actions of Nr5a2 and estrogen are achieved by separate mechanisms.
The canonical intracellular signal in the Wnt/β-catenin pathway, β-catenin, has been shown to bind stably and interact with Nr5a2 via the ligand-binding pocket of the latter [35]. This interaction has important consequences, in that induction of cyclin-mediated proliferation of pancreatic and hepatic cell lines by Nr5a2 is potentiated by β-catenin [16]. The β-catenin signal also functions as a Nr5a2 coactivator in the ovary [36]. Thus, it was no surprise that in the current study, inhibition of nuclear β-catenin activity in cKO granulosa cells drastically depleted the abundance of transcripts for the same cell-cycle genes that were affected by Nr5a2 depletion alone. The β-catenin inhibitor we used, iCRT3, abrogates activation of transcription via Tcf4. The inhibitor, iCRT3, has been shown to have modest effects on other signaling pathways, including signal transducer and activator of transcription and Notch [23]. Thus, the reduction in transcription of Nr5a2 engendered by this treatment indicates that Nr5a2 may be downstream of β-catenin.
4. Conclusion
Herein, we provide evidence that Nr5a2 is a critical regulator of proliferation in ovarian granulosa cells. Follicle development, driven by granulosa cell proliferation, occurs in the cKO mouse model used, indicating that Nr5a2 is not sine qua non for mitosis. Nonetheless, severe impairment of the cell cycle is engendered in cKO animals, interfering with the normal proliferative response to gonadotropins.
Acknowledgments
The authors are grateful to Vickie Roussel for technical support.
Financial Support: This study was funded by grant 14857Z from the Canadian Institutes of Health Research to B.D.M.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Amhr2Cre/+
- Cre-recombinase driven by the anti-Müllerian type II receptor
- BrdU
- bromodeoxyuridine
- BSA
- bovine serum albumin
- cKO
- conditional knockout
- CON
- control
- Cy3
- cyanine-3
- Cyp19a1
- cytochrome P450 19a1
- DAPI
- 4′,6-diamidino-2-phenylindole
- DMSO
- dimethylsulfoxide
- eCG
- equine chorionic gonadotropin
- FACS
- fluorescent-activated cell sorting
- FDR
- false discovery rate
- FSH
- follicle-stimulating hormone
- FSHr
- receptor for follicle-stimulating hormone
- iCRT3
- inhibitor to β-catenin-responsive transcription
- IGF
- insulin-like growth factor
- IgG
- immunoglobulin G
- Kegg
- Kyoto Encyclopedia of Genes and Genomes
- Nr5a2
- nuclear receptor subfamily 5, group A, member 2
- Nr5a2f/f
- Nr5a2 floxed
- PBS
- phosphate-buffered saline
- PCNA
- proliferative cell nuclear antigen
- qPCR
- quantitative polymerase chain reaction.
References and Notes
- 1.Binelli M, Murphy BD. Coordinated regulation of follicle development by germ and somatic cells. Reprod Fertil Dev. 2010;22(1):1–12. [DOI] [PubMed] [Google Scholar]
- 2.McNatty KP, Jeungel JL, Pitman JL. Oocyte-somatic cell interactions and ovulation rate: effects on oocyte quality and embryo yield. Reprod Biol Insights. 2014;7:1–8. [Google Scholar]
- 3.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250(2):358–373. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Cortés ZT, Kimmins S, Monaco L, Burns KH, Sassone-Corsi P, Murphy BD. Estrogen mediates phosphorylation of histone H3 in ovarian follicle and mammary epithelial tumor cells via the mitotic kinase, Aurora B. Mol Endocrinol. 2005;19(12):2991–3000. [DOI] [PubMed] [Google Scholar]
- 6.Pelusi C, Ikeda Y, Zubair M, Parker KL. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008;79(6):1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22(14):1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14(5):250–260. [DOI] [PubMed] [Google Scholar]
- 9.Parker KL, Schimmer BP, Schedl A. Genes essential for early events in gonadal development. EXS. 2001;(91):11–24. [DOI] [PubMed] [Google Scholar]
- 10.Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated size-matched stages of human preantral follicles. Mol Cell Endocrinol. 2015;401:189–201. [DOI] [PubMed] [Google Scholar]
- 11.Lewis SR, Hedman CJ, Ziegler T, Ricke WA, Jorgensen JS. Steroidogenic factor 1 promotes aggressive growth of castration-resistant prostate cancer cells by stimulating steroid synthesis and cell proliferation. Endocrinology. 2014;155(2):358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu P, Oosterveer MH, Stein S, Demagny H, Ryu D, Moullan N, Wang X, Can E, Zamboni N, Comment A, Auwerx J, Schoonjans K. LRH-1-dependent programming of mitochondrial glutamine processing drives liver cancer. Genes Dev. 2016;30(11):1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianco S, Jangal M, Garneau D, Gévry N. LRH-1 controls proliferation in breast tumor cells by regulating CDKN1A gene expression. Oncogene. 2015;34(34):4509–4518. [DOI] [PubMed] [Google Scholar]
- 14.Kramer HB, Lai CF, Patel H, Periyasamy M, Lin ML, Feller SM, Fuller-Pace FV, Meek DW, Ali S, Buluwela L. LRH-1 drives colon cancer cell growth by repressing the expression of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res. 2016;44(2):582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Wu S, Lv S, Wang H, Wang Y, Guo Q. Suppression of liver receptor homolog-1 by microRNA-451 represses the proliferation of osteosarcoma cells. Biochem Biophys Res Commun. 2015;461(3):450–455. [DOI] [PubMed] [Google Scholar]
- 16.Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15(4):499–509. [DOI] [PubMed] [Google Scholar]
- 17.Lin Q, Aihara A, Chung W, Li Y, Chen X, Huang Z, Weng S, Carlson RI, Nadolny C, Wands JR, Dong X. LRH1 promotes pancreatic cancer metastasis. Cancer Lett. 2014;350(1-2):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolin K, Gossen J, Schoonjans K, Murphy BD. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology. 2014;155(5):1931–1943. [DOI] [PubMed] [Google Scholar]
- 19.Sirianni R, Seely JB, Attia G, Stocco DM, Carr BR, Pezzi V, Rainey WE. Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J Endocrinol. 2002;174(3):R13–R17. [DOI] [PubMed] [Google Scholar]
- 20.Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10(6):823–844. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Large MJ, Duggavathi R, DeMayo FJ, Lydon JP, Schoonjans K, Kovanci E, Murphy BD, Murphy BD. Liver receptor homolog-1 is essential for pregnancy. Nat Med. 2013;19(8):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato J. Induction of S phase by G1 regulatory factors. Front Biosci. 1999;4(4):D787–D792. [DOI] [PubMed] [Google Scholar]
- 23.Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci USA. 2011;108(15):5954–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furlong HC, Stämpfli MR, Gannon AM, Foster WG. Cigarette smoke exposure triggers the autophagic cascade via activation of the AMPK pathway in mice. Biol Reprod. 2015;93(4):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerrits H, Paradé MC, Koonen-Reemst AM, Bakker NE, Timmer-Hellings L, Sollewijn Gelpke MD, Gossen JA. Reversible infertility in a liver receptor homologue-1 (LRH-1)-knockdown mouse model. Reprod Fertil Dev. 2014;26(2):293–306. [DOI] [PubMed] [Google Scholar]
- 26.Labelle-Dumais C, Jacob-Wagner M, Paré JF, Bélanger L, Dufort D. Nuclear receptor NR5A2 is required for proper primitive streak morphogenesis. Dev Dyn. 2006;235(12):3359–3369. [DOI] [PubMed] [Google Scholar]
- 27.Bayrer JR, Mukkamala S, Sablin EP, Webb P, Fletterick RJ. Silencing LRH-1 in colon cancer cell lines impairs proliferation and alters gene expression programs. Proc Natl Acad Sci USA. 2015;112(8):2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O’Brien DA, Hess RA, França LR, Kiyokawa H, Cooke PS. Cell-cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod. 2005;72(6):1429–1436. [DOI] [PubMed] [Google Scholar]
- 29.Jirawatnotai S, Moons DS, Stocco CO, Franks R, Hales DB, Gibori G, Kiyokawa H. The cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1 cooperate to restrict proliferative life span in differentiating ovarian cells. J Biol Chem. 2003;278(19):17021–17027. [DOI] [PubMed] [Google Scholar]
- 30.Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology. 2017;158(7):2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brito IR, Saraiva MV, Araújo VR, Celestino JJ, Magalhães-Padilha DM, Lima IM, Van den Hurk R, Figueiredo JR, Silva JR. The effect of IGF-1 and FSH on the in vitro development of caprine secondary follicles and on the IGF-1, IGFR-I and FSHR mRNA levels. Res Vet Sci. 2012;93(2):729–732. [DOI] [PubMed] [Google Scholar]
- 32.Pawshe CH, Rao KB, Totey SM. Effect of insulin-like growth factor I and its interaction with gonadotropins on in vitro maturation and embryonic development, cell proliferation, and biosynthetic activity of cumulus-oocyte complexes and granulosa cells in buffalo. Mol Reprod Dev. 1998;49(3):277–285. [DOI] [PubMed] [Google Scholar]
- 33.Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60(1):51–89. [DOI] [PubMed] [Google Scholar]
- 34.Chand AL, Wijayakumara DD, Knower KC, Herridge KA, Howard TL, Lazarus KA, Clyne CD. The orphan nuclear receptor LRH-1 and ERα activate GREB1 expression to induce breast cancer cell proliferation. PLoS One. 2012;7(2):e31593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yumoto F, Nguyen P, Sablin EP, Baxter JD, Webb P, Fletterick RJ. Structural basis of coactivation of liver receptor homolog-1 by β-catenin. Proc Natl Acad Sci USA. 2012;109(1):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapointe E, Boerboom D. WNT signaling and the regulation of ovarian steroidogenesis. Front Biosci (Schol Ed). 2011;3:276–285. [DOI] [PubMed] [Google Scholar]