Abstract
Schizophrenia typically involves poor social functioning. This may be due, in part, to deficits in theory-of-mind, the cognitive ability to reason flexibly about the mental states of others. Patients also have deficits in social knowledge. It is currently unclear how these two impairments interrelate in schizophrenia. To address this issue, 43 patients with schizophrenia and 25 healthy controls completed two theory-of-mind tests and a novel test of social judgment. This latter measure required participants to judge whether various social behaviors were normal or reasonable in the context in which the behaviors occurred. Whereas patients demonstrated clear deficits in theory-of-mind, they performed similarly to controls when judging socially appropriate behaviors and violations of social norms. Patients, however, were less likely than controls to judge social behavior as reasonable when the behavior was impolite but understandable if the characters’ thoughts were taken into account. This latter difficulty correlated with patients’ performance deficits on the theory-of-mind tasks. Overall, findings suggest that basic social knowledge is intact in schizophrenia, though judgments of social behavior are affected by patients’ theory-of-mind deficits.
Keywords: Psychosis, Schizophrenia, Social cognition, Social knowledge, Social reasoning, Theory of mind
1. Introduction
Schizophrenia typically involves poor social functioning. Patients with schizophrenia, for example, display poorer social skills and report fewer close relationships than patients without schizophrenia (Green et al., 2008, Hooley, 2008). These social impairments can cause considerable suffering to patients and also interfere with functional outcomes, such as education and employment (Couture et al., 2006, Green et al., 2008). The social impairments associated with schizophrenia may result in part from deficits in social cognition — “the mental operations that underlie social interactions, including perceiving, interpreting, and generating responses to the intentions, dispositions, and behaviors of others” (Green et al., 2008, p. 1).
Social cognition can be conceived as comprising both “fluid” and “crystallized” abilities — a distinction commonly used by researchers of intelligence. An example of a “fluid” ability is “theory of mind” (ToM) — the cognitive ability to reason flexibly about the mental states of others in social contexts. ToM is required to negotiate dynamic social interactions and interpret ambiguous social cues, and there is strong evidence that patients with schizophrenia have deficits in this ability (Bora and Pantelis, 2013, Bora et al., 2009). An example of a “crystallized” ability is social knowledge. This declarative knowledge – in the form of understanding roles, protocols, and goals of social interactions – permits rule-based reasoning of social exchanges. Acquired social knowledge of this type is also critical to normal social functioning and previous research suggests that it is similarly deficient in schizophrenia (Green et al., 2008).
Previous research has assessed social knowledge in schizophrenia by asking patients to answer questions about general social issues (e.g., why do you think the divorce rate is going up? Cutting and Murphy, 1990, Muñoz et al., 1992, Upthegrove et al., 2002), recall sequences of typical behaviors in routine social scripts (e.g., going to the supermarket; Chan et al., 1999, Matsui et al., 2006, Matsui et al., 2009), or identify roles, rules, and goals in social scenarios (Addington and Piskulic, 2011, Addington et al., 2006). In all such tasks, patients with schizophrenia perform worse than healthy controls. Performance deficits on these tasks, however, may reflect more general difficulties with abstract thinking and semantic memory (i.e., generalized knowledge about concepts and objects), both of which are well-known to be affected in schizophrenia (Doughty and Done, 2009).
Given these limitations, it is unclear the extent to which social knowledge is impaired in schizophrenia. Social deficits in schizophrenia usually become most apparent around the time of illness onset. It is possible, therefore, that a significant proportion of people who develop schizophrenia have had sufficient ToM ability earlier in their life to acquire adequate social knowledge. These individuals would not have been noticed by clinicians early in their life, unlike young individuals with autism, and, even after the onset of their illness, may retain what social knowledge they acquired earlier. The ability of these individuals, however, to access and/or demonstrate their social knowledge on cognitively demanding tasks may decline with the onset and progression of their illness. This proposal predicts a dissociation between impaired ToM and intact social knowledge if the latter is assessed using less demanding tasks.
To address this possibility, we adapted a social judgment task from autism research (Ellis et al., 1994). The original version of this task required participants to rate behaviors in various social scenarios in terms of how normal or expected the behaviors were. There are two key advantages of this task. First, it does not rely on semantic memory in the same way as previous measures of social knowledge and is also less cognitively demanding. Second, it assesses participants’ application of their knowledge of social norms to judge other people’s behavior. The task therefore seems particularly relevant to understanding social difficulties in people with schizophrenia, since judgments of this type are often mistaken or misguided in these individuals, triggering interpersonal conflict. The primary disadvantage of the original version of this task, however, is that judging some of the behaviors presented in the original version conflated ToM and the application of rule-based social knowledge. To tease these capacities apart, we adapted five of the original stories to ask participants to judge three types of behavior: (1) behavior that is appropriate according to social norms; (2) violations of social norms; and (3) impolite behavior that is understandable if the characters’ thoughts are taken into account (see Appendix for an example). Participants were required to categorize each type of behavior as either normal, unusual, or shocking.
If normative social knowledge is intact in schizophrenia, while ToM is impaired, it was hypothesized that patients would judge the socially appropriate behaviors and the violations of social norms like controls, but would be less likely than controls to judge the impolite but understandable behaviors as normal. It was also expected that this latter difficulty would correlate with patients’ performance deficits on ToM tasks.
2. Method
2.1. Participants
Forty-three patients (24 males, 19 females) of mean age 41.53 years (SD = 9.58; range 24–60) were recruited from the Volunteer Register administered by the Australian Schizophrenia Research Bank (Loughland et al., 2010). Thirty-two patients had a diagnosis of schizophrenia and 11 had a diagnosis of schizoaffective disorder, confirmed using the Diagnostic Interview for Psychosis (DIP; Castle et al., 2006); and consistent with DSM-IV criteria (American Psychiatric Association, 2000). Twenty-five healthy controls (10 males, 15 females) of mean age 41.3 years (SD = 13.8; range 19 to 60) were recruited from the general community and screened using the affective, psychotic, and substance abuse screening modules from the Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID-I; First et al., 1996).
All participants were required to speak English as their first language. Exclusion criteria for both groups included history of head injury (unconscious for greater than one hour), neurological illness, and substance dependence (met criteria for a DSM-IV diagnosis for two or more of the last five years). All participants gave written informed consent. The experiment was approved by the local human research ethics committee and followed principles outlined in the World Medical Association Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects.
2.2. Materials and procedure
Participants completed the following tasks, after which patients were interviewed using the DIP to confirm diagnosis and the Scales for Assessment of Positive and Negative Symptoms of Schizophrenia (SAPS and SANS; Andreasen, 1983, Andreasen, 1984) to rate symptom severity, while controls were screened using the SCID. IQ was estimated using the National Adult Reading Test (Nelson and Willison, 1991). All testing and interviewing were conducted by experienced clinical researchers.
ToM was assessed using two tasks. The first was a verbal story comprehension task based on Harrington et al.’s (2005) ToM task. Participants read four stories with accompanying cartoons and answered questions that required ToM to comprehend either deception or a character’s false belief. For each story, participants predicted the character’s behavior (correct response = 1) and explained why the character would behave in this way (explicitly correct response = 2, partially correct = 1, and incorrect = 0). Scores were summed across stories. Participants also had to answer a factual control question about each story (correct response = 1). These scores were likewise summed across stories.
The second ToM measure was a non-verbal picture-sequencing task (Langdon and Coltheart, 1999, Langdon et al., 2010, Langdon et al., 2014). Participants were shown four picture-cards in a prearranged incorrect order and required to rearrange them to depict a logical sequence of events. There were four types of sequences (four sequences per type): ToM “false-belief stories” that involved going beyond the immediate objective information to infer a character’s mistaken belief; “social-script stories” that controlled for simple social reasoning; “mechanical stories” that controlled for physical cause-and-effect reasoning; and “capture stories” that controlled for inhibition of an obvious but misleading cue. Each sequence scored two points if the first card was positioned correctly, two points if the last card was correct, and one point each for the second and third cards being correct. Scores were averaged across each type of story (range 0–6).
To assess social judgment, participants read five vignettes that each involved three types of social behavior: socially appropriate, violations of social norms, and inappropriate but understandable if character’s thoughts were taken into account. These three types of behavior were presented in a fixed pseudo-random order across stories. Participants categorized each behavior as either normal (or reasonable), unusual (or strange), or shocking (or odd). For each type of social behavior, the percentage of behaviors assigned to these three categories was calculated.
2.3. Statistical analyses
Patients and controls’ demographic variables were compared using independent sample t-tests and chi-square tests. For the ToM story comprehension task, independent sample t-tests were used to compare the performance of patients and controls. For the ToM picture-sequencing task, a 2 (group: patient vs. control) × 4 (story type: false-belief versus the three control conditions) mixed ANOVA was used. If the two-way interaction proved significant, simple contrasts, using independent sample t-tests, were also reported for each story type. For the social judgment task, a 2 (group: patient vs. control) × 3 (condition: socially appropriate, violation of social norm, impolite but understandable) × 3 (response: normal, unusual, shocking) mixed ANOVA was used. If the three-way interaction proved significant, post hoc analysis used separate 2 (group) × 3 (response) ANOVAs for each of the three conditions. Greenhouse–Geisser correction was used if Mauchly’s test indicated that the assumption of sphericity had been violated. The alpha level was set at p = .05 for each task.
To examine relationships with ToM, a composite ToM score was derived from summing the standardized z-scores of the verbal and non-verbal ToM scores. Analysis focused on the Pearson correlations between this composite score and the percentage of “normal” responses per condition in the social judgment task. Correlations between the composite measure of ToM and basic demographics and clinical ratings – specifically, IQ, education, and SAPS and SANS total scores – were also examined. Partial correlations between ToM and “normal” responses in the social judgment task were conducted controlling for any demographic or clinical variables that correlated with ToM. Given the number of correlations, an adjusted alpha level of p = .01 was used.
3. Results
Patients and controls did not differ significantly in age, gender-ratio or NART-estimated IQ, although controls had more years of education (see Table 1). The patient group was characterized by chronic schizophrenia (mean duration of illness = 16.24 years; SD = 7.15; range 4–31 years) with mild symptoms (mean SAPS global rating of 1.9; mean SANS global rating of 1.9).
Table 1.
Basic demographics of patients and controls.
| Patients | Healthy Controls | Significance Test | |
|---|---|---|---|
| Males:Females | 24:19 | 10:15 | χ2(1) = .31 |
| Age (years) | 41.53 ± 9.58 (24–60) |
41.28 ± 13.83 (19–60) |
t(66) = .09 |
| Formal Education (years) | 12.37 ± 2.69 (8–18) |
14.42 ± 2.63 (10–21) |
t(66) = 3.05* |
| NART IQ | 107.07 ± 10.59 (75–128) |
110.76 ± 8.62 (91–124) |
t(66) = 1.48 |
Notes: Continuous data expressed as means ± SD (range in parentheses).
p < .05.
Patients showed significant deficits on both ToM tasks. In the story comprehension task, patients performed worse than controls on ToM questions but not on control questions (see Table 2). In the picture-sequencing task, a 2 (group: patient vs. control) × 4 (story type: false-belief versus the three control conditions) mixed ANOVA revealed a significant interaction of group by story type, F(2.45, 161.83) = 4.26, p = .01. This occurred because patients performed worse than controls on the false-belief stories that required ToM, but not on the control stories (see Table 2 for simple contrasts).
Table 2.
Performance of patients and controls on the two measures of ToM.
| Patients | Healthy Controls | t-value | p-value | |
|---|---|---|---|---|
| Story Comprehension | ||||
| ToM | 7.74 ± 3.27 | 9.76 ± 2.35 | 2.70 | < .01 |
| Control | 3.91 ± .29 | 3.92 ± .28 | .18 | .86 |
| Picture Sequencing | ||||
| False Belief (ToM) | 3.92 ± 1.56 | 5.15 ± .80 | 3.65 | < .01 |
| Social (Control) | 5.69 ± .63 | 5.83 ± .52 | .96 | .34 |
| Mechanical (Control) | 5.49 ± .88 | 5.79 ± .46 | 1.56 | .13 |
| Capture (Control) | 4.12 ± 1.10 | 4.55 ± 1.27 | 1.48 | .14 |
Notes. Data expressed as means ± SD.
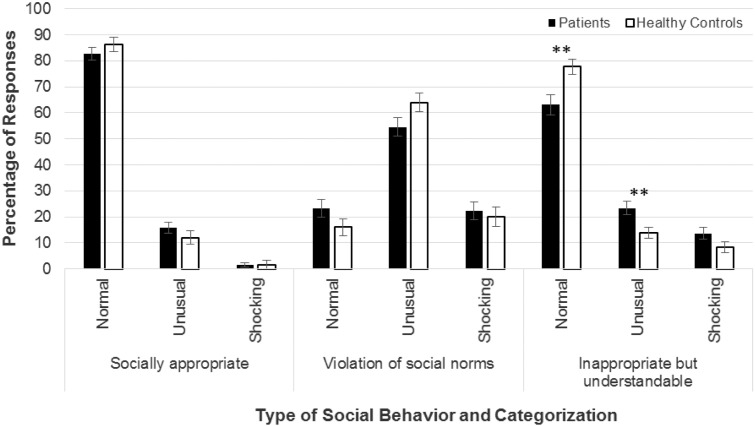
Patients showed a more complicated pattern of responding in the social judgment task. A 2 (group: patient vs. control) × 3 (condition: socially appropriate, violations, inappropriate but understandable) × 3 (response: normal, unusual, shocking) mixed ANOVA revealed a significant main effect of response and two-way interaction of condition by response that were incorporated into a significant three-way interaction, F(2.80, 185.00) = 3.78, p = .01 (see Fig. 1). Post hoc analysis used separate 2 (group) × 3 (response) ANOVAs for each of the three conditions to examine the significant three-way interaction. These revealed only main effects of response for the socially appropriate and violations conditions; that is, across groups, the most common response was “normal” for socially appropriate behaviors and “unusual” for violations. In contrast, there was a significant interaction between group and response in the impolite but understandable condition, F(1.46, 96.49), p = .01. Patients rated fewer behaviors in this condition as “normal” than controls, p < .01.
Fig. 1.
Patients’ and controls’ ratings across the three conditions of the social judgment task. Error bars represent standard errors.
** p < .01.
A composite measure of ToM was derived by summing the standardized z-scores of the verbal and non-verbal ToM scores. This composite measure of ToM correlated positively with the percentage of “normal” responses to inappropriate but understandable behaviors, r(43) = .55, p < .01. There was also a trend for the composite ToM measure to correlate negatively with the percentage of “normal” responses to violations, r(43) = − .30, p = .05. In addition, the composite measure of ToM correlated positively with IQ, r(43) = .45, p < .01, and education, r(43) = .57, p < .01, but not with the SAPS and SANS total scores or any other demographic. The correlation between ToM and the percentage of “normal” responses to inappropriate but understandable behaviors in the social judgment task, however, remained significant partialling out either IQ (p < .01) or years of education (p < .01). In contrast, the correlation between ToM and the percentage of “normal” responses to violations was no longer significant after partialling out either years of education (p = .27) or IQ (p = .22).
4. Discussion
Consistent with previous research (Bora et al., 2009), patients displayed significant deficits in ToM on both verbal and nonverbal measures but not in task control conditions. Despite their ToM deficits, patients did not differ significantly from controls in their judgments of behavior in the social judgment task if the behavior was consistent or clearly inconsistent with social norms. Nevertheless, patients were less likely to judge social behavior that appeared impolite, but was understandable if the story characters’ thoughts and intentions were taken into account, as normal or reasonable. This latter difficulty in categorizing social behavior correlated with ToM independent of either IQ or years of formal education. These findings confirm that patients can recognize when social behavior conforms to normative rules, while ToM deficits impair their ability to consider other people’s thoughts and intentions when judging social behavior.
The ability of patients to correctly identify normative social behavior is also illustrated by the fact that patients performed similarly to controls in the social control condition of the picture sequencing task. This general finding, however, differs from previous research that has shown social knowledge deficits in patients using more cognitively demanding tasks. It appears that while patients may retain knowledge of normative social behavior, they may have difficulty applying this knowledge to answer more abstract questions or complete tasks that require higher-level cognitive abilities. Patients’ intact social knowledge may also be masked by ToM deficits in other tasks, particularly if the tasks require patients to relate their knowledge of social norms to features of social situations that involve other peoples’ thoughts and experiences. Without the ability to take others’ thoughts into account, patients’ tolerance of impolite behavior will be compromised.
The study was limited by the convenience sampling of patients. In addition, as the social judgment task was not explicitly matched in difficulty to the ToM measures, it is unclear the extent to which normative social knowledge can be separated from ToM abilities. It remains possible, for example, that patients performed similarly to controls on the socially appropriate and violations conditions of the social judgment task, but differently on the ToM tasks, due to the relative difficulty of tasks. Despite these limitations, however, the findings suggest that social intelligence in schizophrenia is not globally impaired. Patients may have acquired adequate social knowledge of normative behavior before the onset of their illness. They may continue to be able to demonstrate this knowledge if the circumstances are not too cognitively demanding and do not rely on ToM capacity to understand. Remediation of poor social functioning in schizophrenia may therefore be most productive when it targets patients’ ToM capacities to be sensitive to others in social contexts, rather than simply imparting declarative knowledge about normative social behavior.
Role of Funding Source
This research was funded by salary support to RL and participant reimbursement costs from an Australian Research Council (ARC) Future Fellowship to RL (grant number FT110100631).
Contributors
RL designed the study, supervised data collection, and helped to draft the manuscript. MC analyzed the data and drafted the manuscript. EC helped to design the study and assisted with data collection.
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Acknowledgements
Recruitment of participants for this study was facilitated by the Australian Schizophrenia Research Bank (ASRB), which is supported by the National Health and Medical Research Council of Australia, the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation, and the Schizophrenia Research Institute.
Appendix A. An example vignette from the social knowledge task
Participants categorize italicized sections.
Charlie, 23, had been out of work for several months. On this day his hopes were high because he was on his way to apply for a job which seemed just right for him. As Charlie rode the lift to his interview a stranger said pleasantly, “Nice day, isn’t it?” (SOCIALLY APPROPRIATE)
Just then, Charlie happened to see his reflection in a mirror by the lift buttons. His hair was sticking up in a peculiar way and he had no comb with him. He turned to the friendly stranger and asked, “Do you have a comb I could borrow for a minute please?” (VIOLATION OF SOCIAL NORMS)
The man looked at Charlie and said “Sure thing, I suppose you want to borrow a spare pair of my underwear as well.” (IMPOLITE BUT UNDERSTANDABLE IF THE MAN’S RESPONSE IS UNDERSTOOD AS IRONIC)
References
- Addington J., Piskulic D. Social cognition and functional outcome are separate domains in schizophrenia. Schizophr. Res. 2011;127:262–263. doi: 10.1016/j.schres.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Addington J., Saeedi H., Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. Br. J. Psychiatry. 2006;189:373–378. doi: 10.1192/bjp.bp.105.021022. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Text Revision (DSM-IV-TR) Fourth ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andreasen N.C. University of Iowa; Iowa City, IA: 1983. Scale for the Assessment of Negative Symptoms (SANS) [Google Scholar]
- Andreasen N.C. University of Iowa; Iowa City, IA: 1984. Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- Bora E., Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr. Res. 2013;144:31–36. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr. Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Castle D.J., Jablensky A., Mcgrath J.J., Carr V., Morgan V., Waterreus A. The Diagnostic Interview for Psychoses (DIP): development, reliability and applications. Psychol. Med. 2006;36:69–80. doi: 10.1017/S0033291705005969. [DOI] [PubMed] [Google Scholar]
- Chan A.S., Chiu H., Lam L., Pang A., Chow L.-Y. A breakdown of event schemas in patients with schizophrenia: an examination of their script for dining at restaurants. Psychiatry Res. 1999;87:169–181. doi: 10.1016/s0165-1781(99)00051-7. [DOI] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. The functional significance of social cognition in schizophrenia: a review. Schizophr. Bull. 2006;32:S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J., Murphy D. Impaired ability of schizophrenics, relative to manics or depressives, to appreciate social knowledge about their culture. Br. J. Psychiatry. 1990;157:355–358. doi: 10.1192/bjp.157.3.355. [DOI] [PubMed] [Google Scholar]
- Doughty O.J., Done D.J. Is semantic memory impaired in schizophrenia? A systematic review and meta-analysis of 91 studies. Cogn. Neuropsychiatry. 2009;14:473–509. doi: 10.1080/13546800903073291. [DOI] [PubMed] [Google Scholar]
- Ellis H.D., Ellis D.M., Fraser W., Deb S. A preliminary study of right hemisphere cognitive deficits and impaired social judgments among young people with Asperger syndrome. Eur. Child Adolesc. Psychiatry. 1994;3:255–266. doi: 10.1007/BF01978114. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. New York State Psychiatric Institute, Biometrics Research Department; New York, NY: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders — Patient Edition (SCID-I P, Version 2.0) [Google Scholar]
- Green M.F., Penn D.L., Bentall R., Carpenter W.T., Gaebel W., Gur R.C. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 2008;34:1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L., Langdon R., Siegert R.J., Mcclure J. Schizophrenia, theory of mind, and persecutory delusions. Cogn. Neuropsychiatry. 2005;10:87–104. doi: 10.1080/13546800344000327. [DOI] [PubMed] [Google Scholar]
- Hooley J.M. Interpersonal functioning and schizophrenia. In: Millon T., Blaney P., Davis R., editors. Oxford Textbook of Psychopathology. 2nd ed. Oxford University Press; New York, NY: 2008. [Google Scholar]
- Langdon R., Coltheart M. Mentalising, schizotypy, and schizophrenia. Cognition. 1999;71:43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- Langdon R., Ward P.B., Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr. Bull. 2010;36:321–330. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon R., Still M., Connors M.H., Ward P.B., Catts S.V. Theory of mind in early psychosis. Early Interv. Psychiatry. 2014;8:286–290. doi: 10.1111/eip.12072. [DOI] [PubMed] [Google Scholar]
- Loughland C., Draganic D., Mccabe K., Richards J., Nasir A., Allen J. Australian Schizophrenia Research Bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Aust. N. Z. J. Psychiatry. 2010;44:1029–1035. doi: 10.3109/00048674.2010.501758. [DOI] [PubMed] [Google Scholar]
- Matsui M., Sumiyoshi T., Yuuki H., Kato K., Kurachi M. Impairment of event schema in patients with schizophrenia: examination of script for shopping at supermarket. Psychiatry Res. 2006;143:179–187. doi: 10.1016/j.psychres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Matsui M., Arai H., Yonezawa M., Sumiyoshi T., Suzuki M., Kurachi M. The effects of cognitive rehabilitation on social knowledge in patients with schizophrenia. Appl. Neuropsychol. 2009;16:158–164. doi: 10.1080/09084280903098414. [DOI] [PubMed] [Google Scholar]
- Muñoz R.A., Muñoz L.A., Bias M., Ruiz D. Use of the Social Knowledge Scale (SKS) among patients treated for schizophrenia and psychology students. Ann. Clin. Psychiatry. 1992;4:267–273. [Google Scholar]
- Nelson H.E., Willison J. NFER Nelson; Windsor, UK: 1991. National Adult Reading Test (NART): Test Manual. [Google Scholar]
- Upthegrove R., Oyebode F., George M., Haque M.S. Insight, social knowledge and working memory in schizophrenia. Psychopathology. 2002;35:341–346. doi: 10.1159/000068595. [DOI] [PubMed] [Google Scholar]