Abstract
Objective
To know the predictors of a successful outcome of percutaneous transvenous mitral commissurotomy (PTMC) other than described in the Wilkins scoring system.
Methods
Two hundred fifty-eight consecutive patients were enrolled for this observational study in a tertiary care heart center of Pakistan who had a Wilkins score of ≤8. Patients with more than mild mitral regurgitation (MR) or having a clot in the left atrium were excluded. The Bonhoeffer multi-track system was used as a default technique. Successful PTMC was defined as achieving a mitral valve area (MVA) of ≥1.5 cm2 with no more than mild MR.
Results
Out of 258 PTMC procedures, 197 were successful. The Bonhoeffer multi-track system was used in ~94% cases. Among unsuccessful procedures, 41 patients did not achieve the required valve area, and 21 patients developed more than mild MR, including those 8 patients who did not achieve the required valve area and had more than mild MR. Bigger mean annulus size (33.5±2.6 versus 32.8±2.1 mm; p=0.02) and pre-procedure MVA (0.93±0.1 versus 0.87±0.1 cm2; p=0.002) had a significant effect on successful PTMC. Lower mean preprocedure systolic right ventricular pressure on echo (65.4±19.4 versus 75.3±18 mm Hg; p=0.000) and on cath (74±21.5 versus 81.5±24.6 mm Hg; p=0.002), lower grade of left ventricular dysfunction (p=0.04), and tricuspid regurgitation on echo (p=0.003) also had positive effects on the outcome.
Conclusion
Bigger preprocedure mitral valve annulus size and mitral valve area, and better left and right ventricular hemodynamics are correlated with successful PTMC.
Keywords: percutaneous transvenous mitral commissurotomy, Bonhoeffer multi-track system, predictors
Introduction
Rheumatic mitral stenosis (MS) is one of the common valvular heart diseases in southeast Asia (1, 2). Percutaneous transvenous mitral commissurotomy (PTMC) is an established alternative to surgical mitral commissurotomy and is often a preferred method of treatment in patients with symptomatic moderate to severe mitral stenosis and with suitable anatomy (3-5). The Wilkins scoring system is an established method of predicting the outcome of PTMC (6, 7). However, there are studies that have questioned the accuracy and validity of this scoring system as a predictor of outcomes and that have suggested a more refined and comprehensive assessment in light of recent data (8-11). Moreover, The Wilkins scoring system is mainly based on
the structural changes of the mitral valve apparatus and is lacking in demographic, technical, and other echocardiographic features. Secondly, in the majority of cases (17 out of 22), a single-balloon technique was used by Wilkins et al. (6). Therefore, despite the common incidence in our part of the world, we did not know if there were other predictors of outcome besides that described by Wilkins et al. (6) In particular, if we are using a double-balloon (Bonhoeffer multi-track system) technique, then it is more important to know the other predictors of the outcome in our setup (12).
This study was designed to determine the predictors of a successful outcome of PTMC in patients with symptomatic moderate to severe mitral stenosis by Bonhoeffer multi-track system(12)
Methods
Study design and patients
This observational study was conducted at a tertiary care cardiovascular center of the National Institute of Cardiovascular Diseases, Karachi, Pakistan, during the years 2009 to 2011. A total of 258 consecutive patients presenting with moderate to severe mitral stenosis and with a Wilkins score of ≤8 were included in study. Patients with more than mild mitral regurgitation (MR) and/or a clot in the left atrium (LA) and/or a Wilkins score of >8 were excluded from the study. Patients with concomitant significant valvular disease besides tricuspid regurgitation (TR) were also excluded from the study.
The double balloon (Bonhoeffer multi-track system) was used as the default technique. The single-balloon technique was used as an alternative when the double-balloon technique was not feasible.
Procedure
A right femoral approach was used for catheterization; 6 F and 8 F sheaths were introduced into the femoral artery and vein, respectively. Right heart pressure hemodynamics were checked and recorded with a balloon angiographic catheter. Similarly, left heart pressures were recorded with a 6 F pigtail catheter, and then it was left in the ascending aorta for continuous pressure monitoring and for an anatomical landmark. An 8 F Mullins sheath was introduced into the femoral vein, and the interatrial septum was punctured with a Brockenbrough needle. A Mullins sheath was then introduced into the LA with the dilator. Once the position in the LA was confirmed with the help of a small injection of contrast material, the dilator and Brockenbrough needle were removed. Simultaneous pressure of the LA and LV was recorded with the Mullins in the LA and a pigtail catheter in the LV to obtain the gradient across the mitral valve. A flow-directed end-hole balloon catheter was introduced into the Mullins sheath, and the mitral valve was crossed, followed by the aortic valve, and then it was kept just distal to the arch of aorta. A 0.035” wire with a 6-cm floppy J-tip was then introduced into the balloon catheter and positioned preferably just distal to the arch of aorta to get better support and stability. The balloon catheter, along with the Mullins sheath, was removed, and the skin and interatrial septum were dilated with a 14 F dilator. The balloons of the multi-track system were prepared with contrast, and the first multi-track balloon was introduced and positioned across the mitral valve, and then, the second balloon was advanced over the wire and lined up with the first one. The balloons were then inflated simultaneously under fluoroscopic vision once, twice, or more until the disappearance of the so- called waist (which is formed around the inflated balloon by tight mitral stenosis). The balloons were removed, and pressures of the right and left heart were recorded with the help of multi-track angiographic and pigtail catheters. After the achievement of an acceptable transmitral gradient, the system was removed, and hemostasis was secured.
Data collection and definitions
The study was approved by the hospital research ethics committee. Written informed consent was taken by all eligible patients, and information was collected in the form of a detailed questionnaire, included the following: patient’s demographics; prior history of surgery, commissurotomy, or thromboembolic event; electrocardiographic findings; echocardiographic findings before and after the procedure; and hemodynamic and catheterization data.
Transthoracic echocardiography was performed just after and 24-48 hours after the procedure, and all findings were recorded. The same experienced echocardiographer performed all measurements using the parasternal short and long axis and four-chamber views.
Prospectively collected procedure-related complications included: death, pericardial tamponade, thromboembolism, and moderately severe MR. Procedure-related death was defined as in-hospital death that was directly related to PTMC. MR was graded as follows: mild, moderate, moderately severe, and severe. More than mild MR was defined as a central jet of >4 cm2 or occupying >20% of the LA area on color Doppler echocardiography. Successful PTMC was defined as achieving a post-procedural mitral valve area (MVA) of ≥1.5 cm2 with no more than mild MR.
Statistical analysis
Data were entered and analyzed using SPSS, version 17 (SPSS Inc., Chicago, IL, USA). Mean±SD was computed for all quantitative variables. Frequency and percentages were calculated for all categorical variables. Independent sample t-test was applied to compare means of various quantitative variables, like age, weight, and annulus size, etc, whereas chi-square test was applied for the association between relative frequencies of qualitative variables. Binary logistic regression was performed to determine the significant predictive factors for a successful PTMC outcome. A p value of <0.05 was considered significant.
Results
Out of 258 PTMC procedures, 197 (76.3%) were successful and 61 (23.6%) were unsuccessful. Among unsuccessful procedures, 41 (15.8%) patients did not achieve the required valve area, and 21 (8.1%) patients developed more than mild MR, including those 8 (3.1%) patients who did not achieve the required valve area and had more than mild MR. Tamponade developed in 5 patients (1.9%). One patient died, and two procedures were abandoned.
Table 1 shows the baseline demographic and preprocedural echo and cath features of the successful and unsuccessful procedures of PTMC. A trend of successful PTMC was observed toward taller and heavier patients, but the difference was not significant among the two groups (p=0.05). However, a significant difference was observed between annulus size and preprocedural MVA among the successful and unsuccessful groups.
Table 1.
Baseline demographic and preprocedural echocardiographic and catheterization features of patients undergoing percutaneous transvenous mitral commissurotomy
| Successful n=197 (%) | Unsuccessful n=61 (%) | P value | |
|---|---|---|---|
| Age, years (±SD) | 30 (9.8) | 27.9 (8.8) | 0.12 |
| Height (±SD) | 155.4 (7.5) | 153.2 (8.5) | 0.05 |
| Weight (±SD) | 52 (11.6) | 48.9 (10) | 0.05 |
| Gender | 0.60 | ||
| Male | 55 (27.9) | 15 (24.5) | |
| Female | 142 (72.0) | 46 (75.4) | |
| Previous commissurotomy | 1.00 | ||
| Yes | 05 (2.5) | 01 (1.6) | |
| No | 192 (97.4) | 60 (98.3) | |
| History of CVA | 1.00 | ||
| Yes | 03 (1.5) | 00 | |
| No | 194 (98.4) | 61 | |
| History of atrial fibrillation | 0.37 | ||
| Yes | 14 (7.1) | 02 (3.2) | |
| No | 183 (92.8) | 59 (96.7) | |
| Echo features | |||
| Annulus size, mm (±SD) | 33.5 (2.6) | 32.8 (2.1) | 0.02 |
| MVA, cm2 (±SD) | 0.93 (0.1) | 0.87 (0.1) | 0.002 |
| RV pressure, mm Hg (±SD) | 65.4 (19.4) | 75.3 (18) | <0.001 |
| MPG across MV, mm Hg (±SD) | 16.0 (4.5) | 16.8 (4.7) | 0.22 |
| LA size, mm (±SD) | 47.5 (6.1) | 47.9 (6) | 0.69 |
| Mitral regurgitation | 0.63 | ||
| Yes | 49 (24.8) | 17 (27.8) | |
| No | 148 (75.1) | 44 (72.1) | |
| Tricuspid regurgitation | 0.003 | ||
| Yes | 132 (67.0) | 28 (45.9) | |
| No | 65 (32.4) | 33 (54.0) | |
| LV dysfunction | 0.04 | ||
| No / mild | 186 (94.4) | 53 (86.8) | |
| Moderate / severe | 11 (5.5) | 08 (13.1) | |
| Cath findings (±SD) | |||
| RV systolic pressure, mm Hg | 74 (21.5) | 81.5 (24.6) | 0.02 |
| Mean LA pressure, mm Hg | 32.4 (7.6) | 31.8 (8.6) | 0.60 |
| LVEDP mm Hg | 13.2 (3.9) | 12.4 (3.7) | 0.19 |
| Mean PG across MV, mm Hg | 19.3 (7.7) | 20.1 (8.5) | 0.46 |
| Calcification on fluoroscopy | 0.07 | ||
| No calcium | 185 (93.9) | 53 (86.8) | |
| Moderate / Severe | 12 (6.09) | 08 (13.1) | |
AF - atrial fibrillation, Cath - cathetrization; CVA - cerebrovascular accident; LA - left atrium; LV - left ventricle; LVEDP - left ventricular end-diastolic pressure; MPG - mean pressure gradient; MV - mitral valve; MVA - mitral valve area; RV - right ventricle
Similarly, a significant difference in RV pressure was observed among the two groups, and high RV pressure was correlated with unsuccessful procedures. Moreover, moderate to severe TR was frequently observed among unsuccessful procedures (p=0.003). LV dysfunction was also frequently found with unsuccessful procedures (p=0.003).
Table 2 shows the technical features and postprocedural cath and echo findings of successful and unsuccessful procedures. The PTMC wire was most commonly placed in the ascending aorta and least commonly placed in the LV apex, but this placement did not show any significant difference over the success of the procedure. Regarding balloon sizes, a 14x16-mm balloon set was most successful, although the 14x14-mm balloon set was used most frequently. Similarly, two or more balloon inflations correlated more frequently with successful out comes. Right ventricular (RV) systolic pressure and mean pressure gradient (MPG) across the mitral valve on cath was significantly high in unsuccessful procedures as compared to successful procedures just after the procedure. Similarly, MVA, MPG, and RV pressure were significantly raised on echocardiography in unsuccessful procedures when performed on Day 1 of the procedure.
Table 2.
Technical and postprocedural catheterization and echocardiographic features of patients who underwent PTMC
| Successful n=197 (%) | Unsuccessful n=61 (%) | P value | |
|---|---|---|---|
| Technique of procedure | |||
| Position of wire | 0.87 | ||
| Descending aorta | 66 (33.5) | 19 (31.1) | |
| Ascending aorta | 76 (38.5) | 23 (37.7) | |
| LV apex | 55 (27.9) | 19 (31.1) | |
| Balloon sizes | 0.002 | ||
| Double balloon 14x14 | 120 (60.9) | 34 (55.7) | |
| Double balloon 14x16 | 43 (21.8) | 06 (9.8) | |
| Double balloon 16x16 | 18 (9.1) | 04 (6.5) | |
| Single/graduated balloon | 16 (8.1) | 16 (26.2) | |
| Number of balloon inflations | 0.002 | ||
| Once | 18 (9.1) | 12 (19.6) | |
| Twice and more | 179 (90.8) | 48 (78.6) | |
| Catheterization features (±SD) | |||
| RV systolic pressure, mm Hg | 52.9 (14.9) | 63.2 (21.8) | <0.001 |
| Mean LA pressure, mm Hg | 18.2 (5.2) | 18.8 (6.4) | 0.48 |
| LVEDP mm Hg | 15.6 (4.3) | 14.8 (5.1) | 0.19 |
| Mean PG across MV, mm Hg | 2.8 (2.9) | 4.0 (04) | 0.01 |
| Echocardiographic features | |||
| MVA, cm2 (±SD) | 1.8 (0.2) | 1.4 (0.2) | <0.001 |
| Mean PG across MV, mm Hg | 5.6 (02) | 8.3 (3.6) | <0.001 |
| PA pressure, mm Hg | 35.1 (12.9) | 47 (14.9) | <0.001 |
| Post-PTMC MR | <0.001 | ||
| No/Mild | 197 (100) | 40 (65.5) | |
| Moderate/Severe | 00 | 21 (34.4) | |
| Post-PTMC TR | 0.06 | ||
| No/Mild | 159 (80.7) | 41 (67.2) | |
| Moderate/Severe | 38 (19.2) | 18 (29.5) | |
LA - left atrium; LVEDP - left ventricular end-diastolic pressure; MV - mitral valve; MVA - mitral valve area; MR - mitral regurgitation; PA - pulmonary artery; PG - pressure gradient; PTMC - percutaneous transvenous mitral commissurotomy; RV - right ventricle; TR - tricuspid regurgitation
Table 3 shows the categorical analysis of the PTMC procedure in different groups. The findings strengthened the observation mentioned in Table 1. MVA of <0.9 cm2 and mitral annulus size of <33 mm predicted a greater chance of an unsuccessful procedure. Similarly, the higher the RV pressure, the greater the chance was of predicting an unsuccessful procedure.
Table 3.
Univariate analysis of demographic, echocardiographic, and catheterization features of patients who underwent PTMC
| Successful n=197 (%) | Unsuccessful n=61 (%) | P value | |
|---|---|---|---|
| Age, years | 0.29 | ||
| ≤30 | 117 (59.3) | 41 (67.2) | |
| >30 | 80 (40.6) | 20 (32.7) | |
| BSA, m2 | 0.03 | ||
| ≤1.3 | 28 (14.2) | 16 (26.2) | |
| >1.3 | 169 (85.7) | 45 (73.7) | |
| Height, cm | 0.17 | ||
| ≤150 | 59 (29.9) | 24 (39.3) | |
| >150 | 138 (70.0) | 37 (60.6) | |
| Weight, kg | 0.19 | ||
| ≤45 | 66 (33.5) | 26 (42.6) | |
| >45 | 131 (66.4) | 35 (57.3) | |
| Echo features | |||
| Annulus size, mm | 0.001 | ||
| ≤33 | 99 (50.2) | 45 (73.7) | |
| >33 | 98 (49.7) | 16 (26.2) | |
| MVA, cm2 | 0.04 | ||
| <0.9 | 117 (59.3) | 45 (73.7) | |
| ≥0.9 | 80 (40.6) | 16 (26.2) | |
| MPG across MV, mm Hg | 0.80 | ||
| ≤12 | 45 (22.8) | 13 (6.5) | |
| >12 | 152 (77.1) | 48 (24.3) | |
| RV pressure, mm Hg | 0.002 | ||
| ≤60 | 95 (48.2) | 16 (26.2) | |
| >60 | 102 (51.7) | 45 (73.7) | |
| LA size, mm | 0.88 | ||
| ≤45 | 86 (43.6) | 26 (42.6) | |
| >45 | 111 (56.3) | 35 (57.3) | |
| Cath findings | |||
| RV systolic pressure, mm Hg | 0.006 | ||
| ≤90 | 164 (83.2) | 40 (65.5) | |
| >90 | 33 (16.7) | 21 (34.4) | |
| Mean LA pressure, mm Hg | 0.10 | ||
| ≤25 | 36 (18.2) | 17 (27.8) | |
| >25 | 161 (81.7) | 44 (72.1) | |
| LVEDP mm Hg | 0.55 | ||
| <18 | 165 (83.7) | 53 (86.8) | |
| ≥18 | 32 (16.2) | 08 (13.1) | |
| Mean PG across MV, mm Hg | 0.96 | ||
| <12 | 35 (17.7) | 11 (18.0) | |
| ≥12 | 162 (82.2) | 50 (81.9) | |
LA - left atrium; LVEDP - left ventricular end-diastolic pressure; MV - mitral valve; MPG - mean pressure gradient; MVA - mitral valve area; PTMC - percutaneous transvenous mitral commissurotomy; RV - right ventricle
Table 4 shows the multivariate analysis for factors predicting a successful outcome of PTMC. The variables age, gender, annulus size, and preprocedure echocardiographic and catheterization features were included in the model to evaluate if they were significant predictive factors for a successful PTMC outcome. It was found that annulus size, preprocedure MVA, and RV pressure on echo and cath were individually significant predictors, but the multivariate logistic regression showed that only MVA (p=0.030) and RV pressure (p=0.040) were significant.
Table 4.
Factors predicting successful outcome of PTMC on multivariate analysis
| Variable | Odds Ratio | 95% Confidence Interval for Odds Ratio | P value |
|---|---|---|---|
| Annulus size, mm | 1.103 | 0.976-1.247 | 0.117 |
| Preprocedure MVA, cm2 | 13.02 | 1.15-148.21 | 0.039 |
| Preprocedure RV pressure, mm Hg | 0.978 | 0.959-0.998 | 0.030 |
| Preprocedure RV pressure at cath, mm Hg | 0.996 | 0.981-1.010 | 0.566 |
MVA - mitral valve area; PTMC - percutaneous transluminal mitral valve commissurotomy; RV - right ventricle
Figure 1 depicts the same relation of higher RV pressure with an unfavorable outcome of the PTMC procedure.
Figure 1.
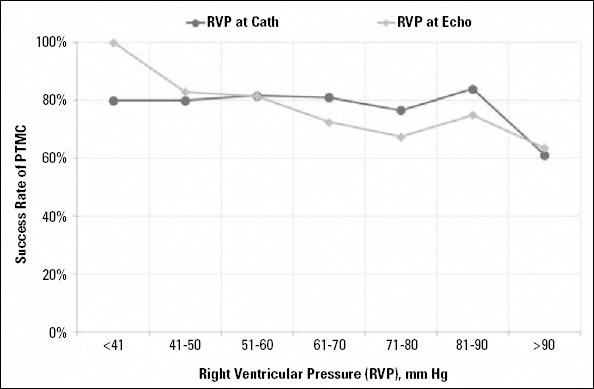
Trend of successful PTMC outcome with preprocedure right ventricular pressure. Note that success rate decreases as RVP increases
Discussion
PTMC is considered the mainstay of treatment in patients with MS (13-15). It is a time-tested, safe, and effective treatment (15-17). Despite the careful selection of patients using Wilkins score and selecting patients with a Wilkins score of <8, PTMC carries a considerable failure rate (18, 19). This led us to search for other predictors of successful PTMC.
Bonhoeffer et al. (12) have described the use of a multitrack system, which is actually a refinement of the double-balloon technique. This technique is one of the two main techniques that are currently used. The other one is the Inoue balloon technique, which was first described in 1984, and is claimed to be the most commonly used technique (20). However, in our institute, the Bonhoeffer (Multi-track) double-balloon technique is used in the majority of cases. To the best of our knowledge, this is the largest dataset showing the experience of the Bonheoffer technique for PTMC procedures in our part of the world. Secondly, this is the first study showing the predictors of the outcome in PTMC procedures other than Wilkins score.
Wilkins score concentrates on mitral valve apparatus and does not consider patient demographics and technical and echocardiographic features, including other systems, like pulmonary artery hypertension (6, 7). According to our findings, there are several other features that may help us in predicting the outcome of the procedure. However, these findings need discussion.
Among demographic features, one important finding is the tendency of a successful PTMC in patients with increased BSA. It is an established fact that the annular dimensions of the mitral valve increase correspondingly with body surface area (21). Although some other mitral valve structures, like interpapillary muscle and papillary muscle annular distance, have the same correlation, MV annular area shows the highest correlation with BSA (21, 22). Hence, the mitral valve shows a steady rise in its diameter with a rise in body surface area (21). This is probably the reason for the tendency of our operator to use a more aggressive approach-i.e., use of a bigger balloon and multiple inflations-in patients with increased BSA to get the optimum result; hence, they got better results.
Among echocardiographic features, we observed in our data that patients with a smaller annular size and valve area showed less favorable results (Table 1 and 3). Mitral annular calcification and valvular size have been reported recently as factors predicting suboptimal outcomes (23, 24). It is a known fact that MVA in rheumatic heart disease (RHD) reduces by 0.1 mm2/year. Therefore, a smaller MVA and annular size reflects more chronic disease and, understandably, more complex histopathological changes in mitral valve apparatus, including subendothelial collagen fibrosis and calcinations (25). This apparently requires a bigger balloon with multiple dilations to get the favorable outcome. In contrast, our operators used a less aggressive approach, and (in general) they used an undersized balloon in patients with a smaller annular size as a bid to prevent MR. Had they used a bigger balloon, would it have made the outcome better? It was not in our scope to answer this question, and study did not have sufficient power to settle this issue. However, we observed in our study that a bigger set of balloons is more successful than a smaller set of balloons (Table 2).
Similarly, increased pulmonary artery and right heart pressures are also a reflection of chronic disease and poor medical management (26). This is understandable in our setting, in which patients from far-flung rural areas do not have access to better medical facilities, and they are left untreated for a longer period of time until they become symptomatic (27-29). Usually, they present with extensive disease, and obviously, their outcome is not as good as in those patients with better right heart hemodynamics. This is the fact that we observed and documented in our data. In fact, we observed that pre-procedural RV pressure on echocardiography and right heart catheterization had a strong relation with the outcome of the procedure (Table 3). The higher the RV pressure was, the poorer the outcome was in our study (Fig. 1).
Left ventricular dysfunction is another echocardiographic finding that predicted a less favorable outcome in our study (Table 1). Although it lost its significance in the multivariate analysis, it can be said that it may have an impact on outcomes, keeping in mind the fact that LV dysfunction is not an uncommon association with mitral stenosis (30). In addition to that, the infarct shows a severe form of disease and further complicates the disease with atrial fibrillation and thrombus formation, which is, again, more commonly seen with mitral stenosis associated with LV dysfunction (30, 31). Hence, with a worse LV geometry and deformed subvalvular apparatus, the outcome of PTMC understandably could not be as favorable as observed with normal LV function.
One surprising technical finding that we observed was the negative relation of the wire positioning on the outcome of the procedure. Ideally, the PTMC wire should be parked in the arch of aorta. It is believed that this position gives better support and alignment to the balloon and makes the procedure easier and quicker. Equivalent results, irrespective of the place of the wire positioning, may be related to our experienced operators in high-volume center, leading to better balloon control.
It may be argued that the overall success rate of the PTMC procedures, ~77% in our study, is not up to the mark. Although the success rates of PTMC procedures in other landmark studies are comparable (18, 19), it should be noted that the postprocedural mean MVA of unsuccessful procedures was 1.4 cm2 (Table 2), whereas it was significantly smaller (0.8 cm2) before the procedure (Table 1) as compared to successful procedures (0.9 cm2). Therefore, though technically speaking, these procedures were unsuccessful, practically most of these patients showed remarkable clinical improvement. It is probably due to the achievement of more than 50% of the valve area from the baseline value in most of these so-called unsuccessful procedures. Secondly, in ~6% of cases, our operators used the single-balloon technique. These were the high-risk and/or pregnant patients in whom the operator wanted to make the procedure simpler and quicker. Hence, they used a single balloon in these patients, despite knowing the suboptimal outcome of this technique (32, 33). Therefore, not surprisingly, the single-balloon technique was found inferior to the double-balloon technique in our study, and it further validates what is already reported in the literature (32, 33).
What are the implications of our findings? Our study suggests that there is a need to look for other demographic, echo, and technique-related predictors of a successful PTMC rather than just sticking to only mitral valve apparatus-related predictors. It also highlights the need for a modified Wilkins score that includes other parameters, like BSA, pulmonary artery pressure, and LV dysfunction.
Study limitations
As the selection of balloon size was absolutely at the discretion of the operators, they did not use any uniform criteria to select the balloon size. Some of our operators used BSA, and others used annular size for the selection of the balloon set. Therefore, the results may not be standardized. Secondly, echocardiography was done just after the procedure, and it was repeated 24-48 hours after the procedure. Most of our patients belonged to far-flung rural areas, and they were followed up in their local hospital. Therefore, we could not record their follow-up echo findings and did not know if there was any long-term impact on the results.
Conclusion
Besides Wilkins score, increased body surface area, bigger preprocedure mitral valve annulus size and mitral valve area, and better left and right ventricular hemodynamics are significantly correlated with successful PTMC. Our study urges the need for percutaneous intervention before the worsening of annulus size and pulmonary artery pressure, along with a suitable mitral valve apparatus. It further suggests the development of a modified Wilkins score incorporating other predictors of successful PTMC.
Acknowledgment
Authors wish to thank Mr. Jaffer bin Baqar for his great help in managing and analyzing data.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - M.T.F.; Design - M.T.F.; Supervision - K.S.Z., S.I.R.; Resource - T.S., T.A.; Material - T.A., J.A.S.; Data collection and/or processing - M.T.F., T.S., T.A.; Analysis and/or Interpretation - M.T.F., N.K.; Literature search - M.T.F.; Writing - M.T.F.; Critical review -N.K.; Other -J.A.S., K.S.Z.
References
- 1.Ullah K, Ahmed SA, Badsha S, Khan A, Kiani MR. Rheumatic heart disease - A study of surgically excised cardiac valves and biopsies. J Coll Physicians Surg Pak. 2002;12:542–5. [Google Scholar]
- 2.Khan RF, Imtiaz Y, Ali H, Khan MU, Ali M, Riaz N, et al. Natural history and relative distribution of different valvular heart disease in Mayo Hospital, Lahore. Ann King Edward Med Coll. 2002;8:90–1. [Google Scholar]
- 3.Orrange SE, Kawanishi DT, Lopez BM, Curry SM, Rahimtoola SH. Actuarial outcome after catheter balloon commissurotomy with mitral stenosis. Circulation. 1997;95:382–9. doi: 10.1161/01.cir.95.2.382. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.Hu X, Zhao Q. Systematic comparison of the effectiveness of percutaneous mitral balloon valvotomy with surgical mitral commissurotomy. Swiss Med Wkly. 2011;141:13180. doi: 10.4414/smw.2011.13180. [DOI] [PubMed] [Google Scholar]
- 5.Rahman F, Akhter N, Anam K, Rashid MA, Uddin MJ, Ahmed CM, et al. Balloon mitral valvuloplasty:immediate and short term haemo- dynamic and clinical outcome. Mymensingh Med J. 2010;19:199207. [PubMed] [Google Scholar]
- 6.Wilkins GT, Weymen AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve:an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abascal VM, Wilkins GT, O’Shea JP, Choong CY, Palacios IF, Thomas JD, et al. Prediction of successful outcome in 130 patients undergoing percutaneous balloon mitral valvotomy. Circulation. 1990;82:448–56. doi: 10.1161/01.cir.82.2.448. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Prendergast BD, Shaw TR, Iung B, Vahanian A, Northridge DB. Contemporary criteria for the selection of patients for percutaneous balloon mitral valvuloplasty. Heart. 2002;87:401–4. doi: 10.1136/heart.87.5.401. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anwar AM, Attia WM, Nosir YF, Soliman Ol, Mosad MA, Othman M, et al. Validation of a new score for the assessment of mitral stenosis using real-time three-dimensional echocardiography. J Am Soc Echocardiogr. 2009;23:13–22. doi: 10.1016/j.echo.2009.09.022. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Soliman OI, Anwar AM, Metawei AK, McGhie JS, Geleijnse ML, Ten Cate FJ. New scores for the assessment of mitral stensosis using real-time three-dimensional echocardiography. Curr Cardiovasc Imaging Rep. 2011;4:370–7. doi: 10.1007/s12410-011-9099-z. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarath Babu D, Ranganayakulu KP, Rajasekhar D, Vanajakshamma V, Pramod Kumar T. Assessment of mitral valve commissural morphology by transoesophageal echocardiography predicts outcome after balloon mitral valvotomy. Indian Heart J. 2013;65:269–75. doi: 10.1016/j.ihj.2013.04.022. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonhoeffer P, Esteves C, Casal U, Tortoledo F, Yonga G, Patel T, et al. Percutaneous mitral valve dilatation with the multi-track system. Catheter Cardiovasc Interv. 1999;48:178–83. doi: 10.1002/(sici)1522-726x(199910)48:2<178::aid-ccd11>3.0.co;2-5. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Palacios I, Block PC, Brandi S, Blanco P, Casal H, Pulido JI, et al. Percutaneous balloon valvotomy for patients with severe mitral stenosis. Circulation. 1987;75:778–84. doi: 10.1161/01.cir.75.4.778. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Nobuyoshi M, Hamasaki N, Kimura T, Nosaka H, Yokoi H, Yasumoto H, et al. Indications, complications and short-term clinical outcome of percutaneous transvenous mitral commissurotomy. Circulation. 1989;80:782–92. doi: 10.1161/01.cir.80.4.782. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Babic UU, Grujicic S, Popovic Z, Djurisisc Z, Pejcic P, Vucinic M. Percutaneous transarterial balloon dilatation of the mitral valve:five year experience. Br Heart J. 1992;67:185–9. doi: 10.1136/hrt.67.2.185. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vahanian A, Michel PL, Cormier B, Vitoux B, Michel X, Slama M, et al. Results of percutaneous mitral commissurotomy in 200 patients. Am J Cardiol. 1989;63:847–52. doi: 10.1016/0002-9149(89)90055-6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Chen CR, Cheng TO, Chen JY, Zhou YL, Mei J, Ma TZ. Long-term results of percutaneous mitral valvuloplasty with the Inoue balloon catheter. Am J Cardiol. 1992;70:1445–8. doi: 10.1016/0002-9149(92)90297-c. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Leon MN, Harrell LC, Simosa HF, Mahdi NA, Pathan AZ, Lopez-Cuellar J, et al. Comparison of immediate and long-term results of mitral balloon valvotomy with the double- balloon versus Inoue techniques. Am J Cardiol. 1999;83:1356–63. doi: 10.1016/s0002-9149(99)00100-9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Palacios IF, Sanchez PL, Harrell LC, Weyman AE, Block PC. Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplsty variables that predict long-term outcome. Circulation. 2002;105:1465–71. doi: 10.1161/01.cir.0000012143.27196.f4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of intravenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. [PubMed] [Google Scholar]
- 21.Rajila Rajendran HS, Seshayyan S, Victor A, Murugesan N, Sundaramurthi I. The study of mitral valve annular dimension in relation to the body surface area in the Indian population. Eur J Cardio-thorac Surg. 2011;39:653–6. doi: 10.1016/j.ejcts.2010.08.052. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 22.Sonne C, Suqenq L, Watanabe N, Weinert L, Saito K, Tsukiji M, et al. Age and body surface area dependency of mitral valve and papillary apparatus parameters:assessment by real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009;10:287–94. doi: 10.1093/ejechocard/jen237. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Salarifar M, Rezvanfard M, Sadeghian H, Safir-mardanloo A, Shafii N. Mitral annular calcification predicts immediate results of percutaneous transvenous mitral commissurotomy. Cardiovasc Ultrasound. 2011;9:29. doi: 10.1186/1476-7120-9-29. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korkmaz S, Aksu T, Şaşmaz H, Çolak A, Yılmaz MB, Güray Y, et al. Acute results of mitral balloon valvuloplasty. Turk Kardiyol Dern Ars. 2011;39:137–42. doi: 10.5543/tkda.2011.01375. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 25.Krymskii LD, Nestaiko GV, Iaroshinskii IuN. Heart valves in rheumatic heart disease in the light of scanning electron microscopy. Kardiologiia. 1975;15:57–61. [PubMed] [Google Scholar]
- 26.Otto CM, Davis KB, Reid CL, Slater JN, Kronzon I, Kisslo KB, et al. Relation between pulmonary artery pressure and mitral stenosis severity in patients undergoing balloon mitral commissurotomy. Am J Cardiol. 1993;71:874–8. doi: 10.1016/0002-9149(93)90844-3. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 27.Rheumatic fever and rheumatic heart disease. World Health Organ Tech Rep Series. 2004;923:1–122. [PubMed] [Google Scholar]
- 28.Shaikh MA. Cardiac valvular lesions in patients with rheumatic heart disease. J Pak Inst Med Sci. 2004;15:862–5. [Google Scholar]
- 29.Rizvi SF, Khan MA, Kundi A, Marsh DR, Samad A, Pasha O. Status of rheumatic heart disease in rural Pakistan. Heart. 2004;90:394–9. doi: 10.1136/hrt.2003.025981. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shikano M, Nakatani S, Kim J, Hanatani A, Hashimura K, Yasumura Y, et al. Impaired left ventricular systolic function in mitral stenosis. J Cardiol. 2003;42:75–9. [PubMed] [Google Scholar]
- 31.Farman MT, Sial JA, Khan N, Rahu QA, Tasneem H, Ishaq M. Severe mitral stenosis with atrial fibrillation - a harbinger of thromboembolism. J Pak Med Assoc. 2010;60:439–43. [PubMed] [Google Scholar]
- 32.Lock JE, Khalilullah M, Shrivastava S, Bahl V, Keane JF. Percutaneous catheter commissurotomy in rheumatic mitral stenosis. N Engl J Med. 1985;313:1515–8. doi: 10.1056/NEJM198512123132405. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 33.Kveselis DA, Rocchini AP, Beekman R, Snider AR, Crowley D, Dick M, et al. Balloon angioplasty for congenital and rheumatic mitral stenosis. Am J Cardiol. 1986;57:348–50. doi: 10.1016/0002-9149(86)90922-7. [CrossRef] [DOI] [PubMed] [Google Scholar]


