Abstract
Objective: The purpose of this study was to investigate the effects of supplementation with a spearmint (Mentha spicata L.) extract, high in polyphenols including rosmarinic acid, on cognitive performance, sleep, and mood in individuals with age-associated memory impairment (AAMI).
Design: Subjects with AAMI (N = 90; 67% female; age = 59.4 ± 0.6 years) were randomly assigned (n = 30/group) to consume 900, 600, or 0 mg/day (two capsules, once daily) spearmint extract for 90 days, in this double-blind, placebo-controlled trial. Assessments were completed for cognition (days 0, 45, and 90), sleep (days 0 and 90), and mood (days 0 and 90) by using the Cognitive Drug Research (CDR) System™, Leeds Sleep Evaluation Questionnaire (LSEQ), and Profile of Mood States (POMS™), respectively.
Results: Quality of working memory and spatial working memory accuracy improved after supplementation with 900 mg/day spearmint extract by 15% (p = 0.0469) and 9% (p = 0.0456), respectively, versus placebo. Subjects consuming 900 mg/day spearmint extract reported improvement in their ability to fall asleep, relative to subjects consuming placebo (p = 0.0046). Overall treatment effects were evident for vigor-activity (p = 0.0399), total mood disturbance (p = 0.0374), and alertness and behavior following wakefulness (p = 0.0415), with trends observed for improvements after spearmint supplementation relative to placebo.
Conclusions: These results suggest that the distinct spearmint extract may be a beneficial nutritional intervention for cognitive health in older subjects with AAMI.
Keywords: : spearmint, polyphenols, rosmarinic acid, working memory, sleep
Introduction
Aging is commonly associated with deficits in cognitive domains, including speed of processing, working memory capacity, and long-term memory. Age-associated memory impairment (AAMI) is a nonpathological condition that is frequently observed as a result of normal brain aging.1 With the number of older adults (>65 years of age) worldwide expected to more than double by the year 2030 and average life span predicted to extend by 10 years by 2050, cognitive decline threatens quality of life and creates challenges for healthcare systems.2 Thus, the need for effective interventions that are aimed at maintaining cognitive health or slowing cognitive decline is critical.
Various nutrients and botanical ingredients have been investigated for their neurocognitive benefits, with studies reporting varying degrees of efficacy.3–9 A few studies have reported positive acute effects on cognition after the consumption of plant extracts from the Lamiaceae family,10–12 which some research suggests may be attributed to the polyphenols contained in these plants and their extracts. Specifically, polyphenols found in plants within the Lamiaceae family, such as rosmarinic and salvianolic acids, have been shown to have anticholinesterase and antioxidant activity, as well as additional neuroprotective and neurotrophic effects both in vitro and in vivo,13–15 all which provide biological plausibility for the potential cognitive benefits of extracts derived from this plant group.
Systemic accumulation of free radical and oxidation end products have been inversely correlated with cognitive function.16 Extracts from plants within Lamiaceae family have been shown to have antioxidant capacity and the ability to reduce systemic and local inflammation.17,18 Specifically, rosmarinic acid (RA) has been shown to promote antioxidant status in both neuronal cells and hippocampal tissue.13,15 In addition, salvianolic acid, an RA dimer and compound detected in the spearmint extract tested, has been shown to promote anti-inflammatory and antioxidative effects in a mouse model of Alzheimer's disease.19 Therefore, it is plausible that other phenolic compounds within the spearmint extract, in addition to RA, may contribute to the observed benefits.
Spearmint (Mentha spicata L.) lines were developed through traditional breeding techniques to produce significantly higher levels of bioactive polyphenols, such as RA,20 to study the effects of these polyphenols on cognitive function. These spearmint lines are distinct from typical commercially available spearmint because they do not contain certain constituents, such as carvone, and they contain 8–9% RA compared with 0–6% RA on a dry weight basis reported for other plants within the Lamiaceae species.20,21 The first indications that the dried aqueous extract from this spearmint could support cognitive performance were obtained by using a senescence accelerated mouse-prone 8 (SAMP8) model of aging.22 When the spearmint extract was administered to SAMP8 mice for 12 weeks at 320 mg/kg body weight, improvements in learning and memory occurred that corresponded to reduced protein carbonyls and 3-nitrotyrosine in the hippocampus. This reduction in oxidation byproducts in the hippocampus is a plausible mechanism of action for the cognitive effects of spearmint extract supplementation. Further, since working memory is reported to be hippocampally dependent,23 it is plausible that spearmint extract could favorably impact working memory. These initial findings were subsequently corroborated by an open-label clinical study in which healthy older subjects with self-reported memory impairment consumed the extract for 30 days. This study confirmed that the extract was well tolerated and improved cognitive performance.24
Based on these positive findings, we hypothesized that chronic daily consumption of the spearmint extract would favorably impact cognition. Therefore, this study investigated the effects of this dried aqueous spearmint extract on cognitive performance, sleep, and mood in healthy older subjects with AAMI.
Materials and Methods
Study design
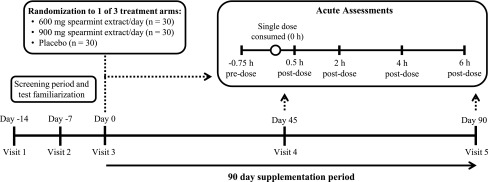
This randomized, double-blind, placebo-controlled study was conducted in accordance with Good Clinical Practice Guidelines, the Declaration of Helsinki,25 and the United States 21 Code of Federal Regulations.26 An Institutional Review Board (Quorum Review IRB, Seattle, Australia) approved the study protocol as shown in Figure 1.
FIG. 1.
Study design overview. This double-blind, placebo-controlled, parallel study included one telephone screen, two screening visits (days −14 and −7), a baseline visit (day 0), and two treatment visits (days 45 and 90). Subjects were randomly assigned one of three treatments: 600 mg spearmint extract, 900 mg spearmint extract, or placebo, which was consumed each day with breakfast over a 90-day treatment period. During baseline and treatment visits, subjects completed the CDR System™ computerized cognitive function test battery (days 0, 45, and 90, at −0.75, 0.5, 2, 4, and 6 h), computerized Profile of Mood States (days 0 and 90 only; at −0.75 h), and computerized Leeds Sleep Evaluation Questionnaire (days 0 and 90 only; at −0.75 h). After their predose assessments, subjects consumed a standardized breakfast and one dose of their assigned study product (0 h) followed by postdose computerized CDR test battery. CDR, Cognitive Drug Research. Figure modified from Lasrado et al.27 and reproduced with permission.
Subjects
Generally healthy men and women were recruited by the Biofortis Clinical Research team by using an established database of volunteers and local advertisements. Study visits were conducted in the clinical research center in Addison, IL, and eligible participants were identified based on the inclusion and exclusion criteria outlined in Table 1.
Table 1.
Subject Inclusion/Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 50–70 Years of age | Uncontrolled hypertension |
| Body–mass index between 18.5 and 35.0 kg/m2 | Abnormal laboratory test results of clinical significance |
| Possessing at least a high school diploma | History or presence of cancer, except nonmelanoma skin cancer |
| Subjects with AAMI were eligible based on the following National Institute of Mental Health criteria,1,45 specified as scoring: ≥25 on the MAC-Q46 ≤29 or ≤9 on the VPA I and II portions of the Wechsler Memory Scale IV, respectively47 ≥24 on the MMSE48 |
History or presence of clinically important cardiac, renal, hepatic, endocrine, pulmonary, biliary, gastrointestinal, pancreatic, or neurologic disorders (including sleep disorders, head injuries, Alzheimer's disease, Parkinson's disease, stroke, inflammatory brain disease) |
| Willing to maintain their habitual diet and exercise routines | History within previous 12 months of alcohol or substance abuse |
| Willing to maintain consistent sleep duration the evening before study visits | History of depression within past 24 months or use of psychotropic medications within 1 month of screening |
| History of heavy smoking (>1 pack/day) within past 3 months | |
| History of heavy caffeinated beverage consumption (>400 mg caffeine/day) within past 2 weeks | |
| Women who were pregnant, lactating, or planning to be pregnant during the study period or of childbearing potential and unwilling to use a medically approved form of contraception | |
| Occupations that resulted in disruption of sleep-wake cycles | |
| Use of medications or supplements known to alter cognitive function within past 2 weeks | |
| Inability to complete or understand the cognitive function practice tests |
AAMI, age-associated memory impairment; MAC-Q, Memory Assessment Clinic Scale Questionnaire; MMSE, Mini Mental State Examination; VPA, Verbal Paired Associates.
Study product and treatment
The water-extracted dry proprietary spearmint (M. spicata L. [aerial parts]) extract contained a minimum of 14.5% RA and 24% total polyphenols (Kemin Foods, L.C., Des Moines, IA).20,27–29 In addition, a compositional analysis of this spearmint extract revealed the presence of other polyphenols and their derivatives, including, but not limited to, RA, lithospermic, caftaric, and salvianolic A and B acids.29 Study capsules contained microcrystalline cellulose (placebo), or 300 or 450 mg of the spearmint extract (Five-Star Pharmacy, Clive, IA). Subjects were instructed to consume two capsules with breakfast equivalent to 0, 600, or 900 mg/day of extract for 90 days. Dose levels were identified based on previous preclinical and clinical studies.22,24,28 Compliance was calculated as a percentage of study product consumed according to the returned quantity of study product and confirmed by a diary that subjects completed daily.
Cognitive assessments
Cognitive performance was assessed by using the Cognitive Drug Research (CDR) System™ (Bracket, LLC, Wayne, PA). This computerized testing battery is validated for populations with AAMI and has been shown to be sensitive to acute and chronic nutritional interventions.4,10,30 The entire assessment was completed on laptop computers, with the exception of the word recall test, which was completed on paper. The cognitive performance assessment was administered at −0.75, 0.5, 2, 4, and 6 h, where t = 0 h was the time of product consumption at each visit (days 0, 45, and 90) based on previously published studies evaluating botanical extracts in both humans and preclinical mouse models of accelerated aging.4,12,22 Practice sessions, allowing each subject to plateau, were completed at screening to overcome any initial practice effects.31 In addition, parallel forms of the test were administered at each session to reduce training effects.32
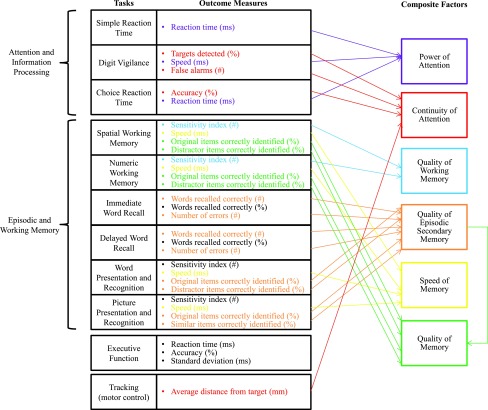
The assessment system consisted of 11 individual tasks to assess attention and information processing, episodic and working memory, executive function, and motor control. The tasks were performed in the following order: immediate word recall, simple reaction time, digit vigilance, choice reaction time, numeric working memory, spatial working memory, delayed word recall, word recognition, picture recognition, tracking, and executive function. These tasks have been previously described in detail,4 with the exception of the executive function task. The executive function task requires judgments about the value or number of digits in identical digit strings. Each battery took 30–45 min and was completed in an environment where temperature, lighting, and noise were controlled. Composite factors were calculated by combining individual task outcome scores as previously described and depicted in Figure 2.4
FIG. 2.
Cognitive performance assessments diagram. The computerized cognitive performance test battery (CDR System; Bracket, LLC, Wayne, PA) is summarized, indicating the tasks, outcome measures from each task, and the outcome measures that contribute to the composite factors. CDR, Cognitive Drug Research. Figure modified from Pengelly et al.12 and reproduced with permission.
Mood assessments
The Profile of Mood States (POMS™ Standard Form) questionnaire was also incorporated into the computerized assessment system and administered at days 0 and 90 during the −0.75 h assessment to evaluate chronic differences in mood.33 Ratings were combined into six factor composites: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, confusion-bewilderment, and a total mood disturbance (TMD) score.33
Sleep assessment
The Leeds Sleep Evaluation Questionnaire (LSEQ) was incorporated into the computerized assessment system and administered at days 0 and 90 at the −0.75 h timepoint. This validated questionnaire is a subjective evaluation of sleep and results in four domains: ease of getting to sleep, quality of sleep, awakening from sleep, and behavior following wakefulness.34
Study procedures
Eligible subjects arrived at the clinic in the morning for each test day (days 0, 45, and 90) after fasting (10–14 h) and body weight, compliance, and vital signs were assessed. Subjects then completed the CDR test battery, POMS questionnaire (days 0 and 90 only), and LSEQ (days 0 and 90 only) at the timepoints previously described. After that, subjects consumed a standard breakfast meal (670 kcal: 59% carbohydrates, 20% protein, 21% fat) with the assigned treatment. Subjects were asked to avoid vigorous physical activity (24 h), alcoholic beverages (24 h), caffeine (10–14 h), and tobacco use (1 h) before and for the duration of all test visits.
Statistical analysis
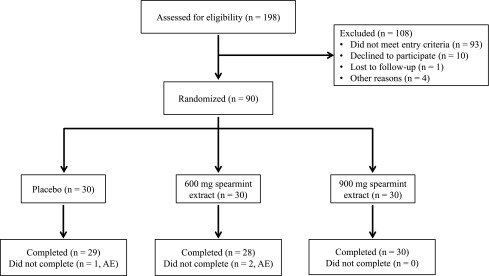
Sample size calculations were performed to test the difference between each active treatment and placebo. An evaluable sample size of 27 subjects per group was expected to provide 80% power (two-sided, α = 0.05) based on ability to detect a 10% difference in quality of memory or power of attention, independent domains of cognitive function utilizing standard deviations from the CDR in healthy individuals older than 65 years of age after Salvia Officinalis supplementation.10 A sample of 30 subjects per group (N = 90) was randomized to account for attrition and noncompliance (Fig. 3). Randomized subjects were stratified based on smoking status.
FIG. 3.
Study flow diagram. A total of 198 subjects were screened. Men and women with age-associated memory impairment were randomly assigned to one of three treatments: 0 (placebo), 600, or 900 mg/day spearmint extract (N = 90; n = 30/group). A total of 29, 28, and 30 subjects completed the trial in the placebo, 600 mg/day spearmint extract, and 900 mg/day spearmint extract groups, respectively. Three subjects withdrew from the study due to adverse events, including knee pain, myalgia, headache, worsening of oily scalp, cystic acne, and heartburn. All adverse events were deemed “not related” with the exception of heartburn, reported by one subject in the 600 mg/day spearmint extract group, which was deemed “probably related” to study product consumption. All available data were included in the intent-to treat population (n = 28–30/group). AE, adverse event(s). Figure modified from Lasrado et al.27 and reproduced with permission.
Statistical analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, NC) on the intent-to-treat (ITT) population, which included all subjects who were randomized into the study, consumed at least one dose of study product, and had at least one post-randomization outcome data point. Baseline comparability of demographic variables and all other outcomes were assessed by the analysis of variance model. Changes from baseline (day 0) for cognitive performance were evaluated by using daily averages of scores at −0.75, 0.5, 2, 4, and 6 h of each visit. For outcome measures administered once on a given test day (POMS and LSEQ), change from baseline (day 0) was used for comparisons. Change scores were evaluated by a mixed-model repeated-measures analysis containing the between-group effect of treatment, the repeated-measures effect of time, and the treatment × time interaction. All tests of significance were completed at α = 0.05, two-tailed without adjustment for multiple comparisons; thus, the possibility of Type I error exists.
Results
Subjects
Subjects were recruited between August 2013 and January 2014 (Fig. 3), with baseline characteristics presented in Table 2. Compliance was 98.1% ± 0.9%, 99.1% ± 0.8%, and 100.1% ± 0.4% for the placebo, 600 mg/day spearmint extract, and 900 mg/day spearmint extract groups, respectively. There were no statistically significant differences in compliance between treatment groups over the 90-day period. A total of 198 subjects were screened; 90 subjects were randomized to treatment, consumed at least 1 dose of study product, and had at least 1 post-randomization outcome data point that constituted the ITT population (Fig. 3). Three participants did not complete the study due to reported adverse events. Complete results from the safety assessments are published elsewhere.27
Table 2.
Baseline Characteristics of Subjects in the Intent-to-Treat Population
| Parameter | Overall | Placebo | 600 mg Spearmint extract | 900 mg Spearmint extract | p* |
|---|---|---|---|---|---|
| Gender, n (%) | 0.4581 | ||||
| Male | 30 (33) | 9 (30) | 8 (27) | 13 (43) | |
| Female | 60 (67) | 21 (70) | 22 (73) | 17 (57) | |
| Ethnicity, n (%) | 0.6125 | ||||
| Hispanic/Latino | 5 (6) | 1 (3) | 1 (3) | 3 (10) | |
| Non-Hispanic/Latino | 85 (94) | 29 (97) | 29 (97) | 27 (90) | |
| Race, n (%) | 0.3453 | ||||
| Non-Hispanic White | 81 (90) | 27 (90) | 25 (83) | 29 (97) | |
| Black/African American | 8 (9) | 3 (10) | 4 (13) | 1 (3) | |
| Asian/Pacific Islander | 1 (1) | 0 (0) | 1 (3) | 0 (0) | |
| Smoking status, n (%)a | 0.9565 | ||||
| Nonsmoker | 51 (57) | 17 (57) | 18 (60) | 16 (53) | |
| Current smoker | 5 (6) | 1 (3) | 2 (7) | 2 (7) | |
| Past smoker | 34 (38) | 12 (40) | 10 (33) | 12 (40) | |
| Age (years), mean (SEM) | 59.4 (0.6) | 58.2 (1.2) | 59.1 (1.0) | 60.8 (1.0) | 0.2482 |
| Body–mass index (kg/m2), mean (SEM) | 26.9 (0.4) | 25.9 (0.7) | 27.1 (0.7) | 27.9 (0.7)b | 0.1368 |
| SBP (mm Hg), mean (SEM) | 121.8 (1.3) | 120.1 (2.3) | 121.1 (2.5) | 124.3 (2.2) | 0.4150 |
| DBP (mm Hg), mean (SEM) | 74.7 (0.9) | 73.2 (1.2) | 73.1 (1.8) | 77.7 (1.6)c | 0.0680 |
| Heart rate (bpm), mean (SEM) | 65.1 (1.0) | 63.1 (1.2) | 64.8 (1.0) | 67.4 (1.8) | 0.1760 |
| MAC-Q score, mean (SEM) | 29.2 (0.3) | 29.1 (0.6) | 29.2 (0.6) | 29.1 (0.5) | 0.9697 |
| MMSE score, mean (SEM) | 28.5 (0.2) | 28.5 (0.3) | 28.6 (0.3) | 28.3 (0.3) | 0.4746 |
| VPA I score, mean (SEM) | 23.7 (0.7) | 23.2 (1.4) | 24.2 (1.3) | 23.8 (0.9) | 0.8886 |
| VPA II score, mean (SEM) | 7.0 (0.2) | 7.0 (0.3) | 6.6 (0.4) | 7.2 (0.3) | 0.5187 |
Participant smoking status was defined as nonsmokers, current smokers (current use or use ≤6 months before screening), and past smokers (use >6 months before screening).
Although the overall comparison of all three groups was not significant, body–mass index (placebo vs. 900 mg/day spearmint extract) did reach significance (p = 0.0482).
Although the overall comparison of all three groups was not significant for DBP, placebo versus 900 mg spearmint did reach significance (p = 0.048).
p-Values for the overall comparison were generated from an analysis of variance model without adjustments for multiple comparisons (n = 30/group and an overall N = 90).
bpm, beats per minute; DBP, diastolic blood pressure; MAC-Q, Memory Assessment Clinic Scale Questionnaire; MMSE, Mini-Mental State Exam; SBP, systolic blood pressure; SEM, standard error of the mean; VPA, Verbal Paired Associates.
Cognitive assessments
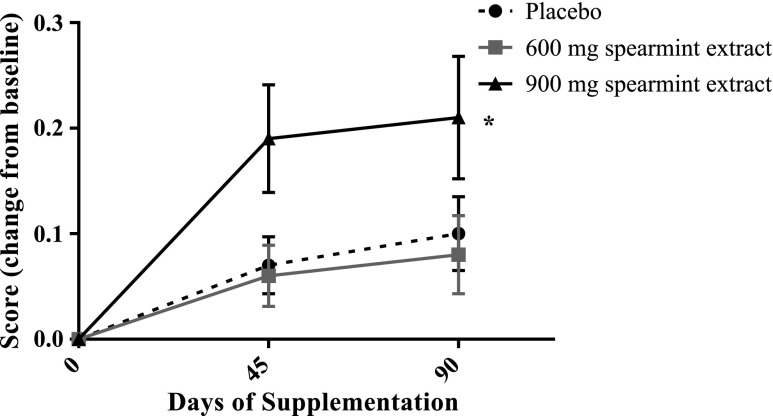
Composite factor scores on each test day are included in Table 3. The CDR battery identified an overall treatment effect in quality of working memory [F(2,84) = 3.25, p = 0.0435] for subjects supplemented for 90 days with spearmint extract (Fig. 4). Pairwise comparisons of the change from baseline indicated that subjects who consumed 900 mg/day extract had improved scores compared with subjects who consumed either 600 mg/day [t(84) = −2.35, p = 0.0212, d = 0.546] extract or placebo [t(84) = 2.02, p = 0.0469, d = 0.473]. The improvement in the 900 mg/day spearmint extract group over the 90-day supplementation period was 22% compared with 5% and 7% in the 600 mg/day and placebo groups, respectively. Similarly, there was an overall treatment effect after 90 days of spearmint extract supplementation [F(2,84) = 3.42, p = 0.0373] in spatial working memory (distractor items correctly identified, Fig. 5). This improvement from baseline was significantly greater for subjects consuming 900 mg/day (17%) extract than subjects consuming either 600 mg/day [3%, t(84) = −2.43, p = 0.0172, d = 0.563] extract or placebo [6%, t(84) = 2.03, p = 0.0456, d = 0.483] over the 90-day supplementation period. No additional statistically significant cognitive effects were detected.
Table 3.
Cognitive Composite Factor Scores Before and After 45 and 90 Days of Supplementation with Spearmint Extract
| Placebo | 600 mg Spearmint extract | 900 mg Spearmint extract | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 45 | Day 90 | Day 0 | Day 45 | Day 90 | Day 0 | Day 45 | Day 90 | |||
| Composite factors | Mean score (SEM) | p* | Directiona | ||||||||
| Power of attention (msec) | 1275.56 (26.46) |
1291.00 (25.90) |
1308.77 (28.48) |
1308.11 (22.23) |
1338.47 (24.73) |
1345.09 (25.22) |
1278.83 (16.87) |
1307.07 (17.85) |
1316.91 (21.11) |
0.9312 | ↑ |
| Continuity of attention (No.) | 66.11 (3.00) |
65.51 (3.13) |
67.80 (2.95) |
67.50 (2.17) |
67.86 (2.26) |
70.35 (1.60) |
66.86 (2.19) |
68.80 (1.68) |
68.38 (2.67) |
0.8881 | ↑ |
| Quality of working memory (No.) | 1.72 (0.04) |
1.79 (0.03) |
1.82 (0.03) |
1.77 (0.03) |
1.83 (0.03) |
1.84 (0.02) |
1.61 (0.06) |
1.80 (0.03) |
1.81b (0.02) |
0.0435 | ↑ |
| Quality of episodic secondary memory (No.) | 126.69 (5.88) |
143.06 (6.06) |
133.64 (9.16) |
133.33 (5.29) |
143.18 (5.98) |
139.91 (6.20) |
127.89 (7.28) |
137.83 (7.37) |
129.25 (7.80) |
0.8082 | ↑ |
| Speed of memory (msec) | 3829.22 (109.90) |
3681.86 (122.25) |
3564.54 (111.07) |
3824.15 (104.82) |
3679.49 (108.44) |
3559.19 (93.16) |
3939.63 (123.86) |
3740.41 (102.85) |
3653.68 (99.13) |
0.6460 | ↑ |
| Quality of memory (No.) | 298.38 (8.30) |
321.51 (8.51) |
315.54 (11.15) |
310.20 (6.71) |
325.22 (7.28) |
323.66 (7.29) |
288.19 (10.33) |
316.93 (8.73) |
309.93 (9.01) |
0.2296 | ↑ |
Bold values indicate overall treatment effects, p < 0.05.
The computerized cognitive performance battery (CDR System™; Bracket, LLC) was administered at baseline (day 0) and after 45 and 90 days of spearmint extract or placebo supplementation.
The arrow notes the direction of change that suggests improvement in the associated outcome.
900 mg/day spearmint extract versus placebo, p = 0.0469; 900 mg/day spearmint extract versus 600 mg/day spearmint extract, p = 0.0212.
p-Values for the overall and pairwise comparisons in the intent-to-treat sample were generated from a mixed-model repeated-measures analysis of variance model based on the average daily difference from baseline (n = 28–30/group).
CDR, Cognitive Drug Research; SEM, standard error of the mean.
FIG. 4.
Quality of working memory scores after 90 days of spearmint supplementation. Supplementation with the spearmint extract at 900 mg/day resulted in improved (overall treatment effect, *p = 0.0435) quality of working memory versus subjects consuming either placebo (p = 0.0469) or 600 mg/day (p = 0.0212). Data are expressed as mean difference from baseline (day 0) for the daily averages ± standard error of the mean (n = 28–30/group).
FIG. 5.
Spatial working memory distractor items correctly identified after 90 days of spearmint supplementation. Subjects supplemented for 90 days with spearmint extract at 900 mg/day had improved (overall treatment effect, *p = 0.0373) accuracy of spatial working memory versus subjects consuming either placebo (p = 0.0456) or 600 mg/day spearmint extract (p = 0.0172). Data shown are the mean difference from baseline (day 0) for the daily averages ± standard error of the mean (n = 28–30/group).
Mood
Administration of the computerized POMS questionnaire identified significant treatment effects in the vigor-activity factor [F(2,82) = 3.35, p = 0.0399, Table 4] and the composite TMD [F(2,82) = 3.42, p = 0.0374] after 90 days of supplementation. However, significant differences in pairwise comparisons between either of the spearmint dose levels and placebo were not evident. A trend for improvement after 900 mg spearmint, relative to placebo, was observed in the vigor-activity factor [t(82) = 1.87, p = 0.646, d = 0.502]. Further, a significant effect in vigor-activity was also evident for subjects administered the 900 versus 600 mg/day dose level of spearmint extract [t(82) = −2.49, p = 0.0149, d = 0.729]. No additional significant findings were observed in the other individual mood parameters from the POMS.
Table 4.
Subjective Ratings of Mood, from the Profile of Mood States Questionnaire, Before and After 90 Days of Supplementation with Spearmint Extract
| Placebo | 600 mg Spearmint extract | 900 mg Spearmint extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 90 | Day 0 | Day 90 | Day 0 | Day 90 | |||
| Mood factors | Mean rating (SEM) | p* | Directiona | |||||
| Tension-Anxiety | 3.53 (0.53) | 3.48 (0.51) | 4.13 (0.59) | 4.39 (0.95) | 6.10 (0.89) | 5.04 (0.62) | 0.3411 | ↓ |
| Depression-Dejection | 1.80 (0.42) | 2.10 (0.59) | 1.30 (0.48) | 2.11 (0.66) | 3.83 (1.14) | 2.32 (0.79) | 0.0862 | ↓ |
| Anger-Hostility | 0.73 (0.35) | 0.86 (0.28) | 0.53 (0.27) | 1.25 (0.54) | 2.07 (0.58) | 1.54 (0.48) | 0.1935 | ↓ |
| Vigor-Activity | 17.47 (1.26) | 18.07 (1.30) | 16.80 (1.42) | 16.11 (1.66) | 15.90 (1.27) | 19.82 (1.17)b | 0.0399 | ↑ |
| Fatigue-Inertia | 3.40 (0.84) | 3.79 (0.93) | 2.50 (0.51) | 3.75 (0.79) | 4.30 (0.68) | 3.86 (0.79) | 0.2111 | ↓ |
| Confusion-Bewilderment | 5.63 (0.67) | 5.10 (0.73) | 4.40 (0.43) | 4.32 (0.53) | 5.37 (0.63) | 5.00 (0.67) | 0.8448 | ↓ |
| Total Mood Disturbance | 2.87 (1.93) | 3.38 (2.58) | 1.87 (1.50) | 6.07 (2.85) | 10.60 (3.49) | 5.04 (2.28)c | 0.0374 | ↓ |
Bold values indicate overall treatment effects, p < 0.05.
The Profile of Mood States (POMS™ Standard Form) questionnaire (65-item) was administered at baseline (day 0) and after 90 days of spearmint extract or placebo supplementation. Subjects were asked to rate how they had been feeling in the past week as follows: not at all (0), a little (1), moderately (2), quite a bit (3), extremely (4).
The arrow notes the direction of change that suggests improvement in the associated outcome.
900 mg/day spearmint extract versus placebo, p = 0.0646; 900 mg/day spearmint extract versus 600 mg/day spearmint extract, p = 0.0149.
900 mg/day spearmint extract versus placebo, p = 0.0832; 900 mg/day spearmint extract versus 600 mg/day spearmint extract, p = 0.0123.
p-Values for the overall and pairwise comparisons in the intent-to treat sample were generated from a mixed-model repeated-measures analysis of variance model based on the difference from baseline in mood ratings (n = 28–30/group).
SEM, standard error of the mean.
Sleep
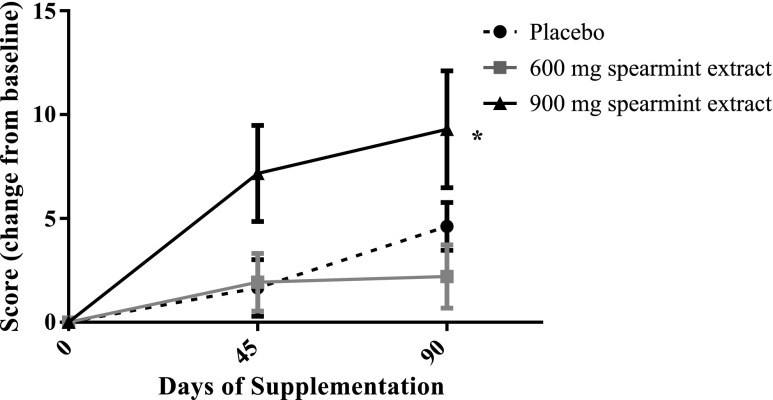
Subjects were administered the LSEQ to evaluate getting to sleep, quality of sleep, ease of awakening from sleep, and alertness and behavior following wakefulness (Table 5). An overall treatment effect [F(2,82) = 4.29, p = 0.0170] was evident in ratings of getting to sleep. Further, between-group comparisons showed that subjects consuming 900 mg/day spearmint extract reported improved ability to get to sleep versus subjects consuming placebo [t(82) = 2.91, p = 0.0046, d = 0.805]. An overall treatment effect was also observed for behavior following wakefulness [F(2,82) = 3.31, p = 0.0415]. The pairwise comparison suggests that subjects consuming spearmint extract at 900 mg/day had improved behavior following wakefulness relative to subjects consuming 600 mg/day of spearmint extract [t(82) = −2.52, p = 0.0137]. No significant differences were observed in quality of sleep or ease of awakening from sleep.
Table 5.
Subjective Ratings of Sleep, from Leeds Sleep Evaluation Questionnaire, Before and After 90 Days of Supplementation with Spearmint Extract
| Placebo | 600 mg Spearmint extract | 900 mg Spearmint extract | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Day 0 | Day 90 | Day 0 | Day 90 | Day 0 | Day 90 | ||
| Sleep factors (mm) | Mean rating (SEM) | p* | Directiona | |||||
| Ease of getting to sleep | 49.83 (0.95) | 45.48 (2.15) | 51.30 (0.87) | 50.36 (1.70) | 47.63 (0.91) | 51.11 (1.36)b | 0.0170 | ↑ |
| Quality of sleep | 50.27 (0.37) | 48.62 (3.20) | 50.97 (1.12) | 52.04 (2.23) | 49.83 (0.57) | 53.25 (1.98) | 0.4267 | ↑ |
| Ease of awakening from sleep | 49.40 (0.40) | 52.69 2.84) | 48.63 (1.29) | 50.54 (2.75) | 52.10 (1.31) | 54.14 (1.76) | 0.9016 | ↑ |
| Alertness and behavior after wakefulness | 52.20 (2.13) | 59.31 (2.89) | 55.03 (2.64) | 53.50 (3.44)c | 52.07 (1.83) | 63.46 (2.78) | 0.0415 | ↑ |
Bold values indicate overall treatment effects, p < 0.05.
The Leed's Sleep Evaluation Questionnaire was administered at baseline (day 0) and after 90 days of spearmint extract or placebo supplementation. Subjects were asked to rate aspects of sleep by using 100 mm visual analog scales flanked with antonyms (i.e., a rating of 50 mm is neutral).
The arrow notes the direction of change that suggests improvement in the associated outcome.
900 mg/day spearmint extract versus placebo, p = 0.0046; 900 mg/day spearmint extract versus 600 mg/day spearmint extract, p = 0.0879.
600 mg/day versus placebo, p = 0.0892; 600 mg/day versus 900 mg/day spearmint extract, p = 0.0137.
p-Values for the overall and pairwise comparisons in the intent-to-treat sample were generated from a mixed-model repeated-measures analysis of variance model based on the difference from baseline in ratings of sleep (n = 28–30/group).
SEM, standard error of the mean.
Discussion
This study shows that supplementation with this dried aqueous spearmint extract at 900 mg/day for 90 days significantly improved quality of working memory and spatial working memory versus placebo in healthy subjects with AAMI. Further, subjects taking the 900 mg daily dose also reported improvements in the ability to fall asleep and demonstrated trends for improved ratings from subjective mood assessments, including vigor-activity and TMD. These data corroborate initial findings from a single-arm trial in which supplementation with 900 mg/day of this extract for 30 days showed improvements in components of working memory.32
Working memory pertains to the ability to use and manipulate information stored within short-term memory. Current evidence indicates that working memory decreases with age, beginning in early adulthood.35 Wesnes reported a decrease in working memory of roughly 10% per decade after the age of 40 in healthy individuals (n = 2282; 18–87 years of age).36 Given this decrease with age, the improvements observed in the current study are promising as quality of working memory and spatial working memory improved by ∼15% and 9% over placebo, respectively. The quality of working memory score at day 0 was lower in the 900 mg/day spearmint group relative to the other treatments; however, evaluation of the change from baseline incorporates this value and suggests improvement over placebo. These data suggest that this extract could improve working memory equivalent to that which may have diminished over a decade of life. The identified improvements in working memory, a hippocampal-dependent task, are further supported by the previously reported reduction in oxidation status in the hippocampus of SAMP8 mice after spearmint extract supplementation.22
To our knowledge, this is the first randomized, double-blind, placebo-controlled study to evaluate the effects of a dried aqueous spearmint extract with high levels of RA (≥14.5%) on cognitive performance after chronic administration in a parallel design. There are indications that compounds present within plants of the Lamiaceae family could be beneficial for cognition as suggested by previous research. Extracts of rosemary (Rosmarinus officinalis L.), sage (Salvia officinalis L.), and lemon balm (Melissa officinalis L.), all members of the Lamiaceae family, have been shown to benefit secondary memory, quality of memory, and speed of memory, respectively, after acute dosing.10–12 However, there have been no published studies with similar plant extracts (aqueous or otherwise), which have reported working memory benefits after chronic supplementation. These previous studies reported acute benefits utilizing a crossover design after a single dose of the extracts.
Reduced sleep efficiency and quality have been correlated with cognitive decline that occurs with age.37 Therefore, the findings showing improvement in getting to sleep with 900 mg/day spearmint supplementation are notable given the age of the subjects (50–70 years) and the consistency of these effects with improvements in working memory. The reported differences in sleep ratings on the LSEQ after 90 days of supplementation for subjects taking 900 mg/day of extract were an average of 7.8 mm greater than the placebo group. This improvement is consistent with effects reported after administration of commonly used sleep aids typically administered before bed.38 While other studies have reported improved quality of sleep after supplementation with extracts from plants within the Lamiaceae family, these studies typically use a blend of extracts administered before bedtime.39,40 Corroboration of these novel findings for spearmint extract using more objective sleep measurement tools is warranted.
Mood may play a role in cognitive decline with aging41; therefore, the potential mood benefits were not surprising given the observed improvements in both working memory and sleep. There are limited data reporting the effects of plant extracts within the Lamiaceae family on mood after oral supplementation. Studies evaluating the effects of lemon balm (M. officinalis L.) extract on mood identified differences in calmness but inconsistent findings in alertness.11,42 In addition, administration of sage (Salvia lavandulaefolia and S. officinalis) in both oil and dried forms resulted in improved measures of calmness, alertness, and contentedness.43,44 A notable difference in the published studies on lemon balm and sage is the inclusion of young adults (18–30 years of age), crossover design, and acute administration. In contrast, the current study included older adults in a parallel design after chronic administration and identified improvements in vigor-activity and TMD.
The major strength of this study is the randomized, double-blind, placebo-controlled, parallel-group study design. Compliance with study product consumption was greater than 98% in all groups. The CDR testing battery utilized is validated for populations with AAMI and shown to be sensitive enough to detect the effects of acute and chronic nutritional interventions. The computerized tests were carried out in a parallel format to reduce practice effects, and training sessions allowed subjects to acclimate to the testing battery. Finally, subjects completed these tools in a supervised environment where lighting and noise were controlled.
The limitations of this study include potential confounding factors such as diet and lifestyle; however, to minimize these factors, subjects were asked to maintain their habitual diet, exercise routines, and sleep duration throughout the study. Cognitive outcomes measured at multiple time points on each test day were analyzed by using average daily values. One criticism of this approach is that utilization of daily averages may allow any acute effects from the predose assessment to confound the chronic analysis. However, since acute differences were not evident for the individual tasks or composite cognitive performance factors at any visit, to improve the reliability of the measurements, outcome scores were averaged across the −0.75, 0.5, 2, 4, and 6 h timepoints. Therefore, the differences measured and reported within this study best reflect what an individual might expect after a chronic dosing regimen. In addition, it is important to note that since this intervention included healthy older individuals, caution should be taken when extrapolating the observed benefits to those with more advanced stages of cognitive dysfunction.
In summary, healthy older subjects with AAMI taking 900 mg/day of the spearmint extract improved their working memory and self-reported ability to get to sleep. Taken together, these findings suggest that this dried aqueous spearmint extract, containing more than 50 phenolic compounds including higher RA relative to native spearmint species, shows promise as an ingredient for improved cognitive performance, sleep and possibly mood, all of which may improve quality of life.
Acknowledgments
The authors would like to thank Barbara Anderson, MS, RD, for her contribution to protocol development; Eleanor Espinosa, BS, and Tressa Mattioli-Lewis, MPH, for their dedication to the coordination of this trial; Kathleen M. Kelley, MD, and Andrea L. Lawless, MD, for their medical oversight; and the entire clinic staff at Biofortis Research Services/Merieux NutriSciences for their commitment to this study. The authors would also like to thank Jim Rogers, PhD, and Maria Molina at Summit Analytical, LLC, for their contribution to the study design and data analysis. Kemin Foods, L.C., funded this study.
Author Disclosure Statement
The authors acknowledge that they have either received research funding from or are employees of Kemin Foods, LC, the manufacturer of the product studied.
References
- 1.O'Brien JT. Age-associated memory impairment and related disorders. Adv Psychiatr Treat 1999;5:279–287 [Google Scholar]
- 2.Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep 2003;52:101–104, 106 [PubMed] [Google Scholar]
- 3.Scholey A, Ossoukhova A, Owen L, et al. . Effects of American ginseng (Panax quinquefolius) on neurocognitive function: An acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl) 2010;212:345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wesnes KA, Ward T, McGinty A, Petrini O. The memory enhancing effects of a Ginkgo biloba/Panax ginseng combination in healthy middle-aged volunteers. Psychopharmacology (Berl) 2000;152:353–361 [DOI] [PubMed] [Google Scholar]
- 5.Vakhapova V, Cohen T, Richter Y, et al. . Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: A double-blind placebo-controlled trial. Dement Geriatr Cogn Disord 2010;29:467–474 [DOI] [PubMed] [Google Scholar]
- 6.Dodd FL, Kennedy DO, Riby LM, Haskell-Ramsay CF. A double-blind, placebo-controlled study evaluating the effects of caffeine and l-theanine both alone and in combination on cerebral blood flow, cognition and mood. Psychopharmacology (Berl) 2015;232:2563–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naeini AM, Elmadfa I, Djazayery A, et al. . The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: A double-blind, randomized, placebo-controlled trial. Eur J Nutr 2014;53:1255–1262 [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Thomas RG, Grundman M, et al. . Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388 [DOI] [PubMed] [Google Scholar]
- 9.Haskell CF, Kennedy DO, Wesnes KA, et al. . A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guarana in humans. J Psychopharmacol 2007;21:65–70 [DOI] [PubMed] [Google Scholar]
- 10.Scholey AB, Tildesley NT, Ballard CG, et al. . An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology (Berl) 2008;198:127–139 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy DO, Wake G, Savelev S, et al. . Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology 2003;28:1871–1881 [DOI] [PubMed] [Google Scholar]
- 12.Pengelly A, Snow J, Mills SY, et al. . Short-term study on the effects of rosemary on cognitive function in an elderly population. J Med Food 2012;15:10–17 [DOI] [PubMed] [Google Scholar]
- 13.Fallarini S, Miglio G, Paoletti T, et al. . Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br J Pharmacol 2009;157:1072–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fale PL, Madeira PJ, Florencio MH, et al. . Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food Funct 2011;2:130–136 [DOI] [PubMed] [Google Scholar]
- 15.Mushtaq N, Schmatz R, Pereira LB, et al. . Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem Funct 2014;32:287–293 [DOI] [PubMed] [Google Scholar]
- 16.Vina J, Lloret A, Orti R, Alonso D. Molecular bases of the treatment of Alzheimer's disease with antioxidants: Prevention of oxidative stress. Mol Aspects Med 2004;25:117–123 [DOI] [PubMed] [Google Scholar]
- 17.Rocha J, Eduardo-Figueira M, Barateiro A, et al. . Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin Pharmacol Toxicol 2015;116:398–413 [DOI] [PubMed] [Google Scholar]
- 18.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res 2006;40:223–231 [DOI] [PubMed] [Google Scholar]
- 19.Lee YW, Kim DH, Jeon SJ, et al. . Neuroprotective effects of salvianolic acid B on an Abeta25–Abeta35 peptide-induced mouse model of Alzheimer's disease. Eur J Pharmacol 2013;704:70–77 [DOI] [PubMed] [Google Scholar]
- 20.Narasimhamoorthy B, Zhao LQ, Liu W, et al. . Differences in the chemotype of two native spearmint clonal lines selected for rosmarnic acid accumulation in comparison to commercially grown native spearmint. Ind Crops Prod 2015;63:87–91 [Google Scholar]
- 21.Shekarchi M, Hajimehdipoor H, Saeidnia S, et al. . Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn Mag 2012;8:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farr SA, Niehoff ML, Ceddia MA, et al. . Effect of botanical extracts containing carnosic acid or rosmarinic acid on learning and memory in SAMP8 mice. Physiol Behav 2016;165:328–338 [DOI] [PubMed] [Google Scholar]
- 23.Axmacher N, Lenz S, Haupt S, et al. . Electrophysiological signature of working and long-term memory interaction in the human hippocampus. Eur J Neurosci 2010;31:177–188 [DOI] [PubMed] [Google Scholar]
- 24.Nieman KM, Sanoshy KD, Bresciani L, et al. . Tolerance, bioavailability, and potential cognitive health implications of a distinct aqueous spearmint extract. Funct Foods Health Dis 2015;5:165–187 [Google Scholar]
- 25.World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2000;284:3043–3045 [PubMed] [Google Scholar]
- 26.United States Department of Health and Human Services. 21CFR50: Protection of human subjects. 2013. Online document at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm Accessed May10, 2017
- 27.Lasrado JA, Nieman KM, Fonseca BA, et al. . Safety and tolerability of a dried aqueous spearmint extract. Regul Toxicol Pharmacol 2017;86:167–176 [DOI] [PubMed] [Google Scholar]
- 28.Lasrado JA, Trinker D, Ceddia MA, Herrlinger KA. The safety of a dry spearmint extract in vitro and in vivo. Regul Toxicol Pharmacol 2015;71:213–224 [DOI] [PubMed] [Google Scholar]
- 29.Cirlini M, Mena P, Tassotti M, et al. . Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016;21:pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wesnes KA, Simpson PM, White L, Pinker S. The Cognitive Drug Research computerized assessment systems for elderly, AAMI & demented patients. J Psychopharmacol 1992;6:108. [DOI] [PubMed] [Google Scholar]
- 31.Wesnes K, Pincock C. Practice effects on cognitive tasks: A major problem? Lancet Neurol 2002;1:473. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt JA, Benton D, Kallus KW. General methodological considerations for the assessment of nutritional influences on human cognitive functions. Eur J Nutr 2005;44:459–464 [DOI] [PubMed] [Google Scholar]
- 33.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service, 1992 [Google Scholar]
- 34.Tarrasch R, Laudon M, Zisapel N. Cross-cultural validation of the Leeds Sleep Evaluation Questionnaire (LSEQ) in insomnia patients. Hum Psychopharmacol 2003;18:603–610 [DOI] [PubMed] [Google Scholar]
- 35.Park DC, Lautenschlager G, Hedden T, et al. . Models of visuospatial and verbal memory across the adult life span. Psychol Aging 2002;17:299–320 [PubMed] [Google Scholar]
- 36.Wesnes K. The Cognitive Drug Research computerised assessment system: Application to clinical trials. In: de Deyn P, Thiery E, D'Hooge R, eds. Memory: Basic Concepts, Disorders and Treatment. Leuven: Uitgeverij Acco, 2003:453–472 [Google Scholar]
- 37.Blackwell T, Yaffe K, Laffan A, et al. . Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: The MrOS sleep study. Sleep 2014;37:655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zisapel N, Nir T. Determination of the minimal clinically significant difference on a patient visual analog sleep quality scale. J Sleep Res 2003;12:291–298 [DOI] [PubMed] [Google Scholar]
- 39.Cerny A, Schmid K. Tolerability and efficacy of valerian/lemon balm in healthy volunteers (a double-blind, placebo-controlled, multicentre study). Fitoterapia 1999;70:221–228 [Google Scholar]
- 40.Taavoni S, Nazem Ekbatani N, Haghani H. Valerian/lemon balm use for sleep disorders during menopause. Complement Ther Clin Pract 2013;19:193–196 [DOI] [PubMed] [Google Scholar]
- 41.Granholm AC, Boger H, Emborg ME. Mood, memory and movement: An age-related neurodegenerative complex? Curr Aging Sci 2008;1:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy DO, Scholey AB, Tildesley NT, et al. . Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol Biochem Behav 2002;72:953–964 [DOI] [PubMed] [Google Scholar]
- 43.Tildesley NT, Kennedy DO, Perry EK, et al. . Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav 2005;83:699–709 [DOI] [PubMed] [Google Scholar]
- 44.Kennedy DO, Pace S, Haskell C, et al. . Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology 2006;31:845–852 [DOI] [PubMed] [Google Scholar]
- 45.Crook T, Bartus RT, Ferris SH, et al. . Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change—Report of a national institute of mental health work group. Dev Neuropsychol 1986;2:261–276 [Google Scholar]
- 46.Crook TH, 3rd, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: The MAC-Q. Int Psychogeriatr 1992;4:165–176 [DOI] [PubMed] [Google Scholar]
- 47.Wechsler D. Wechsler Memory Scale—Fourth Edition. San Antonio, TX: Pearson, 2009 [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]