Abstract
Background
Membranous nephropathy (MN) represents a distinct glomerular disease which has been considered as a major cause of nephrotic syndrome (NS) in adults. Evidences show that the clinicopathological features of MN are various among MN cases. This study aimed to summarize and analyze the clinicopathological features of patients with MN.
Methods
A total of 231 MN patients were recruited in this study. Their clinical and pathological features were collected and analyzed according to their age, gender, pathological stages, and anti-phospholipase A2 receptor (anti-PLA2R) antibodies tests.
Results
Among the 231 MN cases, the ratio of male to female was 1.47 and the mean age was 47.43±14.32 years. Altogether, 163 (70.6%) cases were positive for NS. Their serum antiPLA2R, body mass index, total cholesterol, triglyceride, low density lipoprotein cholesterol, D2, IgA, and IgE were increased, but IgG was decreased. The majority of the patients were middle aged and old aged. In addition, the pathological stage was significantly correlated with gender (P=0.038), creatinine, (P=0.021) and IgE (P=0.003). A total of 74.9% MN patients were found to be positive for anti-PLA2R antibodies, and they were more likely to have abnormal serum indices.
Conclusion
The major clinicopathological characteristics of MN patients are summarized in this study. Male and elder MN cases are likely to have rapid disease progression. Advanced pathological stages and being positive for anti-PLA2R antibodies may be potential indicators for disease activity of MN.
Keywords: pathological features, clinical manifestation, membranous nephropathy
Introduction
Membranous nephropathy (MN) represents a distinct glomerular disease.1 It has been considered as one of the most frequent types of nephrotic syndrome (NS) among adults worldwide, accounting for approximately 25%–40% of all the adults with NS.2,3 MN can be classified into primary MN, also known as idiopathic MN (iMN), and secondary MN (sMN) based on different pathogenesis, and the most common subtype is iMN, accounting for approximately 75% of MN cases.4 sMN could be induced by various conditions, such as malignancies (breast, ovarian, lung, and stomach cancers, and lymphoproliferative disorders), infections (human immunodeficiency virus, malaria, hepatitis B and C viruses), some systemic autoimmune diseases (rheumatoid arthritis and lupus), and the consumption of drugs and toxins.5,6 MN is characterized by non-selective proteinuria, with the nephrotic range of more than 3.5 g/day.7 Patients diagnosed with MN have a high risk of developing end-stage renal disease. Therefore, further understanding of the clinicopathological characteristics and disease progression is crucial for diagnosis and treatment of MN.
According to the statistics, the clinical manifestation of MN is variable among MN cases, as well as in the disease prognosis.8 Data have revealed that MN is common in middle and old age groups with the peak age of 31–60 years.9 The incidence of MN is different between men and women, and the ratio of male to female is approximately 2:1.10 In previous studies, some typical clinical manifestations have been identified in patients with MN, such as NS, edema, hypertension, renal failure, and microscopic hematuria.11 According to the guidelines of pathology, MN cases can be divided into four pathological stages (stages I, II, III, and IV).12,13 Renal dysfunction can usually be detected in patients who have had MN for 5–10 years and pathological stage III or IV.14 Given the diverse clinicopathological characteristics of MN patients, a better understanding of these features is necessary for MN detection and treatment.
In the present study, we retrospectively analyzed the clinicopathological features of patients with MN.
Ethics statement
The research of investigating clinical and laboratory data of in-hospital patients for “Analysis of clinicopathological features for patients with membranous nephropathy” was a retrospective study and has been evaluated; it was confirmed that the protocols were conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Chinese PLA General Hospital. Signed informed consents were obtained from all the participants and their families. All of the clinicopathological features and personal information were made anonymous in the current study.
Methods
Patients
A total of 231 patients who were diagnosed with MN at Chinese PLA General Hospital from January 1, 2013 to March 15, 2016 were enrolled in this study. The diagnoses were based on the pathological evaluation of percutaneous renal biopsy under B ultrasound guidance. Primary MN was identified without secondary causes based on clinical evaluations, such as lupus, chronic infection, tumors, and drug exposure. Moreover, the primary MN individuals were negative for antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, and hepatitis virus tests. If the collected patients presented the following clinical symptoms, they were confirmed as having NS: heavy proteinuria: ≥50 mg/kg/day (or ≥40 mg/m2/h), or a proteinuria/creatininuria ratio >2 (mg/mg); serum ALB <25 g/L; edema. The medical history, clinical and pathological data of the patients were routinely recorded at biopsy. The pathological stage of the patients was also evaluated by two independent pathologists. In addition, the clinical characteristics of the collected patients were also recorded, including age, gender, blood pressure, urine sugar, and body mass index (BMI). We collected the basic characteristics of the patients at biopsy from the medical records. Signed informed consents were obtained from all the participants or their families.
Data collection
All of the patients underwent percutaneous renal biopsy with the guidance of ultrasound, and the collected renal samples were embedded in paraffin. The pathological stage of the renal samples was evaluated by two independent pathologists following the methods detailed by Churg and Ehrenreich.15 Nineteen clinical parameters were tested from the urine and serum specimens, including anti-phospholipase A2 receptor (anti-PLA2R) antibodies, ALT, AST, total protein (TP), ALB, total bilirubin (TB), direct bilirubin (DB), ALP, GGT, glucose (GLU), urea nitrogen (UN), creatinine (Cr), uric acid (Ua), total cholesterol (TC), triglyceride (TG), CK, LDH, high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), and D2. Moreover, immunofluorescence assay was carried out to detect the serum IgA, IgG, IgM, IgE, C3, and C4. For these analyses, Hitachi 7500 electron microscope was adopted for the electron microscopy observations.
Statistical analysis
All the data used were expressed as mean ± SD, and the statistical analyses were conducted with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The comparison for continuous variables between two groups were carried out by Student’s t-test, while chi-square test was used to analyze the categorical variables between two groups. P<0.05 was considered as statistically significant.
Results
General data and clinical manifestation of the MN patients
A total of 231 MN patients including 219 with primary MN and 12 with sMN were included in our study. The sMN cases were all caused by viral hepatitis infection. There were 135 males and 96 females with the ratio of male to female being 1.47. The mean age of the patients was 47.43±14.32 years (age range of 16–79 years). As shown in Figure 1, most of the patients were 41–59 years old, accounting for 45% of the patients. A total of 76 patients (33%) were ≤40 years, and 52 cases (22%) were ≥60 years. Among all these patients, 82 (35.5%) cases had hypertension and 28 (12.1%) had diabetes, while 163 (70.6%) patients suffered from NS. The average results of systolic and diastolic blood pressure were 132.39±22.18 and 81.85±12.21 mmHg, respectively (Table 1).
Figure 1.
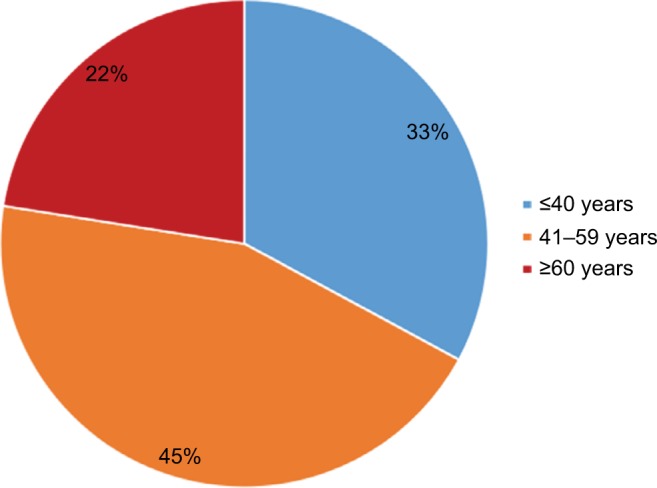
Membranous nephropathy patients stratified according to age.
Note: The majority of the patients were middle and old aged.
Table 1.
Summary of the clinicopathological features of MN patients
| Parameters | Results | Reference range |
|---|---|---|
| Age (years) | 47.43±14.32 | – |
| Gender (male : female) | 135:96 | – |
| Blood pressure (mmHg) | 132.39±22.18/81.85±12.21 | – |
| NS | 163 (70.6%) | – |
| BMI (Kg/m2) | 25.87±4.75 | 18.5–24.9 |
| anti-PLA2R (RU/mL) | 95.45±165.37 | – |
| PLA2R positive (n, %) | 173 (74.9%) | – |
| ALT (U/L) | 21.08±16.48 | 0–40 |
| AST (U/L) | 18.60±9.29 | 0–40 |
| TP (g/L) | 49.44±10.02 | 55–80 |
| ALB (g/L) | 26.92±6.98 | 35–50 |
| TB (µmol/L) | 7.64±3.52 | 0–21 |
| DB (µmol/L) | 1.46±1.14 | 0–8.6 |
| ALP (U/L) | 59.14±16.68 | 0–130 |
| GGT (U/L) | 34.94±48.22 | 0–50 |
| GLU (mmol/L) | 4.95±1.24 | 3.4–6.2 |
| UN (mmol/L) | 5.22±2.42 | 1.8–7.5 |
| Cr (µmol/L) | 75.10±24.66 | 30–110 |
| Ua (µmol/L) | 346.97±95.84 | 104–444 |
| TC (mmol/L) | 7.07±2.34 | 3.1–5.7 |
| TG (mmol/L) | 2.58±1.90 | 0.4–1.7 |
| CK (U/L) | 102.65±139.16 | 2–200 |
| LDH (U/L) | 193.60±57.98 | 40–250 |
| HDL (mmol/L) | 1.44±0.52 | 1–1.6 |
| LDL (mmol/L) | 4.85±2.03 | 0–3.4 |
| D2 (µg/L) | 1.26±2.28 | 0.0–0.5 |
| IgA (mg/dL) | 215.41±84.18 | 70–180 |
| IgG (mg/dL) | 625.48±282.06 | 700–1600 |
| IgM (mg/dL) | 116.30±78.30 | 40–230 |
| IgE (mg/dL) | 126.13±188.48 | 0–100 |
| C3 (mg/dL) | 114.86±24.32 | 90–180 |
| C4 (mg/dL) | 28.19±9.83 | 10–40 |
| Diabetes | 28 (12.1%) | – |
| Viral hepatitis | 12 (5.2%) | – |
| Hepatitis B | 10 (4.3%) | – |
| Hepatitis C | 2 (0.9%) | – |
| Pathological stage (III) | 10 (4.3%) | – |
Notes: –, no data. Data are presented as mean ± SD and n (%). Bold data is statistically significant.
Abbreviations: BMI, body mass index; TC, total cholesterol; Cr, creatinine; DB, direct bilirubin; GLU, glucose; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; MN, membranous nephropathy; NS, nephrotic syndrome; TB, total bilirubin; TG, triglyceride; TP, total protein; Ua, uric acid; UN, urea nitrogen.
All the clinical parameters were summarized in Table 1. According to the analyses in renal tissues and blood samples, we found increased serum anti-PLA2R antibodies with a mean concentration of 95.45±165.37, and 173 (74.9%) MN cases were positive to anti-PLA2R antibodies test. Compared to the normal reference range, the BMI, TC, TG, LDL, D2, IgA, and IgE were increased, but the IgG was decreased in patients with MN. Other clinical features were distributed within the normal range in all the patients with MN.
Effects of age on clinicopathological features of patients with MN
All the patients were classified into two groups based on their age: ≤50 years group (n=125) and >50 years group (n=106). The clinicopathological parameters were compared between the two groups. From the results detailed in Table 2, we found that hypertension, anti-PLA2R antibodies, AST, UN, CK, and IgA were significantly increased in >50 years group, while BMI, TP, ALB, TB, and Ua were significantly decreased in the >50 years group (all P<0.05). However, no difference was observed for the other clinical parameters between the two age groups (all P>0.05).
Table 2.
Clinicopathological features of patients stratified according to age
| Parameters | Age range
|
P-value | |
|---|---|---|---|
| ≤50 years (n=125) | >50 years (n=106) | ||
| Gender (male : female) | 78:47 | 57:49 | 0.185 |
| Hypertension (%) | 24.0 | 49.1 | 0.000 |
| Diabetes (%) | 9.6 | 15.1 | 0.202 |
| Viral hepatitis (%) | 5.6 | 4.7 | 0.763 |
| NS (%) | 65.6 | 76.4 | 0.072 |
| BMI (Kg/m2) | 26.05±5.29 | 25.67±4.01 | 0.010 |
| Pathological stage (III) (%) | 6.4 | 1.9 | 0.093 |
| Anti-PLA2R (RU/mL) | 66.22±104.33 | 128.80±211.13 | 0.001 |
| ALT (U/L) | 21.79±15.16 | 20.44±18.00 | 0.806 |
| AST (U/L) | 17.47±5.93 | 19.98±11.98 | 0.019 |
| TP (g/L) | 50.23±11.19 | 48.54±8.35 | 0.003 |
| ALB (g/L) | 27.68±7.78 | 26.05±5.80 | 0.001 |
| TB (µmol/L) | 7.67±3.79 | 7.62±3.17 | 0.048 |
| DB (µmol/L) | 1.40±0.93 | 1.54±1.35 | 0.401 |
| ALP (U/L) | 54.81±15.64 | 64.34±16.40 | 0.456 |
| GGT (U/L) | 36.11±43.24 | 35.24±56.26 | 0.884 |
| GLU (mmol/L) | 4.87±1.31 | 5.04±1.14 | 0.554 |
| UN (mmol/L) | 4.72±1.75 | 5.83±2.92 | 0.004 |
| Cr (µmol/L) | 72.64±20.32 | 78.02±28.69 | 0.074 |
| Ua (µmol/L) | 356.58±104.41 | 337.34±85.24 | 0.015 |
| TC (mmol/L) | 7.12±2.41 | 7.04±2.28 | 0.318 |
| TG (mmol/L) | 2.54±1.73 | 2.65±2.09 | 0.221 |
| CK (U/L) | 93.17±62.05 | 113.38±193.91 | 0.047 |
| LDH (U/L) | 190.93±62.96 | 198.00±52.83 | 0.228 |
| HDL (mmol/L) | 1.39±0.50 | 1.50±0.54 | 0.787 |
| LDL (mmol/L) | 4.93±2.05 | 4.77±2.01 | 0.566 |
| D2 (µg/L) | 1.11±2.23 | 1.44±2.34 | 0.437 |
| IgA (mg/dL) | 204.71±79.76 | 226.94±88.33 | 0.015 |
| IgG (mg/dL) | 616.03±303.52 | 632.76±257.16 | 0.297 |
| IgM (mg/dL) | 112.79±67.28 | 119.77±89.71 | 0.118 |
| IgE (mg/dL) | 126.67±197.85 | 124.48±177.05 | 0.702 |
| C3 (mg/dL) | 114.64±25.13 | 115.26±23.36 | 0.090 |
| C4 (mg/dL) | 27.89±9.34 | 28.49±10.39 | 0.340 |
Note: Data are presented as mean ± SD unless otherwise stated. Bold data is statistically significant, P<0.05.
Abbreviations: BMI, body mass index; TC, total cholesterol; Cr, creatinine; DB, direct bilirubin; GLU, glucose; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; NS, nephrotic syndrome; TB, total bilirubin; TG, triglyceride; TP, total protein; Ua, uric acid; UN, urea nitrogen.
Clinicopathological characteristics according to gender
To uncover the relationship between the gender of patients and their clinicopathological features, the patients were divided into male group and female group. From the analysis results listed in Table 3, we found that patients with more advanced pathological stage were male (P=0.038). Besides, the ALT, DB, GGT, UN, Cr, Ua, CK, and IgE in males were remarkably higher than that in females (P<0.05 for all). Inversely, the results of anti-PLA2R antibodies and IgM were decreased in males compared with females (both P<0.05).
Table 3.
Clinicopathological features of patients stratified according to gender
| Parameters | Male (n=135) | Female (n=96) | P-value |
|---|---|---|---|
| Age (years) | 47.04±14.70 | 47.95±13.76 | 0.280 |
| Hypertension (%) | 40.0 | 29.2 | 0.090 |
| Diabetes (%) | 11.9 | 12.5 | 0.882 |
| Viral hepatitis (%) | 3.7 | 7.3 | 0.226 |
| NS (%) | 74.1 | 65.6 | 0.165 |
| BMI (Kg/m2) | 26.12±4.35 | 25.54±5.24 | 0.357 |
| Pathological stage (III) (%) | 6.7 | 1.0 | 0.038 |
| Anti-PLA2R (RU/mL) | 77.83±119.78 | 119.55±211.97 | 0.002 |
| ALT (U/L) | 24.21±19.60 | 16.89±9.24 | 0.016 |
| AST (U/L) | 19.48±10.88 | 17.42±6.23 | 0.148 |
| TP (g/L) | 47.99±10.14 | 51.51±9.48 | 0.863 |
| ALB (g/L) | 26.37±7.26 | 27.73±6.50 | 0.271 |
| TB (µmol/L) | 7.93±3.66 | 7.25±3.27 | 0.109 |
| DB (µmol/L) | 1.61±1.33 | 1.26±0.76 | 0.021 |
| ALP (U/L) | 58.85±15.72 | 59.61±17.96 | 0.614 |
| GGT (U/L) | 45.22±61.42 | 22.31±17.34 | 0.000 |
| GLU (mmol/L) | 5.00±1.19 | 4.87±1.30 | 0.662 |
| UN (mmol/L) | 5.83±2.72 | 4.38±1.60 | 0.019 |
| Cr (µmol/L) | 84.73±24.40 | 61.57±17.59 | 0.014 |
| Ua (µmol/L) | 386.04±93.44 | 293.91±71.80 | 0.007 |
| TC (mmol/L) | 7.20±2.46 | 6.93±2.19 | 0.064 |
| TG (mmol/L) | 2.71±2.03 | 2.43±1.71 | 0.290 |
| CK (U/L) | 121.93±171.80 | 75.12±62.21 | 0.019 |
| LDH (U/L) | 196.90±64.39 | 190.34±49.10 | 0.209 |
| HDL (mmol/L) | 1.33±0.51 | 1.59±0.49 | 0.408 |
| LDL (mmol/L) | 4.98±2.14 | 4.68±1.87 | 0.089 |
| D2 (µg/L) | 1.33±2.49 | 1.17±1.96 | 0.361 |
| IgA (mg/dL) | 210.36±83.73 | 221.20±85.23 | 0.466 |
| IgG (mg/dL) | 583.46±288.10 | 679.46±266.66 | 0.428 |
| IgM (mg/dL) | 98.75±64.54 | 139.88±88.99 | 0.022 |
| IgE (mg/dL) | 146.86±204.62 | 96.30±159.14 | 0.021 |
| C3 (mg/dL) | 114.82±22.91 | 115.08±26.19 | 0.294 |
| C4 (mg/dL) | 28.95±10.01 | 27.08±9.49 | 0.513 |
Note: Data are presented as mean ± SD unless otherwise stated. Bold data is statistically significant, P<0.05.
Abbreviations: BMI, body mass index; TC, total cholesterol; Cr, creatinine; DB, direct bilirubin; GLU, glucose; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; NS, nephrotic syndrome; TB, total bilirubin; TG, triglyceride; TP, total protein; Ua, uric acid; UN, urea nitrogen.
Association of histological stage with clinicopathological characteristics of MN patients
In this study, 221 (95.7%) MN patients were pathologically diagnosed with stages I–II, and ten (4.3%) cases were stage III. We also analyzed the clinical characteristics of MN patients according to their pathological stages. In Table 4, the results showed that the patients with more advanced pathological stages were more frequently male than female (P=0.038), moreover, the MN patients with advanced stages were more likely to have increased Cr (P=0.021) and IgE (P=0.003) levels compared with those with stages I–II. There was no significant correlation between pathological stage and other clinical parameters (all P>0.05).
Table 4.
Association of pathological stage with clinicopathological data of MN patients
| Parameters | Pathological stage
|
P-value | |
|---|---|---|---|
| I–II (n=221) | III (n=10) | ||
| Age (years) | 47.57±14.17 | 43.90±17.17 | 0.705 |
| Gender (male : female) | 126:95 | 9:1 | 0.038 |
| Hypertension (%) | 34.8 | 50.0 | 0.327 |
| Diabetes (%) | 12.7 | 0 | 0.230 |
| Viral hepatitis (%) | 5.4 | 0 | 0.449 |
| NS (%) | 70.6 | 70.0 | 0.968 |
| BMI (Kg/m2) | 25.84±4.70 | 26.63±5.92 | 0.364 |
| anti-PLA2R (RU/mL) | 96.66±165.96 | 59.83±148.42 | 0.668 |
| ALT (U/L) | 21.05±16.66 | 23.80±12.89 | 0.909 |
| AST (U/L) | 18.70±9.36 | 17.06±7.25 | 0.856 |
| TP (g/L) | 49.19±9.84 | 55.30±12.14 | 0.548 |
| ALB (g/L) | 26.73±6.91 | 31.35±7.15 | 0.905 |
| TB (µmol/L) | 7.63±3.54 | 7.95±3.12 | 0.902 |
| DB (µmol/L) | 1.45±1.15 | 1.67±0.84 | 0.675 |
| ALP (U/L) | 59.34±16.71 | 55.14±15.48 | 0.535 |
| GGT (U/L) | 35.69±50.50 | 36.34±19.31 | 0.508 |
| GLU (mmol/L) | 4.96±1.25 | 4.52±0.42 | 0.163 |
| UN (mmol/L) | 5.16±2.35 | 6.58±3.54 | 0.214 |
| Cr (µmol/L) | 74.50±23.86 | 88.65±36.66 | 0.021 |
| Ua (µmol/L) | 345.68±96.45 | 393.51±86.54 | 0.197 |
| TC (mmol/L) | 7.10±2.35 | 6.51±2.33 | 0.664 |
| TG (mmol/L) | 2.59±1.93 | 2.55±1.10 | 0.502 |
| CK (U/L) | 103.35±141.29 | 80.06±56.64 | 0.572 |
| LDH (U/L) | 194.00±58.62 | 198.38±58.63 | 0.526 |
| HDL (mmol/L) | 1.45±0.51 | 1.25±0.72 | 0.717 |
| LDL (mmol/L) | 4.87±2.04 | 4.44±1.77 | 0.483 |
| D2 (µg/L) | 1.30±2.32 | 0.45±0.25 | 0.136 |
| IgA (mg/dL) | 216.08±84.79 | 189.18±72.97 | 0.507 |
| IgG (mg/dL) | 617.93±275.25 | 750.10±413.81 | 0.483 |
| IgM (mg/dL) | 117.55±79.21 | 81.73±42.58 | 0.226 |
| IgE (mg/dL) | 119.70±180.77 | 256.20±293.05 | 0.003 |
| C3 (mg/dL) | 115.09±24.17 | 111.22±27.82 | 0.552 |
| C4 (mg/dL) | 28.35±9.89 | 24.14±7.47 | 0.301 |
Note: Data are presented as mean ± SD unless otherwise stated. Bold data is statistically significant, P<0.05.
Abbreviations: BMI, body mass index; TC, total cholesterol; Cr, creatinine; DB, direct bilirubin; GLU, glucose; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; MN, membranous nephropathy; NS, nephrotic syndrome; TB, total bilirubin; TG, triglyceride; TP, total protein; Ua, uric acid; UN, urea nitrogen.
The comparison of clinicopathological characteristics of MN patients based on their anti-PLA2R antibodies test
Based on anti-PLA2R antibodies test, MN patients were divided into PLA2R positive group (173, 74.89%), or negative group (58, 25.11%). Compared to negative group, there were more male patients in positive group (P=0.034), moreover, they were more likely to present NS (P<0.001). Besides, MN patients positive for anti-PLA2R antibodies showed significantly decreased TP (P<0.001), ALB (P<0.001), TB (P=0.005), DB (P=0.001), IgG (P<0.001), and upregulated CH (P=0.003), CK (P=0.020), LDL (P=0.003), and D2 (P=0.032) (Table 5).
Table 5.
Comparison of clinicopathological data of MN patients based on their anti-PLA2R test
| Parameters | Anti-PLA2R
|
P-value | |
|---|---|---|---|
| Positive (n=173) | Negative (n=58) | ||
| Age (years) | 47.37±14.35 | 47.55±14.24 | 0.933 |
| Gender (male : female) | 108:65 | 27:31 | 0.034 |
| Hypertension (%) | 67 (38.73) | 15 (25.86) | 0.076 |
| Diabetes (%) | 18 (10.40) | 10 (17.24) | 0.167 |
| Viral hepatitis (%) | 11 (6.3.6) | 1 (1.72) | 0.301a |
| NS (%) | 133 (76.88) | 30 (51.72) | <0.001 |
| BMI (Kg/m2) | 25.93±4.89 | 25.71±4.30 | 0.759 |
| ALT (U/L) | 20.36±16.17 | 23.60±17.35 | 0.196 |
| AST (U/L) | 18.97±10.04 | 17.60±6.45 | 0.334 |
| TP (g/L) | 47.66±9.63 | 54.78±9.21 | <0.001 |
| ALB (g/L) | 25.67±6.66 | 30.70±6.54 | <0.001 |
| TB (µmol/L) | 7.27±3.10 | 8.75±4.36 | 0.005 |
| DB (µmol/L) | 1.31±0.79 | 1.91±1.75 | 0.001 |
| ALP (U/L) | 58.63±15.54 | 60.75±19.64 | 0.403 |
| GGT (U/L) | 35.86±53.07 | 35.29±37.55 | 0.941 |
| GLU (mmol/L) | 4.93±1.26 | 5.00±1.18 | 0.705 |
| UN (mmol/L) | 5.30±2.33 | 5.02±2.68 | 0.450 |
| Cr (µmol/L) | 75.58±22.72 | 73.70±59.71 | 0.615 |
| Ua (µmol/L) | 352.72±91.73 | 332.92±108.54 | 0.176 |
| TC (mmol/L) | 7.35±2.31 | 6.28±2.29 | 0.003 |
| TG (mmol/L) | 2.70±2.03 | 2.26±1.40 | 0.125 |
| CK (U/L) | 115.05±157.59 | 65.60±38.08 | 0.020 |
| LDH (U/L) | 195.33±51.50 | 190.79±75.82 | 0.614 |
| HDL (mmol/L) | 1.42±0.50 | 1.51±0.58 | 0.277 |
| LDL (mmol/L) | 5.08±2.03 | 4.17±1.89 | 0.003 |
| D2 (µg/L) | 1.45±2.48 | 0.70±1.39 | 0.032 |
| IgA (mg/dL) | 212.78±83.50 | 221.30±97.29 | 0.510 |
| IgG (mg/dL) | 578.53±268.00 | 760.02±284.50 | <0.001 |
| IgM (mg/dL) | 110.46±71.83 | 132.67±93.85 | 0.063 |
| IgE (mg/dL) | 126.51±183.50 | 123.12±203.40 | 0.907 |
| C3 (mg/dL) | 114.23±24.26 | 117.02±24.44 | 0.455 |
| C4 (mg/dL) | 28.72±9.94 | 26.48±9.34 | 0.135 |
Notes:
Case number less than 5 – corrected P-value was used. Data are presented as mean ± SD unless otherwise stated. Bold data is statistically significant, P<0.05.
Abbreviations: BMI, body mass index; TC, total cholesterol; Cr, creatinine; DB, direct bilirubin; GLU, glucose; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; MN, membranous nephropathy; NS, nephrotic syndrome; TB, total bilirubin; TG, triglyceride; TP, total protein; Ua, uric acid; UN, urea nitrogen.
Discussion
MN is a common disease of the urinary system, which is characterized by the accumulation of immune deposits on the outer surface of the glomerular basement membrane.16 Most MN cases are iMN, representing a major cause of idiopathic NS among the adult population.17 Despite various therapeutic strategies, the therapeutic effects are far from satisfactory.18–20 Most of iMN cases will suffer from NS, posing a serious threat to human health.21 And even worse, growing evidences have indicated that MN usually shows high rate of recurrence and long disease course, which may occur at any stage in life.22 So far, the molecular mechanisms underlying the progression of MN remain unclear, and the clinicopathological characteristics are variable in MN cases, bringing a heavy burden for MN diagnosis and treatment.23
In the current study, we investigated and analyzed the clinicopathological characteristics of 231 patients who were pathologically diagnosed with MN. The ratio of male to female in the patients was 1.47 and the mean age was 47.43±14.32. Most patients were 41–59 years old, accounting for 45% of all the cases. Among them, 163 (70.6%) cases were positive for NS, which was in accordance with the data in the previous studies. In addition to these general data, the analysis of clinical parameters indicated that serum anti-PLA2R antibodies, BMI, CH, TG, LDL, D2, IgA, and IgE were increased in the MN patients compared to the normal reference values, but IgG was decreased in MN patients. In subsequent analysis, we analyzed the clinical characteristics of MN patients according to their age. We found significant differences for hypertension, BMI, anti-PLA2R antibodies, AST, TP, ALB, TB, UN, Ua, CK, and IgA between MN patients in the ≤50 years group and those in the >50 years group. MN patients with advanced age were more likely to exhibit poor performance in blood examinations, revealing the aggressive disease progression. The study carried out by Chen et al reported that old age and high blood indices were significantly correlated with poor prognosis of patients with iMN.24 Therefore, timely and effective treatment and medical care is particularly important for older MN patients.
Gender has also been proven to be associated with disease progression in patients with MN.25 In our study, we investigated the clinical characteristics of MN patients based on their gender. The results showed that ALT, DB, GGT, UN, Cr, Ua, CK, and IgE were remarkably higher in males than in females. These results might reveal that male MN patients are more likely to undergo rapid disease progression than female patients. The results are consistent with previous studies. A related study performed among Japanese MN cases demonstrated that male MN cases were more likely to develop end-stage renal diseases, and have a poor prognosis.25 Furthermore, it has been reported that with aging, men presented obviously greater decrements in renal functions than women, moreover, they were more likely to suffer from progressive renal diseases, such as MN, IgA nephropathy, and polycystic kidney disease. The mechanisms underlying the phenomenon might be related to the cell death induced by androgens.26 Inversely, anti-PLA2R antibodies and IgM were decreased in males compared with females. The abnormal results might be attributed to the relatively small sample size, different geographic areas, as well as the detection methods. Thus, further studies will be required to confirm our results.
According to the pathological classification method, MN can be divided into stages I, II, III, and IV. The MN stage I cases have normal glomeruli, which can be observed by light microscopy.27 However, for MN patients with stage II–IV, the capillary wall thicken and the deposits are gradually incorporated into the glomerular basement membrane.28 Pathological stage classification is an effective way to estimate disease progression. In our study, we found that the pathological stage was significantly correlated with patients’ gender, Cr, and IgE. Advanced pathological stage was more likely to be observed in males than in females. Moreover, MN patients with advanced stage usually had increased Cr and IgE compared with those with stages I–II. IgE is an important factor in allergic reactions. Studies have demonstrated that patients with renal diseases showed high serum levels of IgE, moreover, serum IgE level might be significantly correlated with disease activity and clinical outcomes of NS.29,30 However, the functional roles of IgE in the pathogenesis of renal disease remains poorly understood. Further investigations are urgently needed to address the issues.
Anti-PLA2R antibodies is an important factor in the occurrence of MN.31 In general, anti-PLA2R antibodies is considered as a useful biomarker for early diagnosis and prognosis in patients with MN.32 In this study, we compared the baseline characteristics of MN patients according to their anti-PLA2R antibodies test results. The results demonstrated that MN cases who were positive for anti-PLA2R antibodies had significantly decreased TP, ALB, TB, DB, IgG; and upregulated CH, CK, LDL, D2. Furthermore, they were more likely to present NS. The data revealed that patients in the anti-PLA2R antibodies positive group had rapid disease progression. The conclusion is in line with the previous studies.33 The podocyte lesion plays a key role in the etiology of MN. PLA2R is located at the surface of podocytes, and its abnormal expression may reveal the abnormal formation of immune complexes in situ, thus leading to damage in podocytes.34
Conclusion
In conclusion, the clinicopathological characteristics are diverse among MN patients. Male and elderly MN patients are more likely to undergo a rapid disease progression. Furthermore, the clinical characteristics are significantly correlated with pathological stages and anti-PLA2R antibodies level. Due to the relatively small sample size, the results obtained in our study should be verified by well-designed studies with large sample size.
Acknowledgments
This study was supported by: 1) National key R&D Program of China (2016YFC1305500); 2) the National Natural Science Foundation of China (nos. 61471399 and 61671479); 3) Innovation Nursery Fund of PLA General Hospital (no. 15KMZ04); 4) the National Natural Science Foundation of China (81401719).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ronco P, Debiec H. Membranous nephropathy: A fairy tale for immunopathologists, nephrologists and patients. Mol Immunol. 2015;68(1):57–62. doi: 10.1016/j.molimm.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920–923. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 3.Ponticelli C, Passerini P. Can prognostic factors assist therapeutic decisions in idiopathic membranous nephropathy? J Nephrol. 2010;23(2):156–163. [PubMed] [Google Scholar]
- 4.Dai H, Zhang H, He Y. Diagnostic accuracy of PLA2R autoantibodies and glomerular staining for the differentiation of idiopathic and secondary membranous nephropathy: an updated meta-analysis. Sci Rep. 2015;5:8803. doi: 10.1038/srep08803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayalon R, Beck LH., Jr Membranous nephropathy: not just a disease for adults. Pediatr Nephrol. 2015;30(1):31–39. doi: 10.1007/s00467-013-2717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basford AW, Lewis J, Dwyer JP, Fogo AB. Membranous nephropathy with crescents. J Am Soc Nephrol. 2011;22(10):1804–1808. doi: 10.1681/ASN.2010090923. [DOI] [PubMed] [Google Scholar]
- 7.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PloS One. 2014;9(10):e110681. doi: 10.1371/journal.pone.0110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravedi P, Abbate M, Gagliardini E, et al. Membranous nephropathy associated with IgG4-related disease. Am J Kidney Dis. 2011;58(2):272–275. doi: 10.1053/j.ajkd.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Ando M, Yamamoto R, et al. Patient age and the prognosis of idiopathic membranous nephropathy. PLoS One. 2014;9(10):e110376. doi: 10.1371/journal.pone.0110376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol. 2014;9(11):1883–1890. doi: 10.2215/CJN.03850414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Wang GP, Li BM, Chen QK. Clinicopathological analysis of idiopathic membranous nephropathy in young adults. Gen Mol Res. 2015;14(2):4541–4548. doi: 10.4238/2015.May.4.12. [DOI] [PubMed] [Google Scholar]
- 12.Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet. 2015;385(9981):1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 13.Ponticelli C, Passerini P. Management of idiopathic membranous nephropathy. Expert Opin Pharmacother. 2010;11(13):2163–2175. doi: 10.1517/14656566.2010.494599. [DOI] [PubMed] [Google Scholar]
- 14.Praga M, Rojas-Rivera J. Glomerular disease: Predicting outcomes in idiopathic membranous nephropathy. Nat Rev Nephrol. 2012;8(9):496–498. doi: 10.1038/nrneph.2012.161. [DOI] [PubMed] [Google Scholar]
- 15.Churg J, Ehrenreich T. Membranous nephropathy. Perspect Nephrol Hypertens. 1973;1(Pt 1):443–448. [PubMed] [Google Scholar]
- 16.Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy--a modern view. Clin J Am Soc Nephrol. 2014;9(3):609–616. doi: 10.2215/CJN.04160413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartono C, Chung M, Kuo SF, Seshan SV, Muthukumar T. Bortezomib therapy for nephrotic syndrome due to idiopathic membranous nephropathy. J Nephrol. 2014;27(1):103–106. doi: 10.1007/s40620-013-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran TH, J Hughes G, Greenfeld C, Pham JT. Overview of current and alternative therapies for idiopathic membranous nephropathy. Pharmacotherapy. 2015;35(4):396–411. doi: 10.1002/phar.1575. [DOI] [PubMed] [Google Scholar]
- 20.Hofstra JM, Fervenza FC, Wetzels JF. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2013;9(8):443–458. doi: 10.1038/nrneph.2013.125. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Schieppati A, Chen X, et al. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2014;(10):CD004293. doi: 10.1002/14651858.CD004293.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grupper A, Cornell LD, Fervenza FC, Beck LH, Jr, Lorenz E, Cosio FG. Recurrent membranous nephropathy after kidney transplantation: treatment and long-term implications. Transplantation. 2015 Dec 30; doi: 10.1097/TP.0000000000001056. Epub. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Inaba S, Yoshida R, et al. Clinicopathological characteristics of the focal and segmental form of idiopathic membranous nephropathy: comparison with the typical form of this disease. Acta Paediatr Jpn. 1997;39(3):349–353. doi: 10.1111/j.1442-200x.1997.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Chen Y, Shi K, et al. Comparison of prognostic, clinical, and renal histopathological characteristics of overlapping idiopathic membranous nephropathy and IgA nephropathy versus idiopathic membranous nephropathy. Sci Rep. 2017;7(1):11468. doi: 10.1038/s41598-017-11838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiiki H, Saito T, Nishitani Y, et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int. 2004;65(4):1400–1407. doi: 10.1111/j.1523-1755.2004.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.Gandolfo MT, Verzola D, Salvatore F, et al. Gender and the progression of chronic renal diseases: does apoptosis make the difference? Minerva Urol Nefrol. 2004;56(1):1–14. [PubMed] [Google Scholar]
- 27.Rood IM, Merchant ML, Wilkey DW, et al. Increased expression of lysosome membrane protein 2 in glomeruli of patients with idiopathic membranous nephropathy. Proteomics. 2015;15(21):3722–3730. doi: 10.1002/pmic.201500127. [DOI] [PubMed] [Google Scholar]
- 28.Debiec H, Ronco P. Immunopathogenesis of membranous nephropathy: an update. Semin Immunopathol. 2014;36(4):381–397. doi: 10.1007/s00281-014-0423-y. [DOI] [PubMed] [Google Scholar]
- 29.Tan Y, Yang D, Fan J, Chen Y. Elevated levels of immunoglobulin E may indicate steroid resistance or relapse in adult primary nephrotic syndrome, especially in minimal change nephrotic syndrome. J Int Med Res. 2011;39(6):2307–2313. doi: 10.1177/147323001103900629. [DOI] [PubMed] [Google Scholar]
- 30.Tain YL, Chen TY, Yang KD. Implication of serum IgE in childhood nephrotic syndrome. Pediatr Nephrol. 2003;18(12):1211–1215. doi: 10.1007/s00467-003-1269-z. [DOI] [PubMed] [Google Scholar]
- 31.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Huang B, Liu X, et al. Ultrasensitive quantitation of anti-phospholipase A2 receptor antibody as a diagnostic and prognostic indicator of idiopathic membranous nephropathy. Sci Rep. 2017;7(1):12049. doi: 10.1038/s41598-017-12014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu NX, Xie QH, Sun ZX, et al. Renal phospholipase A2 receptor and the clinical features of idiopathic membranous nephropathy. Chin Med J (Engl) 2017;130(8):892–898. doi: 10.4103/0366-6999.204096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Wen S, Zhu X, et al. Diagnostic value of renal phospholipase A2 receptor and serum anti-phospholipase A2 receptor antibody in membranous nephropathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(4):395–399. doi: 10.11817/j.issn.1672-7347.2017.04.005. Chinese. [DOI] [PubMed] [Google Scholar]


