Abstract
Please cite this paper as: Vongphrachanh P, Simmerman JM, Phonekeo D, Pansayavong V, Sisouk T, Ongkhamme S, Bryce GT, Corwin A, Bryant JE. An early report from newly established laboratory‐based influenza surveillance in Lao PDR. Influenza and Other Respiratory Viruses 4(2), 47–52.
Background Prior to 2007, little information was available about the burden of influenza in Laos. We report data from the first laboratory‐based influenza surveillance system established in the Lao People’s Democratic Republic.
Methods Three hospitals in the capital city of Vientiane began surveillance for influenza‐like illness (ILI) in outpatients in 2007 and expanded to include hospitalized pneumonia patients in 2008. Nasal/throat swab specimens were collected and tested for influenza and other respiratory viruses by multiplex ID‐TagTM respiratory viral panel (RVP) assay on a Luminex® 100× MAP IS instrument (Qiagen, Singapore).
Results During January 2007 to December 2008, 287 of 526 (54·6%) outpatients with ILI were positive for at least one respiratory virus. Influenza was most commonly identified, with 63 (12·0%) influenza A and 92 (17·5%) influenza B positive patients identified. In 2008, six of 79 (7·6%) hospitalized pneumonia patients were positive for influenza A and four (5·1%) were positive for influenza B. Children <5 years represented 19% of viral infections in outpatients and 38% of pneumonia inpatients.
Conclusion Our results provide the first documentation of influenza burden among patients with febrile respiratory illness and pneumonia requiring hospitalization in Laos. Implementing laboratory‐based influenza surveillance requires substantial investments in infrastructure and training. However, continuing outbreaks of avian influenza A/H5N1 in poultry and emergence of the 2009 influenza A(H1N1) pandemic strain further underscore the importance of establishing and maintaining influenza surveillance in developing countries.
Keywords: Influenza, Laos
Introduction
Influenza virus infection causes predictable and sometimes severe seasonal epidemics of respiratory illness in temperate climates characterized by substantial morbidity in all age groups and significant mortality in the elderly. 1 , 2 Antigenic and genetic analysis of influenza A (H3N2) viruses indicate that influenza viruses continually circulate in Southeast Asia via a network of temporally overlapping epidemics that seed viruses in temperate regions. This finding underscores the importance of establishing laboratory‐based surveillance in Southeast Asia to improve global antigenic forecasting and vaccine strain selection. 3 , 4 While data are limited, recent studies also suggest a significant burden of disease from influenza in Southeast Asia. 5 , 6 , 7 Influenza surveillance systems with laboratory confirmation provide data to improve understanding of influenza disease burden and seasonality in tropical developing countries, support the development of prevention and treatment strategies, and improve global pandemic preparedness. 8 , 9 , 10
Access to rapid and accurate influenza laboratory diagnostics is essential to identify new influenza virus strains with pandemic potential and rapidly conduct outbreak investigation and containment efforts. 11 On at least three occasions in the past century shifts in the influenza virus genome have generated pandemic strains resulting in hundreds of millions of illnesses and tens of millions of deaths. 12 , 13 Beginning in East and Southeast Asia in 2003, repeated outbreaks of highly pathogenic avian influenza A/H5N1 in poultry have been documented with 263 fatal human infections recorded worldwide as of December 11, 2009. 14 , 15 Unexpectedly in early 2009, a novel reassortant influenza A(H1N1) virus of swine origin emerged in the Americas and rapidly spread to more than 90 countries causing the WHO to declare a pandemic on June 11th.. Through its newly established surveillance system, Laos identified its first case of pandemic influenza A(H1N1) infection on June 18, 2009.
Background
Lao PDR is a landlocked country in Southeast Asia with a population of 5 759 000 and a gross national per capita income of $1,740 USD in 2006. 16 The 2006 life expectancy at birth was 57 years and 40·8% of the population was younger than 15 years of age. 17 The public health infrastructure in Laos is in poor condition and annual health spending per capita was $85 in 2006. 17 Generally, Laos has a subtropical climate but the northern provinces and higher elevations bordering China and Myanmar are more temperate. The capital city Vientiane is situated at 171 meters above sea level and the average temperature in June is 29°C with 85% relative humidity and 24°C with 78% relative humidity in December. 18 Influenza vaccination is not routinely available in public health care facilities in Laos.
Since 2003, Lao PDR has experienced recurrent avian influenza A (H5N1) outbreaks in domestic poultry with two confirmed fatal human cases. 19 , 20 These events focused political and media attention on the limited capacity of Lao PDR to detect and respond to such threats and ultimately resulted in new contributions from international donors. Under the leadership of the Lao PDR Ministry of Health (MOH), advances in the infectious disease surveillance system and improved laboratory diagnostic capacity for respiratory pathogens have been achieved. In December 2006, Lao PDR obtained the in‐country diagnostic capacity to analyze specimens for influenza viruses as part of a national AI outbreak response program and established an ILI surveillance system in the capital city of Vientiane. The objectives of the ILI surveillance program were to improve understanding of influenza disease burden and seasonality, to monitor circulating virus strains for new variants with pandemic potential, and to enable the early detection and response to influenza outbreaks. In July 2008, surveillance was expanded to include hospitalized pneumonia patients to better understand the epidemiology of severe influenza infections and identify groups at highest risk for serious complications. We describe findings from the first 2 years of influenza surveillance and discuss challenges and priorities for future influenza surveillance and disease burden research in a severely resource limited environment.
Methods
Surveillance for influenza‐like illness
Beginning in January 2007, ILI surveillance was conducted in children and adults seeking care at general outpatient and emergency departments of two public and one private hospital in Vientiane. Influenza‐like illness was defined using the WHO case definition of fever ≥38°C, plus cough and/or throat pain. The surveillance hospitals are centrally located and the catchment population is mainly urban residents of Vientiane. During 2007, the system aimed to collect specimens from four ILI patients per hospital per week, however collection rates varied as new administrative and operational procedures were implemented. In 2008, the sampling approach was adjusted to collect respiratory swab specimens from all patients that met the ILI case definition during one clinic day each week at each hospital. 21
Surveillance was conducted 1 day per week during the hours of 8 am to 3:30 pm. A surveillance nurse screened incoming patients for any report of fever, cough or sore throat. If these were present, an oral temperature was measured using a mercury thermometer. If the patient met the WHO ILI case definition, a nasal and throat specimen was collected using two flocked swabs, placed together in universal viral media (Becton Dickinson, Sparks, MD, USA), and stored at 4°C until transported to the laboratory the same day. On receipt at the National Center for Laboratory and Epidemiology laboratory, samples were assigned anonymous identification numbers, transferred to a biosafety cabinet, and aliquoted for long‐term storage at −80°C. Beginning in 2008, the number, age and gender of all patients seeking outpatient care were recorded each week in the participating wards of the three sentinel hospitals. In addition, the number, age and gender of patients presenting with ILI symptoms were summarized. These data permitted monitoring overall trends in ILI activity for 2008 (Figure 1), but unfortunately were not available for 2007.
Figure 1.
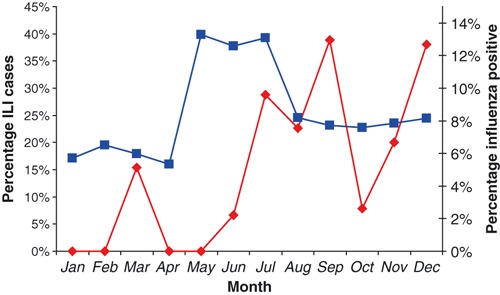
Proportion of outpatients meeting the ILI case definition (blue, n = 161; total outpatient consultations in the three sentinel hospitals) and percentage of laboratory‐confirmed influenza positive cases (red, n = 322; total ILI specimens tested) by month in 2008.
Surveillance for acute lower respiratory infection
Surveillance activities were expanded in August 2008 to include hospitalized pneumonia patients in a single surveillance hospital. We attempted to collect respiratory swab specimens from all eligible cases in the internal medicine, pediatric, and intensive care ward 7 days per week. Patients were screened on admission to these units for signs and symptoms of acute lower respiratory infection (ALRI). In children aged <14 years, the ALRI case definition was fever ≥38°C on admission and cough with dyspnea, tachypnea or shortness of breath. For patients ≥14 years, the case definition was the same except tachypnea was not included and chest pain, dyspnea, and/or adventitious breath sounds were required. Diagnostic screening for tuberculosis and HIV was conducted for suspect cases according to hospital policy. Patients with active tuberculosis, HIV positive or otherwise compromised immunity, and patients who reported an onset of symptoms 14 or more days prior to admission were excluded. Respiratory clinical swabs were collected and handled in the same manner as the ILI outpatient surveillance system. Using the ward log books, the number, age and gender of patients admitted to the Internal Medicine and Pediatric wards with a preliminary diagnostic assessment suggesting lower respiratory infection were recorded each day, as well as the number, age and gender of those who met the ALRI case definition.
Diagnostic testing
Respiratory swab specimens were tested for presence of respiratory viral pathogens using the Luminex RVP (ID‐Tag™RVP ) assay (Bioscience Corp, Toronto, ON, Canada) according to manufacturer’s instructions and as described elsewhere. 22 RNA extractions were performed on specimens prior to freezing. Subsequently, the RNA underwent a single freeze‐thaw cycle during analysis. The RVP primer mix contains 16 primer pairs designed to simultaneously amplify highly conserved regions of viral types and subtypes, specifically influenza A/H1, A/H3 and A/H5, influenza B, RSV A, RSV B, coronavirus (229E, OC43, SARS, NL63, HKU1), parainfluenza virus 1–4, metapneumovirus, adenovirus and enterovirus/rhinoviruses. The RVP assay uses a single primer pair targeting a conserved region of 5′‐UTR to detect viruses of the closely related enterovirus and rhinovirus genera.
Results
Influenza‐like illness
From January 2007 to December 2008, 541 respiratory swab specimens from ILI outpatients were received. Fifteen specimens were excluded from analysis due to improper storage or incomplete lab forms. A total of 526 specimens were tested; 204 in 2007; 322 in 2008. One hundred and sixty (30·4%) specimens were from children <5 years of age, 342 (65%) were from patients 5–49 years, and 24 (4·6%) were from patients 50 years and older. During 2007, 90 (44·1%) of ILI patients tested positive for influenza and in 2008, 65 (20·2%) were influenza positive (Table 1). Among influenza positive specimens, 46 (51·1%) were influenza type B positive in 2007 and 46 (70·8%) were type B positive in 2008. Among influenza A positive specimens, the H3 subtype was predominant in 2007 (41/90; 45·6%) while the H1 subtype was more common in 2008 (17/65; 26·2%).
Table 1.
Distribution of influenza subtypes and other viruses among Influenza‐like illness (ILI) outpatients by year
| 2007 | 2008 | Total ILI | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Influenza A (H1) | 3 (3·3) | 17 (26·2) | 20 (12·9) |
| Influenza A (H3) | 41 (45·6) | 2 (3·1) | 43 (27·7) |
| Influenza A (H5) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Influenza B | 46 (51·1) | 46 (70·8) | 92 (59·4) |
| Total Flu A & B | 90 (44·1) | 65 (20·2) | 155 (29·5) |
| Other viral etiologies | 44 (21·6) | 96 (29·8) | 140 (26·6) |
| Negative | 70 (34·3) | 161 (50·0) | 231 (43·9 |
| Total ILI cases tested | 204 (100·0) | 322 (100·0) | 526 (100·0) |
Influenza accounted for 29% of all pathogen detections and was the most common virus identified in ILI patients 5 years of age and older. (Table 2). Influenza was detected in 9% (11/127) of the 0–2 year age group, and 22% (9/42) of 3–4 year age group. Enteroviruses–rhinoviruses were present in 38% (48/127) of cases in the 0–2 year age group and 29% (12/42) of cases in the 3 to 4‐year‐old age group. Eleven cases of metapneumovirus, four RSV infections, three parainfluenza viruses, three adenovirus, and a single coronavirus infection were identified in children <2 years of age. Co‐infections with two or more viruses were found in 3·2% (17/526) of ILI patients. Fifteen of the 17 were enterovirus–rhinovirus co‐infections (five with human metapneumovirus; three with influenza A/H1; three with influenza B; two with RSV A; one with Parainfluenza 3; one with adenovirus). There was one co‐infection of influenza A/H1 with HPMV and one co‐infection with influenza B and coronavirus.
Table 2.
Etiology of Influenza‐like illness (ILI) cases by age group, 2007–2008*
| Virus identified | Age group in years | Total | |||||
|---|---|---|---|---|---|---|---|
| 0–2 | 3–4 | 5–17 | 18–49 | 50–64 | >65 | ||
| Influenza A | 4 (3%) | 4 (10%) | 18 (12%) | 33 (17%) | 2 (11%) | 2 (33%) | 63 (12%) |
| Influenza B | 7 (6%) | 5 (12%) | 38 (26%) | 41 (21%) | 1 (6%) | 0 (0%) | 92 (17%) |
| Entero/Rhinovirus | 48 (38%) | 12 (29%) | 23 (16%) | 18 (9%) | 2 (11%) | 0 (0%) | 103 (20%) |
| Metapneumovirus | 11 (13%) | 2 (5%) | 3 (2%) | 3 (2%) | 0 (0%) | 0 (0%) | 19 (4%) |
| Parainfluenza (1–4) | 3 (2%) | 2 (7%) | 4 (3%) | 3 (2%) | 0 (0%) | 0 (0%) | 12 (2%) |
| RSV (A and B) | 4 (4%) | 3 (10%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (17%) | 9 (2%) |
| Adenovirus | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (1%) |
| Coronavirus | 1 (1%) | 0 (0%) | 0 (0%) | 2 (1%) | 0 (0%) | 0 (0%) | 3 (1%) |
| Negative | 39 (31%) | 12 (29%) | 60 (41%) | 95 (48%) | 13 (72%) | 3 (50%) | 222 (42%) |
| Total | 127 (100%) | 42 (100%) | 151 (100%) | 199 (100%) | 18 (100%) | 6 (100%) | 543 (100%) |
*Column total exceeds the total number of patients enrolled (n = 526) due to mixed infections with two pathogens in 17 patients.
During February and March 2007, an outbreak of avian influenza A(H5N1) in poultry occurred in Vientiane and the surrounding area. During this period of heightened awareness among Laotian clinicians, surveillance specimens were submitted in greater numbers and 35 of 142 (24·6%) were positive for influenza type B. An increase in the proportion of outpatients seeking care for ILI was observed during the rainy season months of May through July of 2008, as evidenced by collection of aggregate data from the three participating sentinel hospitals (Figure 1, blue line). However, no corresponding increase in laboratory‐confirmed influenza was detected during this period (Figure 1, red line). Whether the 2008 pattern of ILI infections is a true reflection of influenza seasonality in Lao PDR will require continued monitoring in the coming years.
Acute lower respiratory infection
From August to December 2008 respiratory swab specimens were obtained from 88 hospitalized ALRI patients. Specimens from nine patients were excluded due to improper storage or delays in transport. Thirty‐nine (49·3%) were from patients <5 years of age, 10 (12·7%) were 5–17 years, 19 (24·1%) were between 18 and 49 years and 11 (13·9%) were 50 years or older. Among the 79 hospitalized ALRI patients, 10 (12·7%) tested positive for either influenza A or B. Fourteen (17·7%) ALRI patients were co‐infected with enterovirus–rhinovirus and 1 or 2 additional viruses (Table 3). There were four cases of triple infection with enterovirus–rhinovirus and human metapneumovirus plus either adenovirus or an influenza virus. All ALRI patients with influenza were 5 years of age or older. Two of the influenza positive cases were from children aged 5–17 years, three cases were from the 18–49 year age group, four cases were from 50–64 year age group, and one case was over 65 years of age. Four of 10 influenza‐positive ALRI cases were co‐infected with other respiratory viruses (two with enterovirus–rhinovirus, two with enterovirus–rhinovirus plus HPMV) and one case was also HIV positive. All 10 influenza‐positive ALRI cases had negative blood cultures for bacterial respiratory pathogens while two were positive for murine scrub typhus by IgM serology. The two ALRI patients >65 years required mechanical ventilation and one experienced seizures. The average length of hospital stay among all patients was 7·6 days (range 3–17 days). All patients were discharged alive.
Table 3.
Etiology of ARLI cases by age group, 2007–2008*
| Virus identified | Age group in years | Total | |||||
|---|---|---|---|---|---|---|---|
| 0–2 | 3–4 | 5–17 | 18–49 | 50–64 | >65 | ||
| Influenza A | 0 (0%) | 0 (0%) | 1 (6%) | 1 (5%) | 3 (25%) | 1 (50%) | 6 (6%) |
| Influenza B | 0 (0%) | 0 (0%) | 1 (6%) | 2 (10%) | 1 (8%) | 0 (0%) | 4 (4%) |
| Entero/Rhinovirus | 19 (50%) | 2 (29%) | 5 (31%) | 7 (33%) | 2 (17%) | 1 (50%) | 36 (38%) |
| Metapneumovirus | 4 (11%) | 1 (14%) | 6 (38%) | 2 (10%) | 2 (17%) | 0 (0%) | 15 (16%) |
| Parainfluenza (1–4) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| RSV (A and B) | 5 (13%) | 2 (29%) | 1 (6%) | 1 (5%) | 0 (0%) | 0 (0%) | 9 (9%) |
| Adenovirus | 2 (5%) | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) | 3 (3%) |
| Coronavirus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Negative | 7 (18%) | 2 (29%) | 2 (13%) | 7 (33%) | 4 (33%) | 0 (0%) | 22 (23%) |
| Total pathogens detected | 38 (100%) | 7 (100%) | 16 (100%) | 21 (100%) | 12 (100%) | 2 (100%) | 96 (100%) |
*Number of pathogens detected exceeds the total number of patients enrolled (n = 79) due to mixed infections (nine patients; co‐infected with two viruses; four patients co‐infected with three viruses).
Five representative influenza‐positive clinical specimens from 2007 were sent to the WHO Influenza Collaborating Center in Tokyo for viral culture, molecular and antigenic analysis. Two H1N1, two H3N2 viruses and one type B virus were isolated. These viruses were subjected to Hemagluttanin Inhibition (HI) testing using WHO reference antisera. Two H1N1 viruses were found to be antigenically similar to A/Brisbane/59/2007, two H3N2 viruses were concluded to be A/Brisbane/10‐like, and a type B isolate was from the B/Victoria‐lineage and antigenically similar to B/Hiroshima/1/2005.
Discussion
Influenza viruses were the most common viral respiratory pathogen among outpatients presenting with ILI at three hospitals in Vientiane during 2007 and 2008. In 2007 and 2008, influenza virus infections were identified in 44·1% (90/204) and 20·2% (65/322) of all ILI cases, respectively. Influenza was also confirmed in 12·6% of hospitalized pneumonia patients. These findings are consistent with reports from elsewhere in East and Southeast Asia 5 , 23 , 24 and contribute to a growing body of evidence that influenza is an important cause of both outpatient febrile respiratory illness and pneumonia in the region.
The National Center for Laboratory and Epidemiology is currently working toward the geographic expansion of ILI and ALRI surveillance networks beyond the national capital. Five additional ILI surveillance hospital sites and at least one pneumonia surveillance hospital site are planned with corresponding increases in laboratory capacity. Using influenza disease burden data obtained from both Laos and Thailand, in 2009 the Laos MOH established a national seasonal influenza control strategy. Groups at highest risk for serious complications such as pregnant women, young children, persons with underlying disease and health care providers 25 have been designated as priority groups for influenza vaccination. This new policy will be adjusted as additional data from expanded influenza surveillance activities in Laos become available. Finally, newly established laboratory and outbreak response capabilities have also begun to demonstrate the frequency and severity of influenza outbreaks in remote areas of Laos. During 2008 and 2009, seasonal influenza A(H1) and A(H3) strains were identified in three separate febrile respiratory illness outbreak investigations in ethnic minority communities in remote northern regions.
Under the leadership of the Laos MOH and with international funding support, substantial progress has been made to expand laboratory and epidemiological capacity for influenza surveillance. In less than 2 years, this effort has already demonstrated significant public health value in terms of outbreak response and an improved understanding of the burden of disease. Data from multiple years and additional provinces are needed to support the newly established influenza control program and complement ongoing work in other regional countries to advance scientific knowledge of the epidemiology of influenza in tropical and subtropical Southeast Asia.
Conflict of interest
The authors report no known conflicts of interest. This research was conducted as part of the authors’ usual employment. No author received outside support or funding to conduct this research. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their respective employers.
Acknowledgements
Support for influenza surveillance activities in Lao PDR comes from the World Health Organization, United States Centers for Disease Control and Prevention, US Navy Medical Research Unit‐Jakarta, Asian Development Bank and the Institute Pasteur. The contribution of JE Bryant was supported by Agence française de développement, SISEA/ Pasteur Project.
References
- 1. Poehling KA, Edwards KM, Weinberg GA et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 2. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States.[see comment]. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 3. Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008; 320:340–346. [DOI] [PubMed] [Google Scholar]
- 4. Rambaut A, Pybus OG, Nelson MI, et al. The genomic and epidemiological dynamics of human influenza A virus. Nature 2008; 453:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simmerman J, Uyeki T. The burden of influenza in East and South‐East Asia: a review of the English language literature. Influenza Other Respi Viruses 2008; 2:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks WA, Goswami D, Rahman M, et al. (2009) Influenza is a Major Contributor to Childhood Pneumonia in a Tropical Developing Country. Pediatric Infectious Diseases Journal, 29, doi: 10.1097/INF.0b013e3181bc23fd [DOI] [PubMed] [Google Scholar]
- 7. Wong CM, Chan KP, Hedley AJ, et al. Influenza‐associated mortality in Hong Kong. Clin Infect Dis 2004; 39:1611–1617. [DOI] [PubMed] [Google Scholar]
- 8. Katz MA, Tharmaphornpilas P, Chantra S, et al. Who gets hospitalized for influenza pneumonia in Thailand? Implications for vaccine policy. Vaccine 2007; 25:3827–3833. [DOI] [PubMed] [Google Scholar]
- 9. Simmerman JM, Lertiendumrong J, Dowell SF, et al. The cost of influenza in Thailand. Vaccine 2006; 24:4417–4426. [DOI] [PubMed] [Google Scholar]
- 10. Brooks WA. A four‐stage strategy to reduce childhood pneumonia‐related mortality by 2015 and beyond. Vaccine 2009; 27:619–623. [DOI] [PubMed] [Google Scholar]
- 11. Kitphati R, Apisarnthanarak A, Chittaganpitch M, et al. A nationally coordinated laboratory system for human avian influenza A (H5N1) in Thailand: program design, analysis, and evaluation. Clin Infect Dis 2008; 46:1394–1400. [DOI] [PubMed] [Google Scholar]
- 12. Whitley RJ, Monto AS. Seasonal and pandemic influenza preparedness: a global threat. J Infect Dis 2006; 194(Suppl 2):S65–S69. [DOI] [PubMed] [Google Scholar]
- 13. Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chunsuttiwat S. Response to avian influenza and preparedness for pandemic influenza: Thailand’s experience. Respirology 2008; 13(Suppl 1):S36–S40. [DOI] [PubMed] [Google Scholar]
- 15. Gauthier‐Clerc M, Lebarbenchon C, Thomas F. Recent expansion of highly pathogenic avian influenza H5N1: a critical review. Ibis 2007; 149:202–214. [Google Scholar]
- 16. Statistical Information System (WHOSIS). http://www.who.int/whosis/en/ (Accessed 15 December 2009). [Google Scholar]
- 17. Central Intelligence Agency . The World Factbook Laos.(2009) Washington DC. Available at https://www.cia.gov/library/publications/the‐world‐factbook/geos.html (Accessed 15 December 2009). [Google Scholar]
- 18. British Broadcasting Corporation (2009) BBC Weather. Laos, Vientiane. Available at http://www.bbc.co.uk/weather/world/country_guides/results.shtml?tt=TT002550 (Accessed 15 December 2009). [Google Scholar]
- 19. Witt CJ, Malone JL. A veterinarian’s experience of the spring 2004 avian influenza outbreak in Laos. Lancet Infect Dis 2005; 5:143–145. [DOI] [PubMed] [Google Scholar]
- 20. Puthavathana P, Sangsiriwut K, Korkusol A, et al. Avian influenza virus (H5N1) in human, Laos. Emerg Infect Dis 2009; 15:127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Western Pacific Regional Office, World Health Organization (2009) Guide to Harmonizing Virological and Epidemiological Influenza Surveillance. Manila. Available at http://www.wpro.who.int/internet/resources.ashx/CSR/Publications/GuideToHarmonizingInfluenzaSurveillance‐revised2302.pdf (Accessed 15 December 2009). [Google Scholar]
- 22. Mahony J, Chong S, Merante F, Yaghoubian S, et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead‐based assay. J Clin Microbiol 2007; 45:2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simmerman JM, Chittaganpitch M, Levy J, et al. (2009) Incidence, Seasonality and Mortality Associated with Influenza Pneumonia in Thailand; 2005‐2008. PLoS ONE. 4(111):e7776. doi: 10.137journal.pone.0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beckett CG, Kosasih H, Ma’roef C, et al. Influenza surveillance in Indonesia: 1999‐2003. Clin Infect Dis 2004; 39:443–449. Epub 2004 Jul 2022. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report . Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). 2008. Morbidity & Mortality Weekly Report Early Release 57. [PubMed]


