Abstract
Please cite this paper as: Gonzales et al. (2010) Low‐pathogenic notifiable avian influenza serosurveillance and the risk of infection in poultry – a critical review of the European Union active surveillance programme (2005–2007). Influenza and Other Respiratory Viruses 4(2), 91–99.
Background Since 2003, Member States (MS) of the European Union (EU) have implemented serosurveillance programmes for low pathogenic notifiable avian influenza (LPNAI) in poultry. To date, there is the need to evaluate the surveillance activity in order to optimize the programme’s surveillance design.
Objectives To evaluate MS sampling operations [sample size and targeted poultry types (PTs)] and its relation with the probability of detection and to estimate the PTs relative risk (RR) of being infected.
Methods Reported data of the surveillance carried out from 2005 to 2007 were analyzed using: (i) descriptive indicators to characterize both MS sampling operations and its relation with the probability of detection and the LPNAI epidemiological situation, and (ii) multivariable methods to estimate each PTs RR of being infected.
Results Member States sampling a higher sample size than that recommended by the EU had a significantly higher probability of detection. Poultry types with ducks & geese, game‐birds, ratites and “others” had a significant higher RR of being seropositive than chicken categories. The seroprevalence in duck & geese and game‐bird holdings appears to be higher than 5%, which is the EU‐recommended design prevalence (DP), while in chicken and turkey categories the seroprevalence was considerably lower than 5% and with that there is the risk of missing LPNAI seropositive holdings.
Conclusion It is recommended that the European Commission discusses with its MS whether the results of our evaluation calls for refinement of the surveillance characteristics such as sampling frequency, the between‐holding DP and MS sampling operation strategies.
Keywords: Avian influenza, European Union, LPAI, risk factors, surveillance
Introduction
Avian influenza (AI) is a contagious viral disease of various avian species. Avian influenza virus (AIv) strains of the subtypes H5 and H7 can be either of low or high pathogenicity. Highly pathogenic strains can cause high and progressive mortality in commercial poultry flocks and can lead to large outbreaks with severe economical consequences to poultry industries of affected countries. Highly pathogenic AI (HPAI) epidemics occurred either as a consequence of direct introduction of HPAI virus (UK 2007 1 ) or mutations from H5 or H7 Low Pathogenic AI (LPAI) viruses. The latter likely happened in the USA 1983, 2 Mexico 1994, 3 Italy 1999–2000, 4 Chile 2002, 5 The Netherlands 2003 6 , 7 and UK 2008. 8 Based on the risk of LPAI strains changing to HPAI, the World Organization for Animal Health (OIE) 9 considers all AIv of the H5 or H7 subtypes a notifiable disease: Highly pathogenic notifiable avian influenza (HPNAI) and Low pathogenic notifiable avian influenza (LPNAI).
In accordance with the OIE, early detection, notification, control and eradication of LPNAI has become compulsory in the European Union (EU). 10 Early detection is mainly based on passive surveillance, which in general has proven effective for HPNAI strains (e.g. UK and Germany 2007 11 ). Introduction of these strains into poultry flocks induces clear clinical signs 12 , 13 and increased mortality in most poultry species. 14 However, incursions with LPNAIv may remain undetected by passive surveillance, as signs could go unnoticed. As a consequence, the EU bases detection of LPNAI on active (serological) surveillance programmes, 15 , 16 which should comply with the following objectives: (i) detecting sub‐clinical infections with LPAI of subtypes H5 and H7 thereby complementing early detection systems and subsequently preventing possible mutation of these viruses to HPAI, (ii) detecting infections of LPAI H5 and H7 subtypes in specifically targeted poultry populations at specific risk of infection due to their husbandry system or species reared, and (iii) contributing to the demonstration of a free status of a certain country, region or compartment from NAI in the framework of international trade according to OIE rules. 15 , 16
The EU recognizes the application of random, risk based (targeted) or both surveillance strategies, for the implementation of serological surveillance by its MS. 15 , 16 Risk based surveillance may be targeted to the poultry populations at specific risk and linked to factors such as type of production (free range production, backyard flocks), multi‐age or multi‐species flocks, use of surface water, poultry life span, and other relevant factors, such as local trade patterns including live bird markets, holdings with poor biosecurity and possible direct or indirect contact with wild birds. 9
Serological surveillance of poultry populations in MS were first carried out in 2003. 17 The objective was to perform an initial screening to get a first idea of the extent of LPAI H5 and H7 introductions in different species of poultry as a precursor study for possible EU‐wide surveillance programme. Since then, the number on MS implementing surveillance and the number of samples tested have increased over the past years. 18 However, despite the increase in participation of MS in the programme and the overall large amount of samples processed, there are still gaps in our knowledge of the epidemiological situation of LPNAI infections in the EU. For example, there is no quantitative estimation of the risk of each poultry type (PT) of being infected with LPNAI. This risk has been, so far, only qualitatively assessed. 19 Additionally, there is the need to evaluate the current surveillance results in order to optimize the programme’s surveillance design. 16 , 17 This paper analyses the EU surveillance activity carried out from 2005 to 2007, in particular addressing: (i) the MS adherence to the recommended sample size, (ii) the degree of concordance of the observed prevalence and the a priori prevalence assumed in the surveillance design, and (iii) the risk of each PT of being infected with LPNAI in the programme.
Methods
Sampling design
For serological surveillance, the EU sampling design targets the detection of at least one infected holding based on an a priori prevalence [design prevalence (DP)] of AI infected holdings of 5% or more and a confidence level of 95% for each PT (layers, chicken breeders, broilers, etc.) except turkey, duck, geese and quail holdings. For the latter four PTs the sampling design is based on a confidence level of 99%. The number of birds to be sampled within a holding must ensure the detection of at least one positive bird assuming a DP of 30% and a confidence level of 95%. In simple numbers, at least 5 to 10 birds (except ducks, geese and quail) should be sampled per holding, and if there is more than one house within a holding it is recommended to sample at least five birds per house (this would increase the sample size accordingly to the number of houses in the holding). In case of ducks, geese and quails it is recommended to sample at least 40 to 50 birds per holding. 15 , 16 This higher number of birds sampled is due to the expected lower sensitivity of serological tests when testing these bird species. 20
Data analysis
The data used for the analyses are those submitted in 2005, 2006 and 2007 by MS to the European Commission and published in the official LPNAI surveillance reports. 18 , 21 , 22
To summarize and describe MS sampling operations, the ratio between the total number of holdings sampled by each MS for each PT and the minimum sample size required by the EU for each specific PT (ratio = holdings sampled/required sample size) was used as a descriptive indicator. A ratio equal to one indicates that the MS sampled the minimum required number of holdings, a ratio higher than 1 means that the MS sampled more than required and a ratio lower than 1 means that the MS sampled less holdings than required. The relation between this ratio and the probability of finding at least one positive holding was evaluated using a logistic regression model. The results of the model were interpreted as (adjusted by the covariates year and PT) summary odds ratios (sOR), of detecting at least one positive holding when the ratio >1 compared to a ratio ≤1 (Table 1). The goodness of fit of the model was assessed by residual analysis.
Table 1.
Description of the variables used in the multivariable (Logistic and Poisson) regression models
| Variable | Type | Description |
|---|---|---|
| Poultry type (PT) | Categorical | 1 = chicken breeders, 2 = commercial layers, 3 = broilers, 4 = turkey fatteners, 5 = turkey breeders, 6 = backyard, 7 = ducks & geese, 8 = game‐birds, 9 = ratites, 10 = others† |
| Year | Categorical | Year of each survey: 2005, 2006 and 2007 |
| Samples | Numerical | Total number of samples taken by each MS for each PT |
| Ratio sampled/required | Categorical | 1 = Ratio >1 0 = Ratio ≤1‡ |
| Member States§ | Categorical | Every Member State (MS) of the EU |
| Result | Numerical | Number of positive holdings for each PT and MS each year of survey This was the response variable in the Poisson model |
| Detection | Categorical | 1 = Detected 0 = Not detected This was the response variable in the logistic model |
†Member States reported as others: pheasants, partridges, Zoo birds, quails, ostrich, Passeriformes, pigeons, ornamental birds and guinea fowl.
‡An initial evaluation showed no difference between Ratio = 1 and Ratio < 1, therefore these were joined as one factor for the final logistic model evaluating the relation between the amount of sampling and the probability of detection.
§Member States were initially included as a categorical covariate in both, the logistic and the Poisson models. They were removed from the final models since no significant differences (between MS) were observed for this variable and the models fitted better when this variable was excluded.
The seroprevalence of LPNAI in poultry holdings was modeled using a Poisson regression model where ln(number of holdings positive/holdings sampled)j = b0 + bi (poultry type) + bk (year of surveillance). Table 1 shows a description of the variables used in the model. In this model, ln(number of holdings positive)j is the natural log of the number of PTj positive holdings in MSj. Ln(holdings sampled)j is the natural log of the total number of PTj holdings sampled in MSj. The estimated parameters were b0 as the intercept, bi as the regression coefficients (RC) of PTi, bk as the RC for Yeark. The parameters were exponentially expressed (eRC) and the results interpreted as the prevalence ratio (PR) or relative risk (RR). 23 , 24 The significance of the RCs was estimated by the Wald test. The goodness of fit of the model was assessed by residual analysis. Turkey breeders as well as all the data from France were removed from the final model, because in the residual analysis they were outliers.
To characterize the situation of poultry with respect to LPNAI in the EU, we calculated the observed seroprevalence in MS, but also the one tailed exact 95% Fisher’s upper confidence limits (UCL). The latter results were expressed as the maximum expected seroprevalence of LPNAI in each PT.
All the statistical analyses were performed using the software packages ‘R’ 25 for fitting the Poisson and logistic models and WinPepi 26 for the UCL estimations.
Results
Member State’s surveillance sampling operation
The surveillance activities were carried out by each MS not in an homogenous way, main differences included the sample size and the selected PTs. Member States such as Denmark, Italy, Spain, Portugal, The Netherlands, Bulgaria and Romania sampled higher numbers of holdings (some MS like The Netherlands even sampled all the poultry holdings included in the surveillance) than the number requested by the EU guidelines (Table 2a,b).
Table 2.
Number of holdings and poultry types (PT) sampled during the surveillance performed from 2005 to 2007 by European Union Member States (MS) (a) at least one LPNAI positive holding was detected, (b) no LPNAI positive holding was detected
| Member State (MS) | 2005 | 2006 | 2007 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. PT sampled | No. holdings sampled (targeted holding population) | Median ratio holdings sampled/required‡ | No. positive holdings | No. PT sampled | No. holdings sampled (targeted holding population) | Median ratio holdings sampled/required | No. positive holdings | No. PT sampled | No. holdings sampled (targeted holding population) | Median ratio holdings sampled/required | No. positive holdings | |
| (a) At least one LPNAI positive holding was detected | ||||||||||||
| Belgium (BE) | 6 (2)† | 355 | 0.8 | 8 | 7 (3) | 749 (894) | 0.9 | 4 | 9 (6) | 722 (760) | 1.0 | 7 |
| Czech Republic (CZ) | 3 (3) | 91 (220) | 1.0 | 5 (4) | 127 (240) | 1.0 | 5 (4) | 158 (221) | 1.2 | 10 | ||
| Denmark (DK) | 5 (3) | 283 | 1.0 | 6 (4) | 611 (533) | 1.8 | 13 | 5 (4) | 1286 (1190) | 3.4 | 10 | |
| Finland (FI) | 6 (4) | 238 (1891) | 1.0 | 6 (1) | 193 (1211) | 0.6 | 2 | 9 (5) | 143 (1148) | 1.0 | 1 | |
| France (FR) | 5 (3) | 959 (7349) | 2.2 | 59 | 7 (5) | 1075 (9438) | 1.9 | 45 | 7 (6) | 1195 (45419) | 1.6 | 50 |
| Germany (DE) | 4 (3) | 396 (104884) | 1.6 | 3 | 9 (4) | 1024 | 3.1 | 3 | 8 (4) | 850 | 1.0 | 3 |
| Italy (IT) | 7 (6) | 2373 (3195) | 4.0 | 7 (7) | 2335 (2818) | 4.0 | 2 | 10 (9) | 10239 (148436) | 11.6 | 27 | |
| Netherlands (NL) | 6 (6) | 5396 (2672) | 10.4 | 5 (5) | 6623 (2448) | 16.5 | 1 | 6 (5) | 2913 (2853) | 5.8 | ||
| Poland (PL) | 5 (4) | 501 (816) | 1.6 | 2 | 6 (4) | 458 (725) | 1.2 | 1 | 6 (6) | 630 (1447) | 2.0 | 2 |
| Portugal (PT) | 6 (6) | 488 (2884) | 1.3 | 8 (7) | 3094 (239287) | 1.2 | 8 (8) | 775 (237738) | 1.5 | 4 | ||
| Romania (RO) | 9 (9) | 86056 (2017701) | 2.5 | 1 | ||||||||
| Spain (ES) | 9 (8) | 2370 (13587) | 3.0 | 1 | 10 (9) | 7390 (32094) | 5.1 | 17 | 10 (7) | 5254 (36531) | 2.6 | 12 |
| Sweden (SE) | 6 (3) | 169 (651) | 1.0 | 7 (6) | 181 (491) | 1.0 | 8 (4) | 203 (612) | 1.0 | 3 | ||
| United Kingdom (UK) | 7 (4) | 438 | 1.0 | 1 | 7 (4) | 452 (4055) | 1.0 | 3 | 7 (2) | 340 (4439) | 0.7 | 10 |
| (b) No LPNAI positive holding was detected | ||||||||||||
| Austria (AT) | 4 (3)† | 177 (1768) | 1.0 | 4 (3) | 188 (1781) | 1.0 | 4 (4) | 177 (1749) | 1.1 | |||
| Bulgaria (BG) | 10 (8) | 11849 (696011) | 7.2 | |||||||||
| Cyprus (CY) | 8 (2) | 71 | 0.4 | 8 (4) | 168 (8723) | 0.9 | 8 (3) | 131 (8755) | 0.7 | |||
| Estonia (EE) | 1(1) | 13 (13) | 1.0 | 2 (1) | 18 (13) | 1.0 | 3 (1) | 23 (3575) | 0.4 | |||
| Greece (EL) | 6 (2) | 158 | 0.6 | 7 (3) | 360 | 1.4 | 9 (4) | 240 (149547) | 0.9 | |||
| Hungary (HU) | 4 (3) | 527 (2785) | 1.6 | 5 (4) | 2679 (2075) | 1.4 | 9 (6) | 2676 | 1.0 | |||
| Ireland (IE) | 5 (4) | 305 (398) | 1.3 | 5 (4) | 306 (397) | 1.2 | 7 (4) | 275 (394) | 1.0 | |||
| Latvia (LV) | 5 (1) | 38 (43) | 0.7 | 6 (6) | 133 (42) | 1.0 | 6 (6) | 119 | 1.0 | |||
| Lithuania (LT) | 8 (0) | 42 (174281) | 0.4 | 7 (1) | 175 | 0.5 | 6 (6) | 107 (33) | 2.7 | |||
| Luxembourg (LU) | 2 (2) | 12 (12) | 1.0 | 2 (2) | 15 (15) | 1.0 | 4 (2) | 40 (771) | 0.9 | |||
| Malta (MT) | 1 (1) | 63 (305) | 1.2 | 1 (1) | 71 (83) | 1.3 | 1 (0) | 30 (305) | 0.6 | |||
| Slovak Republic (SK) | 7 (1) | 134 (299) | 0.5 | 9 (4) | 430 (54827) | 0.9 | 10 (7) | 332 (332) | 1.3 | |||
| Slovenia (SI) | 5 (4) | 187 (279) | 1.0 | 5 (3) | 150 (267) | 1.0 | 6 (4) | 149 (860) | 1.0 | |||
(†)Number of PT sampled to an equal or higher number of holdings than the required statistical sample size as stated in the EU surveillance guidelines.
‡The ratio of the number of holdings sampled/required sample size was estimated for each PT and the median of these ratios is reported per MS as a descriptive summary of the MS sampling operations.
The ratio holdings sampled/required sample size was used for describing MS sampling operations and their relation with the probability of detection. The results of the logistic model, showed a significant relation between the ratio >1 and the probability of detecting at least one positive holding. The estimated OR for the ratio >1 was 6·3 (95% CI: 3·22–16·30) compared to the ratio ≤1, which means that the odds for detection of a positive holding were 6·3 times higher when the number of holdings sampled were higher than the EU required sampled size.
Table 2a,b summarize each country sampling operation for 2005–2007 surveillance. Member States were grouped in two groups: MS which detected at least one positive holding in at least 1‐year surveillance (n = 14) (Table 2a) and those which did not detect any positive holding (n = 13) (Table 2b). Most MS with positive findings had a median overall ratio of holdings sampled/required sample size higher than 1 (approximately 50% of PTs targeted for sampling, were sampled in higher numbers than the required sample size), while most MS with only negative results had a median ratio equal or lower than 1 (Figure 1).
Figure 1.
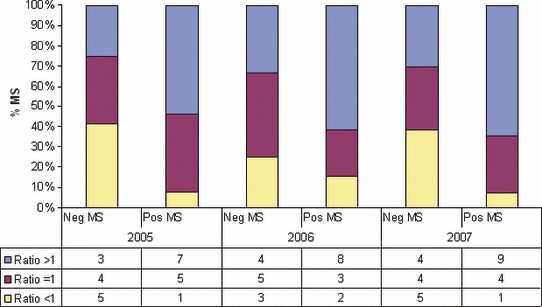
Proportion of Member States (MS) with ratio holdings sampled/required sample size less than 1, equal to 1 and higher than 1, each year of surveillance. Comparing MS with negative findings versus MS with positive findings. Numbers in the table represent the number of MS with the corresponding ratio.
LPNAI in poultry and the PT risk of being infected
Since 2005, an increase in the number of holdings sampled by MS and an increase in the number of positive holdings were observed for most PTs (Table 3). A multivariable analysis which included each year of surveillance and the targeted PTs showed an increase in the overall PR of LPNAI in poultry, in 2006 and 2007 compared to 2005 (Table 4). When MS (including France) were also included as a variable in this analysis (Table 1), no significant differences in the PR between MS were observed. This variable was removed from the final model, in order to improve the fit of the model.
Table 3.
Number of Member States (MS) targeting each poultry type (PT) and number of holdings sampled during 2005–2007 surveys in the EU
| Poultry type (PT) | 2005 | 2006 | 2007 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MS sampling each PT | No. holdings sampled | No. positives (highest prevalence %)¶ | MS sampling each PT | No. holdings sampled | No. positives (highest prevalence %)¶ | MS sampling each PT | No. holdings sampled | No. positives (highest prevalence %)¶ | |
| Chicken breeders | 15 (12)† | 2489 | 3 (4·2) | 17 (11) | 2130 | 1 (0·1) | 22 (17) | 2646 | |
| Laying hens | 25 (22) | 5869 | 1 (1·5) | 25 (22) | 8537 | 5 (1·5) | 27 (24) | 9554 | 9 (0·5) |
| Broilers | 7 (3) | 1967 | 11 (6) | 2383 | 18 (11) | 2875 | 2 (0·5) | ||
| Turkey fatteners | 20 (13) | 2058 | 21 (13) | 1981 | 1 (0·6) | 22 (16) | 3765 | 2 (1·8) | |
| Turkey breeders | 10 (4) | 251 | 10 (5) | 150 | 15 (10) | 409 | |||
| Backyard flocks | 3 (1) | 247 | 9 (8) | 9051 | 2 (0·6) | 15 (12) | 99901 | 7 (4·2) | |
| Ducks & Geese | 21 (14) | 1795 | 68 (16·7) | 22 (16) | 2176 | 62 (33·3) | 23 (16) | 4096 | 92 (23·7) |
| Game birds | 13 (5) | 756 | 18 (8) | 1500 | 12 (2·3) | 21 (14) | 1927 | 9 (10·0) | |
| Ratites | 17 (5) | 352 | 1 (11·1) | 17 (8) | 448 | 2 (2·2) | 18 (7) | 325 | |
| Others‡ | 3 (3) | 441§ | 1 (0·6) | 1 (1) | 649 | 6 (0·9) | 9 (6) | 1414 | 14 (1·3) |
| Total | 25 | 15784 | 25 | 29005 | 27 | 126912 | |||
MS, member states.
†Number of PT sampled to an equal or higher number of holdings than the required statistical sample size as stated in the EU surveillance guidelines.
‡MS reported as others: pheasants, partridges, Zoo birds, quails, ostrich, Passeriformes, pigeons, ornamental birds and guinea fowl.
§France sampled 156 free‐range chicken holdings which were reported as others.
¶The highest apparent prevalence observed by PT in a specific MS.
Table 4.
Relative risk (RR)† and accessory 95% confidence intervals of poultry holdings being infected with LPNAI. RR are summarized by poultry type and year of survey
| Variable | RR | 95% CI | P |
|---|---|---|---|
| Chicken breeders | 1·00‡ | ||
| Layers | 1·08 | 0·39–3·79 | 0·89 |
| Broilers | 0·25 | 0·01–1·70 | 0·22 |
| Turkey fatteners | 1·64 | 0·49–6·27 | 0·43 |
| Backyard | 0·46 | 0·14–1·74 | 0·21 |
| Dunk & Geese | 18·82 | 7·80–61·84 | 1·14 × 10−8 |
| Game‐birds | 6·98 | 2·55–24·39 | 5·19 × 10−4 |
| Ratite | 4·80 | 0·94–21·80 | 0·04 |
| Others | 12·80 | 4·79–44·25 | 3·84 × 10−6 |
| Year 2005 | 1·00‡ | ||
| Year 2006 | 2·03 | 1·16–3·76 | 0·02 |
| Year 2007 | 2·15 | 1·27–3·88 | <0·01 |
†Relative risks or prevalence ratios 24 were estimated by fitting a multivariate Poisson regression model to the EU surveillance results from 2005 to 2007. Survey results were the response variable, poultry type as well as year of survey were used as explanatory variables and the number of holdings sampled was used as an offset. The final model was selected after following standard procedures of model evaluation.
‡Reference categories.
Positive holdings were detected in all PTs but turkey breeders (Table 3). Turkey breeders could not be included in the final Poisson model because the data of this PT did not fit the model. The results of the model, where “Chicken breeders” were used as reference, showed significantly higher RRs for waterfowl PTs, ratites and “others” while no significant differences in the RR was observed between chicken (breeders, layers, and broilers) and turkey PTs (Table 4).
LPNAI apparent prevalence in poultry compared to the programme’s design prevalence
The EU situation with respect to LPAI in poultry was characterized taking the point estimates and the 95% UCL for the seroprevalence. In many cases the absence of positive detections was linked to a lower sample size, which led to high and uninformative UCL, therefore we only used the positive results for these characterization. Table 3 shows the highest estimated seroprevalence for each PT each year. For most PTs the estimated seroprevalence is lower than 5%, which is the a priori prevalence used for the surveillance design. Only, ducks & geese, ratites and game‐birds had estimated prevalences higher than 5%. Most of the UCLs for ducks & geese as well as game birds were higher than 5%. For the rest of PTs, UCLs were generally lower than 5% and in case of chicken layers the highest estimated UCL, within the 3 years surveillance, was 3·5%; which was observed in free range chickens in France 2005 (reported as “others”) (Figure 2).
Figure 2.
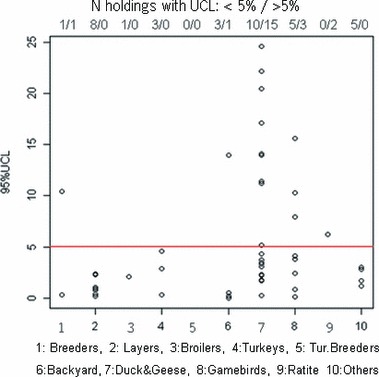
95% upper confidence limit (UCL) of the prevalence of Member States (MS) with detected positive holdings, plotted by PT. The line marks the current EU programme’s 5% design prevalence. The numbers at the top show the number of estimated UCL lower than 5% and higher than 5% for each PT.
Discussion
The overall Sensitivity (Se) of a surveillance system is a function of both the sampling scheme and the accuracy of the detection methods being employed. 27 In the context of the EU LPNAI surveillance: sampling procedures (targeted PTs and risk regionalization), sample size, sampling frequency and the applied diagnostic assays will determine the Se of the system.
Member States had different sampling procedures, and were grouped in those with only negative findings and those which were able to detect LPNAI positive holdings. The latter group included MS with risk based surveillance programmes (Italy, Spain, Denmark, The Netherlands, etc.) which, in addition to sampling holdings in the whole country, carried out enhanced surveillance in identified high risk areas or PTs. 28 , 29 , 30 , 31 For example, Denmark and The Netherlands sampled some specific PTs more than once a year (for instance free range layers and game‐birds were sampled up to four times per year). 28 , 32 Additionally, most MS with positive findings sampled more holdings than required by the EU (ratio holdings sampled/required sample size >1) (Figure 1) which was significantly associated with a higher probability of detection. On the contrary MS with only negative findings, sampled less or just the required number of holdings in each PTs. The latter may suggest that the sampling operations of these MS was not enough for detection.
Targeted surveillance to PTs with higher risk of being infected may improve the Se of the programme. In the EU targeted sampling has been based on qualitative evaluations which indicated waterfowl (ducks, geese, and game birds) as the poultry category with higher risk of being infected. 19 Our results not only confirm these qualitative observations but also provide quantitative estimations of the RR of waterfowl PTs of being infected with LPNAI compared to chicken and turkey poultry categories. The higher risk observed in waterfowl might be related to the species relatedness with migratory wild waterfowl, their long lifespan and the fact that these PTs are mainly kept outdoors which is associated with a higher risk of exposure to AIVs from the environment and wild fauna. 19 , 33 , 34 Commercial layers were the most targeted PT. This PT was targeted assuming a higher risk due to their lifespan and their production characteristics (e.g. marketing of eggs), which sustained transmission during HPAI 35 and LPNAI 36 outbreaks. Our results do not show a significant higher risk for this PT compared to chicken breeders, which are expected to have lower risk due to the higher biosecurity measures implemented in this type of production. Outdoor or free range keeping of poultry is considered a major risk factor for introduction of AI into poultry. 19 , 33 For this reason, MS targeted backyard flocks with increasing numbers every surveillance‐year, and also targeted free range layer and broiler holdings in 2007. 18 Due to the absence of sufficient data (differentiation of holdings as outdoor or indoor was only done in 2007) it was not possible to include an outdoor/indoor evaluation in the model. The low RR estimated for backyard and broilers, although they were not significant, might raise the question of the value of sampling these PTs. The short lifespan of broilers explains their low RR and it has been observed that virus introduction into backyard flocks occurred at a lower rate than into commercial holdings during the H7N7 HPNAI in 2003 in The Netherlands 37 and apparently this PT may not play a risk in the transmission of AI to commercial farms in the EU. 19 , 37 Therefore, it might be advisable to consider sampling these PTs and refine their sample size according to the risk they represent to each MS poultry industry.
It was observed that the expected prevalence in waterfowl PTs appears to be similar or higher than 5%, which is the EU programme’s between holdings DP. However, the expected prevalence in chicken and turkey PTs appears to be lower. The latter might explain the observed lower odds for detection when sampling the EU required holding sample size (ratio ≤ 1). If we take into account that some MS (n = 6) with only negative findings sampled a few or no duck & geese holdings, 18 , 21 , 22 and the fact that no difference was observed in the PR between all MS when fitting the Poisson model, it can be expected that many of these negative MS might be detecting LPNAI introductions when using a lower than 5% DP.
Frequency of sampling is a key element of the overall surveillance Se. The current EU sampling frequency of once a year inherently will result in missed or in delayed detections, which might lead to the development of undesired mutations. The rapid mutation (2–3 weeks after introduction) observed in the last H7N7 HPNAI outbreak in the UK 8 as well as evidence of multiple incursions (different times) of different LP or HPNAI, in Italy 2007 30 and UK 2007, 11 highlights the need for early detection. This can be achieved by a higher sampling frequency. Strategies such as that of Denmark and The Netherlands, where PTs considered of higher risk and/or located in higher risk areas are sampled in higher frequency than others, may contribute to improve the probability of early detection.
To conclude, the estimates of PT RRs for LPNAI reported in this study can be used for the risk based design of targeted surveillance, 38 quantitative evaluations of the Se of surveillance programmes (e.g. scenario tree models 39 ) and risk assessments. It is recommended that the European Commission discusses with its MS whether the results of our evaluation calls for refinement of the surveillance characteristics such as sampling frequency, the between‐holding DP (for chicken and turkey PTs), and MS sampling operation strategies.
Acknowledgements
All MS are gratefully acknowledged for sending their surveillance results to the European Commission and the Community Reference Laboratory (CRL) for Avian Influenza, Weybridge (UK). We thank the CRL and Uta Hesterberg for kindly providing this surveillance data for analysis in this study and we acknowledge their valuable observations. We also thank Dr. Maria Pittman, DG – SANCO, Unit D1‐Animal Health and Standing Committees for providing us further information regarding 2006 AI detections by MS. This work was supported by the EU research project 044429 FLUTEST: improved diagnosis and early warning systems for AI outbreak management and The Foundation for Economic Structure Strengthening (FES), in The Netherlands: FES Program on Avian Influenza.
References
- 1. DEFRA . Outbreak of highly pathogenic H5N1 avian influenza in Suffolk in January 2007. A report of the epidemiological findings by the National Emergency Epidemiology Group, DEFRA 5 April 2007. Available at http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/ai/documents/epid_findings070405.pdf (Accessed 18 May 2008).
- 2. Bean WJ, Kawaoaka Y, Wood JM, Pearson JE, Webster RG. Characterization of virulent and avirulent A/Chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNA’s in nature. J Virol 1985; 54:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia M, Crawford JM, Latimer JW, Rivera‐Cruz E, Perdue ML. Heterogenecity in the haemagglutinin gene and emergence of the highly pathogenic phenotype among recent H5N2 avian influenza viruses from Mexico. J Gen Virol 1996; 77:1493–1504. [DOI] [PubMed] [Google Scholar]
- 4. Capua I, Maragon S. The avian influenza epidemic in Italy, 1999–2000. Avian Pathol 2000; 29:289–294. [DOI] [PubMed] [Google Scholar]
- 5. Rojas H, Moreira R, Avalos P, Capua I, Maragon S. Avian influenza in poultry in Chile. Vet Rec 2002; 151:188. [PubMed] [Google Scholar]
- 6. Elbers ARW, Fabri THF, De Vries TS, De Wit JJ, Pijpers A, Koch G. The highly pathogenic avian influenza A virus (H7N7) epidemic in the Netherlands in 2003: lessons learned from the first five outbreaks. Avian Dis 2004; 47:914–920. [DOI] [PubMed] [Google Scholar]
- 7. Stegeman A, Bouma A, Elbers ARW et al. Avian influenza A virus (H7N7) epidemic in The Netherlands in 2003: course of the epidemic and effectiveness of control measures. J Infect Dis 2004; 190:088–095. [DOI] [PubMed] [Google Scholar]
- 8. DEFRA . Highly pathogenic avian influenza, H7N7, Oxfordshire, June 2008. Situation at 12.30pm Wednesday 2nd July. Available at http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/ai/documents/epireport‐080711.pdf (Accessed 12 July 2008).
- 9. World Organization for Animal Health (OIE) . Guidelines on Surveillance for Avian Influenza; In Terrestrial Animal Health Code, 16th edn Paris: OIE, 2007. [Google Scholar]
- 10. European Council . Council directive 2005/94/EC of 20 December 2005 on community measures for the control of avian influenza and repealing directive 92/40/EEC. OJEU 2005; L 10: 2016. [Google Scholar]
- 11. World Organization for Animal Health (OIE) . WAHID‐interface, summary of immediate notifications and follow‐ups–2007. Available at http://www.oie.int/wahid‐prod/public.php?page=disease_immediate_summary&selected_year=2007 (Accessed 28 March 2008).
- 12. Elbers ARW, Koch G, Bouma A. Performance of clinical signs in poultry for the detection of outbreaks during the avian influenza A (H7N7) epidemic in The Netherlands in 2003. Avian Pathol 2005; 34:181–187. [DOI] [PubMed] [Google Scholar]
- 13. Elbers ARW, Kamps B, Koch G. Performance of gross lesions at postmortem for the detection of outbreaks during the avian influenza A virus (H7N7) epidemic in The Netherlands in 2003. Avian Pathol 2004; 33:418–422. [DOI] [PubMed] [Google Scholar]
- 14. Elbers ARW, Holtslag JB, Bouma A, Koch G. Within‐flock mortality during the high‐pathogenicity avian influenza (H7N7) epidemic in The Netherlands in 2003: implications for an early detection system. Avian Dis 2007; 51:304–308. [DOI] [PubMed] [Google Scholar]
- 15. European Commission . Guidelines on the implementation of survey programmes for avian influenza in poultry and wild birds to be carried out in the Member States in 2007. Available at http://ec.europa.eu./food/animal/diseases/controlmeasures/avian/guidel_ai_surv_wb_poul_2007_en.pdf (Accessed 1 September 2008).
- 16. European Commission . Commission Decision 2007/268/EC of 13 April 2007 on the implementation of surveillance programmes for avian influenza in poultry and wild birds to be carried out in the Member States and amending Decision 2004/450/EC. OJEU 2007; L 115:2003. [Google Scholar]
- 17. European Commission . Commission Decision 2002/649/EC of 5 August 2002 on the implementation of surveys for avian influenza in poultry and wild birds in the Member States. OJEU 2002; L213:2038. [Google Scholar]
- 18. Hesterberg U, Young N, Cook A, Brown I. Annual Report on Surveillance for Avian Influenza in Poultry in the EU During 2007. Brussels: European Commission. Health & Consumer Protection Directorate‐General, Animal Health and Standing Committees; Available at http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/res_surv_wb_annual_07_en.pdf (Accessed 8 November 2008). [Google Scholar]
- 19. EFSA . Scientific report on animal health and welfare aspects of avian influenza. Annex to the EFSA Journal 2005; 266:1–21. [Google Scholar]
- 20. Starick E, Beer M, Hoffmann B et al. Phylogenetic analyses of highly pathogenic avian influenza virus isolates from Germany in 2006 and 2007 suggest at least three separate introductions of H5N1 virus. Vet Microbiol 2008; 128:243–252. [DOI] [PubMed] [Google Scholar]
- 21. Hesterberg U, Young N, Wootton L, Cook A, Brown I. Annual Report of the Avian Influenza Surveillance in Poultry carried out by Member States in 2006. Brussels: European Commission. Health & Consumer Protection Directorate‐General, Animal Health and Standing Committees; Available at http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/res_surv_wb_annual_06_en.pdf (Accessed 19 June 2008). [Google Scholar]
- 22. O’Connor JL, Powell LF, Stewart I, Brown IH. A Report on Surveys for Avian Influenza in Poultry in Member States During 2005. Brussels: European Commission. Health & Consumer Protection Directorate‐General, Animal Health and Standing Committees; Available at http://ec.europa.eu/food/animal/diseases/controlmeasures/avian/res_ai_surv_poultry_2005_en.pdf (Accessed 5 June 2008). [Google Scholar]
- 23. Breslow NE, Day NE. Statistical Methods in Cancer Research. Volume II – The Design and Analysis of Cohort Studies. Lyon: International Agency for Research on Cancer, 1987. [PubMed] [Google Scholar]
- 24. Thrusfield M. Veterinary Epidemiology. Oxford: Blackwell Science, 1995. [Google Scholar]
- 25. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing, 2005. Available at http://www.r‐project.org (Accessed 5 January 2009). [Google Scholar]
- 26. Abramson J. WINPEPI (PEPI‐for‐Windows): computer programs for epidemiologists. Epidemiol Perspect Innov 2004; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thurmond MC. Conceptual foundations for infectious disease surveillance. J Vet Diagn Invest 2003; 15:501–514. [DOI] [PubMed] [Google Scholar]
- 28. Danish Veterinary and Food Administration . Plan for implementation of survey programmes for avian influenza in poultry, game birds for restocking and wild birds to be carried out in Denmark in 2007 and application for financial contribution. Available at http://ec.europa.eu/food/animal/diseases/eradication/programme2007/2007_ai_da.pdf (Accessed 15 September 2008).
- 29. Rodriguez AA, Izquierdo MP, Sierra MMJ, Heras CA. Medidas de vigilancia y contencion de la influenza aviar en aves. Implicaciones para la salud publica. Rev Esp Salud Publica 2006; 80:621–630. [DOI] [PubMed] [Google Scholar]
- 30. Istituto Zooprofilatico Sperimentale delle Venezie . Low pathogenicity avian influenza in Italy: the epidemiological situation. Available at http://www.izsvenezie.it/dnn/Portals/0/AI/AI_Report_30_10_07.pdf (Accessed 15 May 2008).
- 31. European Commission . Commission Decision 2005/734/EC of 19 October 2005 laying down biosecurity measures to reduce the risk of transmission of highly pathogenic avian influenza caused by Influenza virus A subtype H5N1 from birds living in the wild to poultry and other captive birds and providing for an early detection system in areas at particular risk. OJEU 2005;L 274: 2105. [Google Scholar]
- 32. Elbers ARW, De Wit JJ, Hulsbergen HBA, Van Der Spek AN, Fabri T, Koch G. Avian Influenza Surveillance in Poultry in The Netherlands between 2004 and 2006. Beijing, China: Proceedings of the 15th World Veterinary Poultry Association Congress, 10–15 September 2007, 138. [Google Scholar]
- 33. Koch G, Elbers ARW. Outdoor ranging of poultry: a major risk factor for the introduction and development of high‐pathogenicity avian influenza. NJAS 2006; 54:179–194. [Google Scholar]
- 34. Halvorson DA, Kelleher CJ, Senne DA. Epizootiology of avian influenza: effect of season on incidence in sentinel ducks and domestic turkeys in Minnesota. Appl Environ Microbiol 1985; 49:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas ME, Bouma A, Ekker M, Fonken AJM, Stegeman A, Nielen M. Risk factors for the introduction of high pathogenicity avian influenza virus into poultry farms during the epidemic in The Netherlands. Prev Vet Med 2005; 69:1–11. [DOI] [PubMed] [Google Scholar]
- 36. Nishiguchi A, Kobayashi S, Yamamoto T, Ouchi Y, Sugizaki T, Tsutsui T. Risk factors for the introduction of avian influenza virus into commercial layer chicken farms during the outbreaks caused by a low‐pathogenic H5N2 virus in Japan in 2005. Zoonoses Public Health 2007; 54:337–343. [DOI] [PubMed] [Google Scholar]
- 37. Bavinck V, Bouma A, Van Boven M, Bos MEH, Stassen E, Stegeman JA. The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in The Netherlands in 2003. Prev Vet Med 2009; 88:247–254. [DOI] [PubMed] [Google Scholar]
- 38. Alban L, Boes J, Kreiner H, Petersen JV, Willeberg P. Towards a risk‐based surveillance for Trichinella spp. in Danish pig production. Prev Vet Med 2008; 87:340–357. [DOI] [PubMed] [Google Scholar]
- 39. Martin PAJ, Cameron AR, Greiner M. Demonstrating freedom from disease using multiple complex data sources: 1: a new methodology based on scenario trees. Prev Vet Med 2007; 79:71–97. [DOI] [PubMed] [Google Scholar]


