Abstract
Smoking prevalence in schizophrenia is significantly elevated relative to other clinical and to non-clinical groups. The cognitive self-medication hypothesis attributes this to the beneficial effects of nicotine on illness-related cognitive deficits. Significant effects of nicotine have been observed on visual spatial working memory (VSWM), sustained attention (Continuous Performance Test — Identical Pairs; CPT-IP) and prepulse inhibition (PPI). It remains unclear whether these neurophysiological and neurocognitive effects of nicotine influence self-reported smoking motivation.
To explore the relationship between nicotine effects on cognition and self-reported smoking motivation in schizophrenia and non-psychiatric control smokers, the impact of smoking abstinence and reinstatement was examined across three cognitive indices (VSWM, CPT-IP, PPI) and compared to self-reported smoking motivation (Modified Reasons for Smoking Scale revised to include ‘cognitive motivators’). Cognitive function was assessed after ‘typical’ smoking and overnight abstinence. Schizophrenia smokers (but not controls) demonstrated significantly less error on the VSWM task in the smoking relative to abstinent condition. Control (but not schizophrenia) smokers, showed evidence of CPT-IP improvement in the smoking relative to abstinent condition. The overall profile of smoking motivation was comparable between groups. However, significant relationships between subjective and objective indices of smoking related cognitive change were observed for controls.
Differential effects of nicotine on cognition have been hypothesised to influence the pattern and persistence of smoking in schizophrenia. These preliminary findings indicate that evidence for such effects is apparent even in small samples — particularly for VSWM. This is the first study to show that neurocognitive effects of smoking may influence self-reported smoking motivation.
Keywords: Motivation, Schizophrenia, Cigarette smoking, Cognitive function, Attention, Working memory
Smoking prevalence in schizophrenia is up to five times higher than other clinical and non-clinical groups (de Leon and Diaz, 2005). Commonalities in the neurobiological substrates of nicotine transmission and the pathology of schizophrenia have led to the proposal that nicotine may be used to ‘self-medicate’ illness related deficits (Kumari and Postma, 2005). Schizophrenia is characterised by pathological aberrations in the receptor system responsible for mediating the effects of nicotine (Leonard, 2003). Nicotinic cholinergic receptor (nAChR) abnormalities, in turn, have been implicated in the pathogenesis of cognitive dysfunction (Martin and Freedman, 2007). Of particular interest to the current study, accumulating evidence implicates nAChR dysfunction in the expression of several putative endophenotypic markers of schizophrenia, including sensory gating, sustained attention and spatial working memory (Sacco et al., 2004). Conversely, these domains show “strong evidence” for nicotinic modulation in schizophrenia (see Table 3, Sacco et al., 2004). Specifically, nicotine has been found to transiently ameliorate deficits in auditory sensory gating (Adler et al., 1993; George et al., 2006), sustained attention (Dépatie et al., 2002, Sacco et al., 2005) and visual spatial working memory (VSWM; Sacco et al., 2005). Evidence for facilitatory effects of nicotine in non-smokers with schizophrenia (Barr et al., 2008) and clinically unaffected relatives (Adler et al., 1992) indicates that these findings are largely independent of withdrawal (and other potential confounds, including chronic exposure to nicotine and psychotropic agents; Adler et al.,1992). However, there is a paucity of research directly assessing self-reported smoking motivation in schizophrenia (Forchuk et al., 2002, Gurpegui et al., 2007, Barr et al., 2008, Galazyn et al., 2010) and potential cognitive motivators have been largely neglected. Therefore, the degree to which these cognitive effects subjectively motivate smoking remains unclear.
Table 3.
Non-parametric correlations between self reported nicotine dependence (FTND) and MRSS-R subscale scores for schizophrenia and control smokers.
| Schizophrenia |
Control |
|||
|---|---|---|---|---|
| Factor | r | p-value | r | p-value |
| Cognitive | .230 | .082 | .325 | .041⁎ |
| Automatic | .216 | .103 | .499 | .001⁎⁎ |
| Addictive | .397 | .0028 | .549 | < .001⁎⁎ |
| Stimulation | .336 | .010⁎ | .309 | .052 |
| Sedative | .384 | .003⁎⁎ | .477 | .002⁎⁎ |
| Social | .451 | < .001⁎⁎ | .122 | .455 |
| Indulgent | .171 | .200 | − .084 | .607 |
| Sensorimotor Manipulation | .171 | .198 | − .037 | .822 |
Significant at p < .05.
Significant at p < .01.
The aims of the current study were three-fold. Firstly, we assessed self-reported smoking motivators, in particular, the perceived importance of cognitive motivators amongst outpatient smokers with schizophrenia and a non-psychiatric comparison group. Secondly, we evaluated the relative impact of smoking abstinence and re-instatement on three tasks measuring putative endophenotypic markers of schizophrenia (sustained attention, visual spatial working memory and sensory gating). Finally, we explored the relationship between self-reported motivations for smoking (i.e. perceived function of smoking) and objective indices of the physiological impact of nicotine on cognitive functioning.
1. Method
This study consisted of two experimental sessions. A ‘self-report only’ component was also introduced to assess smoking motivation amongst individuals unable or unwilling to participate in experimental sessions. This study was conducted in accordance with the ethical standards (3.10, 8.01, 8.02 and 8.06) of the American Psychological Association. Written, informed consent was obtained from all participants. Study protocol was approved by the University of Newcastle and Hunter New England Human Research Ethics Committees.
1.1. Participants
Twenty-eight smokers (≥ 15 cigarettes per day), 16 outpatients who met DSM-IV criteria (DIP; Castle et al., 2006) for schizophrenia (n = 10) or schizoaffective disorder (n = 6) and 12 community controls without a personal history of psychosis/major affective disorders (DIP; Castle et al., 2006) participated in experimental sessions. Exclusion criteria included neurological condition (e.g. stroke, epilepsy), significant head injury/trauma and evidence of alcohol or illicit substance dependence within the last six months. An additional 71 smokers, 43 outpatients with schizophrenia/schizoaffective disorder and 28 non-psychiatric controls provided self-report data only.
1.2. General procedures
Experimental participants attended two morning appointments (approximately one week apart), one after abstaining from nicotine from at least 11 pm (e.g. Adler et al.1993; ‘abstinent’), the other in the context of usual smoking behaviour (‘smoking’). The order of cognitive assessment was randomly allocated from one of six testing orders, generated using a Latin square design. Appointment order (abstinent first vs. second) was alternated within each of the six testing orders. Self-reported smoking motivation was assessed during the ‘smoking’ appointment. Self-report only participants completed a questionnaire pack assessing demographics, smoking history, self-reported dependence and smoking motivation.
1.3. Measures and instruments
1.3.1. Cigarette and substance use measures
Self-reported smoking status (abstinent vs. smoking) was verified at the beginning of experimental appointments with single breath, expired CO using a Bedfont Micro Smokerlyzer (Air-met Scientific). For the abstinent session a CO reading of ≤ 12 ppm (e.g. Kumari and Gray, 1999) was required. Withdrawal symptoms were assessed using the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999). Self-reported nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND; Steinberg et al., 2005).
1.4. Smoking motivation
Self-reported smoking motivation was assessed using a revised version of the Modified Reasons for Smoking Scale (MRSS; Tate et al., 1994). Thirteen items from the Situation × Trait Adaptive Response Smoking Motivation Questionnaire (STAR-SMQ; Gilbert et al., 2000) were combined with the MRRS into a single, comprehensive measure of self-reported smoking motivations (MRRS-Revised). Participants were required to rate each item using a five-point Likert scale (1 = Never, 5 = Always; Forchuk et al., 2002).
1.5. Cognitive assessment
1.5.1. VSWM
VSWM was assessed using a computerized task designed in accordance with published specifications (George et al., 2006). Participants moved a cursor to the location of a previously observed dot with performance indexed by the average distance (error, in cm) between the cursor and the actual location of target presentation, averaged across 16 trials. Higher scores indicate poorer performance.
1.5.2. Sustained attention
Sustained attention was assessed using the Continuous Performance Task — Identical Pairs version (CPT-IP: number trial only; Michie et al., 2000). Outcome measures included hits (correct responses to targets), false alarms (catch and filler trials), response time (ms) and discrimination [dL; Cornblatt et al., 1988], calculated for blocks 1 & 2 of the task separately to provide sensitivity to changes over the testing period.
1.5.3. PPI
The PPI paradigm was programmed according to published specifications (George et al., 2006). Auditory stimuli were presented binaurally over headphones. Acoustic startle response was measured via eye-blinks using electromyographic activity (EMG) collected using Scan 4.3 (Neuroscan Inc, Biomedics). EMG activity was acquired continuously (A/D rate of 250 Hz) and filtered with a 0.1–30 Hz bandpass filter and 5-Hz notch filter. EMG data were inspected to determine responsiveness of participants to the startle alone stimuli. Participants were classified as ‘acoustic startlers’ if they demonstrated a mean startle response of ≥ 25 μV in the smoking session (George et al., 2006), with this criterion applied to the mean of the startle alone trial across the first three blocks only (where habituation effects are weakest; Braff et al., 1992) resulting in the rejection of one schizophrenia participant.
Spontaneous and voluntary eye blinks in these data sets were then excluded according to previously published criteria (Braff et al., 1992), with 12.5% of trials in controls and 13.89% of trials in schizophrenia participants discarded (comparable to George et al., 2006). Peak amplitude, peak latency and mean amplitude (over 50–200 ms) values were extracted for analysis. PPI was defined as the difference in startle magnitude (as a percentage) between pulse trials preceded by a prepulse and pulse alone trials [1−(pp/p)×100; where pp represents the mean amplitude to pulse trials preceded by a prepulse, and represents the mean amplitude over pulse alone trials].
2. Results
Statistical analysis was performed using SPSS v 16.0. An alpha level of .05 was used for all statistical tests.
2.1. Demographic and clinical characteristics
Table 1, Table 2 display results of independent samples t-tests or X2 analyses comparing schizophrenia versus control participants on demographic, clinical and smoking variables. Schizophrenia participants demonstrated lower estimated full scale IQ (although within average limits; Table 1) and higher self-reported nicotine dependence (Table 2).
Table 1.
Demographic and clinical characteristics for experimental (EXP) and self-report participants (S-R)a.
| Schizophrenia Smokers |
Control Smokers |
p-value | |||
|---|---|---|---|---|---|
| EXP (n = 16) |
S-R (n = 43) |
EXP (n = 12) |
S-R (n = 28) |
||
| Recruitment Source | |||||
| CTNMH Volunteer Database | 50% | 63% | – | – | NA |
| ASRB Volunteer Database | 19% | 22% | – | – | NA |
| HMRI Volunteer Database | – | – | 25% | – | NA |
| Media | 19% | – | 25% | 7% | NA |
| Flyers | 12% | 9% | 50% | 93% | NA |
| Schizophrenia Fellowship | – | 6% | – | – | NA |
| Ageb | 43 (10.8) | 43 (9.78) | 37.6 (11.9) | 37.68 (8.77) | .025⁎ |
| Gender (% Male) | 56.3 | 48.8 | 50.0 | 35.7 | < .001 |
| WTAR Estimated Full-Scale IQb | 99.1 (7.2) | 104.3 (5.7) | .05⁎ | ||
| Self-reported age of illness onsetb | 21.87 (6.09) | 19.08 (4.22) | NA | NA | .11 |
| Duration | 21.13 (9.19) | 24.97 (9.67) | NA | NA | .19 |
| Antipsychotic classc | NA | NA | – | ||
| Atypical | 12 | 35 | NA | NA | |
| Typical | 2 | 9 | NA | NA | |
| CPZ equivalents (mg/day)b | 363.62 (293.66) | 612.05 (466.98) | NA | NA | .03⁎ |
Note. ASRB = Australian Schizophrenia Research Bank; CTNMH = Centre for Translational Neuroscience and Mental Health Research; CPZ = chlorpromazine; HMRI = Hunter Medication Research Institute; WTAR = Weschler Test of Adult Reading.
Values are expressed as a percentage of the group unless otherwise specified.
Values are expressed as mean (SD).
Values are expressed as the total number of participants.
Significant at p < .05.
Table 2.
Smoking variables for experimental and self-report participantsa.
| Schizophrenia Smokers |
Control Smokers |
p-value | |||
|---|---|---|---|---|---|
| EXP (n = 16) |
S-R (n = 43) |
EXP (n = 12) |
S-R (n = 28) |
||
| Smoking onset (1st full cigarette) | 12.9 (4.7) | 13.6 (3.8) | 14.2 (2.3) | 13.6 (3.2) | .77 |
| Age of daily smoking onset | 16.6 (5.3) | 17 (4.5) | 16.1 (2.9) | 16.9 (3.9) | .53 |
| Years since onset of daily smoking | 26.4 (10.2) | 25.9 (10.5) | 21.5 (13.3) | 20.8 (9.4) | .13 |
| Smoking behaviour in last 12 monthsb | |||||
| Abstinent (≥ 1 month) | 12.5 | 17.9 | 41.7 | 14.3 | .18 |
| Changed to lower nicotine cigarette | 18.8 | 22.1 | 16.7 | 28.6 | .79 |
| Reduced average daily cigarettes | 31.3 | 48.7 | 8.3 | 53.6 | .04* |
| Smoking behaviour unchanged | 25 | 25 | 25 | 32.1 | .92 |
| Lifetime number of quit attempts | 5.8 (6.8) | 8.9 (13.6) | 4 (2) | 3.8 (4.9) | .24 |
| Longest period of abstinence (weeks)b | .09 | ||||
| < 1 | 12.5 | 25 | 0 | 27.5 | |
| 1–4 | 25 | 20.8 | 25 | 22.5 | |
| 4–28 | 37.5 | 31.3 | 58.3 | 25 | |
| 29–52 | 25 | 13.5 | 0 | 17.5 | |
| > 52 | 0 | 9.4 | 16.7 | 7.5 | |
| FTND Total Score | 6.8 (1.8) | 7.2 (1.7) | 5.3 (1.6) | 5.8 (2.1) | .003⁎⁎ |
| FTND Dependence Categoryb | .005⁎⁎ | ||||
| Mild | 25 | 17.6 | 50 | 65 | |
| High | 43.8 | 41.2 | 50 | 20 | |
| Very High | 31.3 | 41.2 | 0 | 15 | |
| Duration (min) between cigarettes | 60.94 (55.92) Range = 10–210 |
36.89 (22.36) Range = 5–90 |
54.79 (19.3) Range = 30–90 |
66.66 (49.02) Range = 15–240 | .017⁎ |
Note. FTND = Fagerström Test for Nicotine Dependence (self-report inventory).
Values are expressed as mean (SD) unless otherwise specified.
Values are expressed as a percentage of the group.
Significant at p < .05.
Significant at p < .01.
2.2. Cognitive assessment
Due to small group numbers analysis of all cognitive data was restricted to within-subjects comparisons.
2.2.1. VSWM
Paired-samples t-tests demonstrated that schizophrenia [t(15) = 2.93, p = .01, Cohen’s d = 0.6], but not control (p = .81) smokers demonstrated significantly better performance during typical smoking, relative to short-term (i.e. overnight) abstinence (Fig. 1).
Fig. 1.
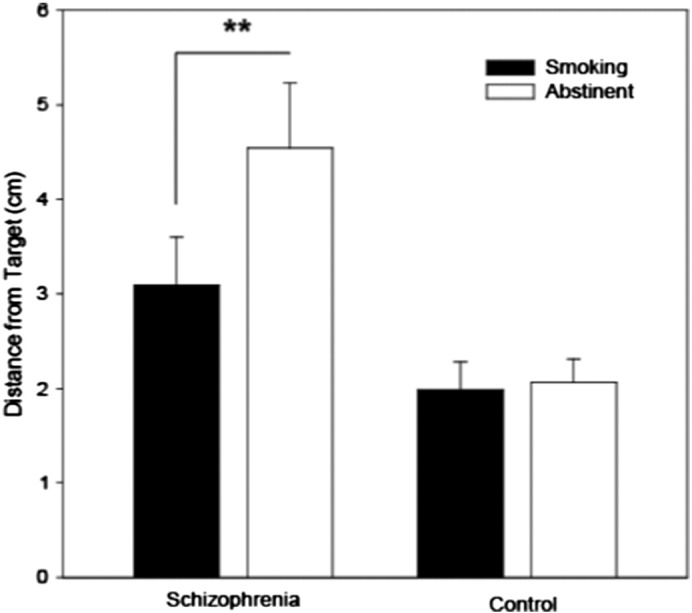
VSWM error demonstrated by schizophrenia and control smokers as a function of smoking status.
** p = .01.
2.2.2. CPT-IP
Trial block (one vs. two) and smoke status (abstinent vs. smoking) were entered into RMANOVA’s performed on each of the CPT-IP performance indices. False alarms to filler trials, catch trials and response time measures were unaffected by smoke status in both groups (all p’s > .17; data not presented).
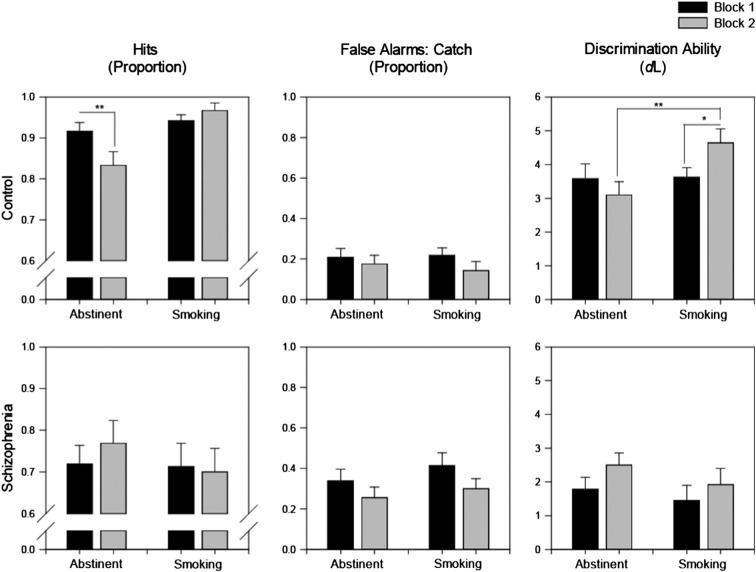
The proportion of hits, proportion of false alarms and discrimination ability (as a function of smoke status and block) appear in Fig. 2. An RMANOVA performed on control hits confirmed an interaction between smoke status and block, reflecting decline in performance over blocks [F(1,11) = 11.99, p = .005]. The main effect of smoke status F(1,11) = 15.82, p = .002], but not block (p = .15) reached significance. Paired samples t-tests confirmed that the proportion of hits significantly declined between blocks one and two in the abstinent [t(11) = 2.80, p = .017; Cohen’s d = 0.86] but not smoking appointment (p = .19). Conversely, schizophrenia hits appear relatively stable across blocks, irrespective of appointment (confirmed by RMANOVA all p’s > .36).
Fig. 2.
Proportion of hits, proportion of false alarms and discrimination ability (DL) demonstrated by schizophrenia and control smokers as a function of smoking status.
* p = < .05.
** p = < .01.
In controls, RMANOVA yielded a significant smoke status × block interaction [F(1,11) = 7.89, p = .017] and main effect of smoke status [F(1,11) = 4.82, p = .050]. Discrimination ability significantly improved across blocks in the smoking [paired t(11) = − 2.69, p = .021; Cohen’s d = 0.8], but not abstinent appointments (p = .27), with sensitivity in block two of ‘smoking’ significantly higher than block two of ‘abstinent’ [t(11) = − 3.37, p = .006; Cohen’s d = 1.09]. Conversely, the discrimination ability of schizophrenia smokers appears to marginally improve between blocks one and two, irrespective of smoking status. However, RMANOVA’s performed on the dL data of schizophrenia participants did not reach significance (all p’s > .08).
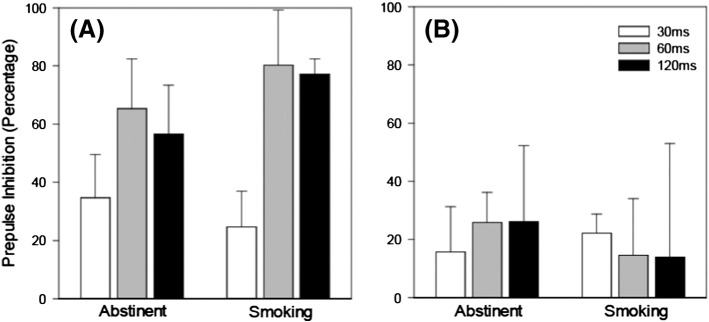
2.2.3. PPI
PPI across the three prepulse intervals (as a function of smoke status) appears in Fig. 3. RMANOVA’s revealed no significant effect of smoke status for either group (all p’s > .19). A significant effect of trial type was detected for control [ε = 0.71, F(2,20) = 19.48, p < .001], but not schizophrenia participants. In controls, PPI was significantly larger in the 60 ms [t(10) = 5.64, p < .001; Cohen’s d = 1.00] and 120 ms [t(10) = 7.65, p < .001; Cohen’s d = 1.09] conditions relative to the 30 ms condition (no difference was detected between the 60 ms and 120 ms conditions,p = .54).
Fig. 3.
Mean percentage prepulse inhibition across trial type for control (A) and schizophrenia (B) smokers as a function of smoke status.
2.3. MRSS-R
Ninety-eight participants (schizophrenia = 58, control = 40) returned the MRSS-R (one from the schizophrenia group was excluded due to excessive missing data). Mean MRSS-R subscale scores were entered into a 2 (Group: schizophrenia vs. control) × 8 (Motive: cognitive vs. automatic vs. addictive vs. stimulation vs. sedative vs. social vs. indulgent vs. sensorimotor manipulation) RMANOVA, with group as a between-subjects factor and motive as a within-subjects factor. Separate ANCOVA’s and Spearman Rank correlations were conducted to determine whether self-reported nicotine dependence (as indexed by FTND scores) influenced self-reported motivation to smoke.
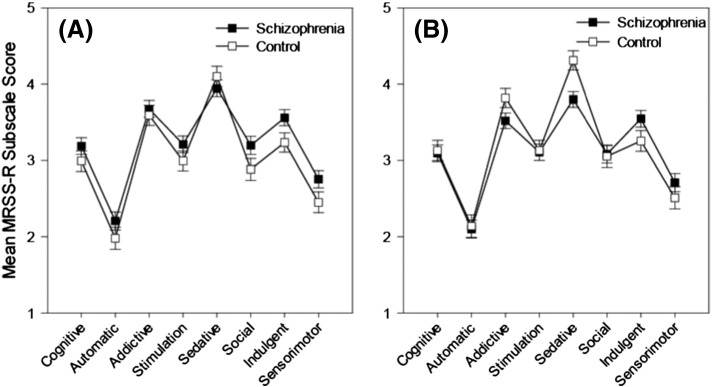
Cognitive motives represent an ‘occasional’ reason for smoking in both groups (Fig. 4a). An RMANOVA conducted on the MRSS-R data produced a significant main effect of motive [ε = .83; F(7,672) = 71.09, p < .001] but no motive × group interaction (p = .26) or main effect of group (p = .11).
Fig. 4.
Mean MRSS-R subscale scores as a function of clinical group for original (A) and FTND covariatea (B) analysis.
Note. FTND = Fagerström Test for Nicotine Dependence (self-report inventory). Responses were scored according to the following scale: 1 = Never, 2 = Seldom, 3 = Occasionally, 4 = Frequently, 5 = Always. aCovariates appearing in the model are evaluated at the following values: FTND = 6.4490.
Cognitive motives continued to represent an ‘occasional’ reason for smoking in both groups after co-varying for the effect of FTND (Fig. 4b). FTND was a significant co-variate [F(1,95) = 23.94, p < .001]. Entering FTND as a covariate in an RMANCOVA produced a significant main effect of motive [ε = .85; F(7,665) = 5.8, p < .001] along with a significant motive × group interaction [ε = .85; F(7,665) = 2.8, p = .01], with stronger endorsement of sedative motivators amongst controls [t(96) = 3.11, p < .01]. The main effect of group remained non-significant (p = .67). Correlations between FTND and MRSS-R subscale scores are presented at the end of this section in Table 3.
2.4. Relationship between cognitive function and self-reported smoking motivation
Difference scores were calculated for each of the primary cognitive performance indices (Smoking minus Abstinent). Analyses were conducted separately for each group. Significant correlations were detected between self-reported cognitive smoking motivation and CPT-IP measures only and in controls only.
Amongst controls, there was a significant positive correlation between block two hits and the ‘cognitive’ (r = .67, p = .02) motivator (Fig. 5). That is, stronger endorsement of ‘cognitive’ items was associated with a larger smoking-related increase in block two hit rate.
Fig. 5.
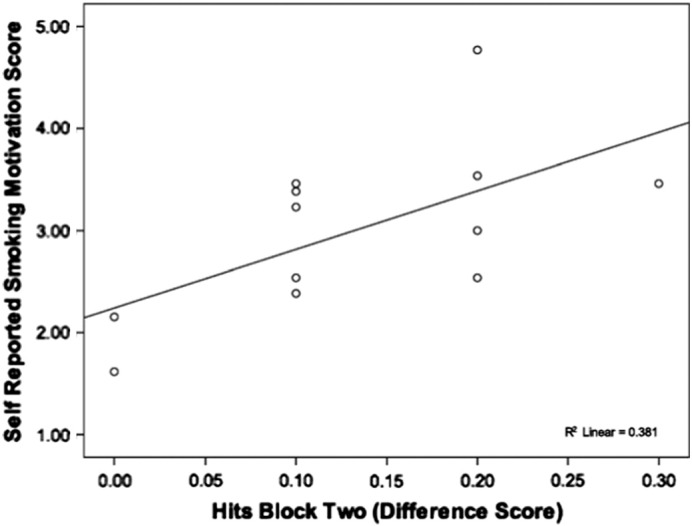
Scattergrama of difference scores for CPT-IP hits (block two) in relation to self-reported ‘cognitive’ smoking motivation scores in non-psychiatric smokers.
aGenerated from two tailed spearman rank correlate.
3. Discussion
To our knowledge, this is the first study to attempt a multi-level evaluation of subjective and objective cognitive effects of nicotine. Deficits within the three cognitive domains assessed are suspected endophenotypic markers for schizophrenia, and as such, are particularly pertinent to understanding the relationship between nicotine and cognition within this illness. Consistent with prior research (Sacco et al., 2005, Smith et al., 2006), smoking vs abstinence had a clear and significant impact on the VSWM function of smokers with schizophrenia. Relative to overnight abstinence, VSWM performance in the smoking appointment was characterised by significantly smaller error. In contrast, the VSWM performance of control smokers remained consistent across appointments. This study provides further support for the sensitivity of VSWM (dys)function to manipulation of nicotine use in smokers with schizophrenia.
Despite apparent impairments in CPT-IP performance, contrary to prediction, sustained attention in schizophrenia smokers was unaffected by the smoking/abstinence manipulation. Conversely, amongst controls, both hits and discrimination ability were significantly higher in the smoking relative to abstinent appointment. The absence of smoking related change in our schizophrenia group is inconsistent with existing evidence (Dépatie et al., 2002, Levin et al., 1996, Sacco et al., 2005, Smith et al., 2006). A notable difference between our study and Dépatie et al. (2002) is the route of nicotine administration (smoking vs. transdermal nicotine patch). Nicotine nasal spray demonstrates a similar pharmacokinetic profile to smoking (Zevin et al., 1998), and consistent with our findings, there is some evidence that CPT-IP performance of smokers with schizophrenia is unaffected by nicotine nasal spray (Sherr et al., 2002). Furthermore, Levin et al. (1996) and Sacco et al. (2005) both used Conner’s CPT. In Conner’s CPT, selective attention and response inhibition are more strongly emphasised (Dépatie et al., 2002), whereas CPT-IP (used in the current study) is a more sensitive assessment of sustained attention and working memory processes (Dépatie et al., 2002, Michie et al., 2000). One potential implication is that, in schizophrenia, the neural networks mediating selective attention and response inhibition (i.e. Conner’s CPT) may demonstrate a differential response to nicotine, relative to sustained attention and working memory (i.e. CPT-IP). Comparing the effect of nicotine across different CPT task versions in a single study may help to clarify apparent discrepancies in the literature.
Contrary to expectation, PPI in smokers with schizophrenia was unaffected by smoking status. In light of existing evidence (e.g. Adler et al., 1993; George et al., 2006; Olincy et al., 2006; Woznica et al., 2009) it would be premature to interpret the current findings as evidence for an absence of effect. The observed discrepancy between the current findings and existing evidence may result from differences in the proximity between smoking and assessment (due to counterbalancing in the current study) and the use of left, relative to right eye EMG. For example, Cadenhead et al. (2000) demonstrated that, as a group, smokers exhibited higher PPI for right but not left eye EMG (Cadenhead et al., 2000). The relative effect of nicotine on right vs. left eye EMG represents an interesting area for future research. Utilisation of bilateral recording procedures may help to more clearly elucidate whether smoking related effects in schizophrenia demonstrate evidence of hemispheric asymmetry.
In summary, we predicted that smoking related change on three putative endophenotypic markers would be strongest for smokers with schizophrenia. This hypothesis was supported by evidence from a computerised version of the VSWM task, but not CPT-IP or PPI. Importantly, change in VSWM function was specific to the schizophrenia group and occurred irrespective of the factors that may have contributed to null effects on CPT-IP (e.g. nicotine dose) and PPI (e.g. temporal proximity to nicotine). Evidently, VSWM function may be especially sensitive to smoking related performance change in schizophrenia.
Informed by the cognitive self-medication hypothesis, we predicted that smokers with schizophrenia would offer a higher level endorsement of cognitive motivators for smoking and furthermore, that smokers who reported stronger cognitive motivations for smoking would also demonstrate a stronger impact of nicotine on cognitive functioning. Our data suggest that, on the measures used here, there is no inflation of cognitive motives in schizophrenia relative to non-psychiatric smokers. According to the Primary Addiction Hypothesis (Chambers et al., 2001), addiction is conceived to be a primary symptom of the schizophrenia syndrome, due to fundamental commonalities in the neurobiology of schizophrenia and addiction (Chambers et al., 2001). Therefore, our self-report data could be interpreted as evidence for smoking in schizophrenia as a biologically driven process (addiction vulnerability) that is independent from subjective awareness.
However, we did find an interesting relationship between cognitive motivators and smoking related change in the sustained attention performance of controls. It is noteworthy that the cognitive task with which self-reported cognitive motives did correlate could be argued to be the most general and most easily definable in lay terms — that is, sustained attention. This finding could be interpreted as having dual implications: that if we wish to assess correlations between motivation and cognition we require tasks that measure general abilities that map well to lay-description of cognitive effects; and that if we wish to better understand the pathways by which nicotine affects cognition, we may require more specific tasks where the contributions of various cognitive operations can be delineated.
The current study is limited by a small sample size, meaning that we were restricted to within subjects comparisons on cognitive measures and correlational analyses were underpowered. Although the reporting of within subjects effects of nicotine versus abstinence in prior research does not enable us to calculate power for PPI and VSWM, the effect size derived from published findings for CPT-IP (Cohen’s dz = 0.89; Dépatie et al., 2002) supports the expectation for relatively ‘large’ effects of nicotine (based on Cohen, 1988) on this task. For schizophrenia participants, post-hoc power analysis using Gpower (α = .05, two-tailed, dependent means; Faul et al., 2007) demonstrated adequate statistical power (.80) for detecting moderate to large effects (≥ .75) of nicotine on CPT-IP performance.
If we had not observed any significant effects then one might reasonably question that the significance of the results might be limited by sample size. However, what we did see was a significant effect on VSWM in patients despite no significant effect on CPT-IP and PPI. This result then replicates published findings in an independent laboratory and adds to the evidence that this measure appears particularly sensitive to nicotine effects in schizophrenia. Moreover, unlike several prior investigations (e.g. George et al., 2006; Olincy et al., 2006; Sacco et al., 2005) we did not experimentally manipulate the nicotinic agonist/antagonist administration. It was therefore possible to counterbalance the smoking and abstinent appointment and thereby reduce the contribution of any practice or order effects. This design distinguishes the current study from prior research in the area (e.g. Levin et al., 1996, Sacco et al., 2005, George et al., 2006; Postma et al., 2006) and should be considered in interpreting differences in smoking related effects in the current study, relative to prior research.
It is also worth noting that the abstinent criterion adopted in the current study (< 12 ppm) is consistent with some (e.g. Kumari et al., 1996; Kumari and Gray, 1999), but higher than others (e.g. Sacco et al., 2005; Smith et al., 2002). Given that the mean CO level for both participant groups during the abstinent appointment (control = 8.78; schizophrenia = 8.75) is comparable to studies adopting the more stringent criterion (e.g. Sacco et al., 2005), surreptitious smoking is unlikely to have contributed to our findings. However, future research should consider employing more definitive methods for verifying abstinence (e.g. plasma nicotine levels).
In summary, task choice in this study was based on those for which existing data supported nicotine effects in schizophrenia (see Sacco et al., 2004). Whether these are the most sensitive indices and indeed the specific components of task performance facilitated by nicotine has yet to be determined. Developing sensitive indices of nicotine-induced cognitive enhancement is an important precursor to attempts to link changes in performance on these tasks to self-report motives, as is the challenge of developing more intuitive descriptions of how ability changes might manifest in everyday life. Findings from the current study highlight several methodological considerations for this type of research and promising avenues for future study. Improved understanding of the subjective, neurophysiological and neurocognitive effects of nicotine represents an important step for developing improved smoking cessation strategies.
Role of funding source
Funding for this research was provided by a scholarship awarded to the chief author by the Schizophrenia Research Institute, NSW, Australia. The funder did not have any further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Contributors
All authors contributed and have approved the final manuscript.
Conflict of interest
No conflict of interest to declare.
Acknowledgments
We gratefully acknowledge the infrastructure support from the University of Newcastle and the Schizophrenia Research Institute and thank Gavin Cooper for programming support. We would also like to acknowledge our participants, without whom this research could never have been conducted. This paper is based on a doctoral dissertation submitted by Alison Beck in partial fulfilment of the requirements of a Professional Doctorate (Clinical Psychology).
References
- Adler L.E., Hoffer L.J., Griffith J., Waldo M.C., Freedman R. Normalisation by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol. Psychiatry. 1992;32(7):607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Adler L.E., Hoffer L.D., Wiser A., Freedman R. Normalisation of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiatry. 1993;150(12):1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Barr A.M., Procyshyn R.M., Hui P., Johnson J.L., Honer W.G. Self-reported motivation to smoke in schizophrenia is related to antipsychotic drug treatment. Schizophr. Res. 2008;100(1–3):252–260. doi: 10.1016/j.schres.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Braff D.L., Grillon C.A., Geyer M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Cadenhead K.S., Swerdlow N.R., Schafer K.M., Diaz M., Braff D.L. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: Evidence of inhibitory deficits. Am. J. Psychiatry. 2000;157(10):1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Castle D.J., Jablensky A., McGrath J.J. The diagnostic interview for psychosis (DIP): development, reliability and applications. Psychol. Med. 2006;36:69–80. doi: 10.1017/S0033291705005969. [DOI] [PubMed] [Google Scholar]
- Chambers R.A., Krystal J.H., Self D.W. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol. Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Cornblatt B.A., Risch N.J., Faris G., Friedman D., Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatr. Res. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- de Leon J., Diaz F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dépatie L., O'Driscoll G.A., Holahan A.-L.V. Nicotine and behavioural markers for of risk for schizophrenia: a double-blind, placebo controlled, cross-over study. Neuropsychopharmacology. 2002;27(6):1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Forchuk C., Norman R., Malla A. Schizophrenia and the motivation for smoking. Perspect. Psychiatr. Care. 2002;38(2):41–49. doi: 10.1111/j.1744-6163.2002.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Galazyn M., Steinberg M.L., Gandhi K.K., Piper M., Williams J.M. Reasons for smoking among individuals with schizophrenia. Schizophr. Res. 2010;122(1–3):268–269. doi: 10.1016/j.schres.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T.P., Termine A., Sacco K.A. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: involvement of nicotinic receptor mechanisms. Schizophr. Res. 2006;87(1–3):307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Gilbert D.G., Sharpe J.P., Ramanaiah N.V., Detwiler F.R.J., Anderson A.E. Development of a Situation × Trait Adaptive Response (STAR) model-based smoking motivation questionnaire. Personal. Individ. Differ. 2000;29(1):65–84. [Google Scholar]
- Gurpegui M., Martínez-Ortega J.D., Aguilar M.C., Diaz F.J., de Leon J. Subjective effects and the main reason for smoking in outpatients with schizophrenia: a case–control study. Compr. Psychiatry. 2007;48:186–191. doi: 10.1016/j.comppsych.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kumari V., Gray J.A. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology. 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Kumari V., Postma P. Nicotine use in schizophrenia: the self medication hypothesis. Neurosci. Biobehav. Rev. 2005;29(6):1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Kumari V., Checkley S.A., Gray J.A. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology. 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Leonard S. Consequences of low levels of nicotinic acetylcholine receptors in schizophrenia for drug development. Drug Dev. Res. 2003;60:127–136. [Google Scholar]
- Levin E.D., Wilson W., Rose J.E., McEvoy J. Nicotine–haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15(5):429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Martin L.F., Freedman R. Schizophrenia and the α7 nicotinic acetylcholine receptor. Int. Rev. Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- Michie P.T., Kent A., Stienstra R., Castine R., Johnston J., Dedman K. Phenotypic markers as risk factors in schizophrenia: neurocognitive factors. Aust. N. Z. J. Psychiatry. 2000;34:S74–S85. doi: 10.1080/000486700226. (Suppl.) [DOI] [PubMed] [Google Scholar]
- Olincy A., Harris J.G., Johnson L.L., Pender V., Kongs S., Allensworth D. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch. Gen. Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Postma P., Gray J.A., Sharma T., Geyer M., Mehrotra R., Das M. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology. 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- Sacco K.A., Bannon K.L., George T.P. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J. Psychopharmacol. 2004;18(4):457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco K.A., Termine A., Seyal A.A. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62(6):649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Sherr J.D., Myers C., Avila M.T., Elliott A., Blaxton T.A., Thaker G. The effects of nicotine on specific eye tracking measures in schizophrenia. Soc. Biol. Psychiatry. 2002;52(7):721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- Smith R.C., Singh A., Infante M., Khandat A., Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smith R.C., Warner-Cohen J., Matute M. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Steinberg M.L., Williams J.M., Steinberg H.R., Krejci J.A., Ziedonis D.M. Applicability of the Fagerström Test for Nicotine Dependence in smokers with schizophrenia. Addict. Behav. 2005;30:49–59. doi: 10.1016/j.addbeh.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Tate J.C., Pomerleau C.S., Pomerleau O.F. Pharmacological and non-pharmacological smoking motives: a replication and extension. Addiction. 1994;89:321–330. doi: 10.1111/j.1360-0443.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Welsch S.K., Smith S.S., Wetter D.W., Jorenby D.E., Fiore M.C., Baker T.B. Development and validation of the Wisconsin Smoking Withdrawal Scale. Expert Clin. Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Woznica A.A., Sacco K.A., George T.P. Prepulse inhibition deficits in schizophrenia are modified by smoking status. Schizophr. Res. 2009;112:86–90. doi: 10.1016/j.schres.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Zevin S., Gourlay S.G., Benowitz N.L. Clinical pharmacology of nicotine. Clin. Dermatol. 1998;16(5):557–564. doi: 10.1016/s0738-081x(98)00038-8. [DOI] [PubMed] [Google Scholar]