Abstract
Background
Patients with schizophrenia show impairments in social information processing, such as recognising facial emotions and face identity.
Goal
The aim of this study was to explore whether these impairments represent specific deficits or are part of a more general cognitive dysfunction.
Method
Forty-two patients with schizophrenia and 42 matched controls were compared on facial emotion and face identity recognition versus (non-social) abstract pattern recognition, using three tasks of the Amsterdam Neuropsychological Tasks (ANT) program.
Results
Patients were slower than controls in social information processing as well as in (non-social) abstract pattern recognition. Patients were also less accurate than controls in processing social information, but not in recognition of abstract patterns. Differences between patients and controls were most substantial for facial emotion recognition compared to both face identity recognition (speed) and non-social pattern recognition (speed and accuracy). Finally, differences between patients and controls were largest for the recognition of negative emotions.
Conclusion
Compared to controls patients with schizophrenia displayed more difficulties in processing of social information compared to non-social information. These results support the hypothesis that facial emotion recognition impairment is a relatively distinct entity within the domain of cognitive dysfunction in schizophrenia.
Keywords: Schizophrenia, Social cognition, Neurocognition, Emotion perception, Face recognition
1. Introduction
For adequate social interaction a quick and proper apprehension of social information is required. Patients with schizophrenia experience problems in the processing of such information. It has been repeatedly demonstrated that patients have substantial problems with recognizing emotions in facial expressions of others (Kohler et al., 2010, Marwick and Hall, 2008), not only compared to healthy control subjects (Kohler et al., 2000, Turetsky et al., 2007, Van’t Wout et al., 2007) but also compared to patients with other psychiatric disorders (Addington and Addington, 1998, Weniger et al., 2004). Facial emotion recognition is part of the domain of social cognition, which further includes social perception and knowledge, theory of mind and attributional bias (Green et al., 2005, Penn et al., 2008).
Based on the differences between patients with schizophrenia and healthy controls on both social cognitive and neurocognitive tasks, there is growing evidence that impairments in social cognition should be considered an independent construct (Mehta et al., 2013, Penn et al., 2000, Pinkham, 2003, Sergi et al., 2007, Van Hooren et al., 2008) with suggestions for separate neural pathways (Phillips et al., 2003). On the other hand there appears to be an overlap between social cognitive and neurocognitive impairments (Addington and Addington, 1998, Kohler et al., 2000, Oerlemans et al., 2013, Poole et al., 2000, Van Rijn et al., 2011), indicative of a more generalized cognitive deficit.
Social cognition appears to explain more variance in functional outcome parameters such as community functioning, compared to neurocognition (Fett et al., 2011). Some authors have proposed that social cognition may act as a mediator in the relation between neurocognition and functional outcome in patients with schizophrenia (Addington et al., 2006, Barbato et al., 2013, Sergi et al., 2007).
Within the domain of social information processing there is a debate on the specificity of facial emotion recognition in relation to the recognition of facial identity. While in some studies no differential deficits between these abilities were found (Addington and Addington, 1998, Pomarol-Clotet et al., 2010, Sachs et al., 2004), other studies have shown specific deficits in facial affect recognition in patients with schizophrenia (Kosmidis et al., 2007, Kucharska-Pietura et al., 2005, Penn et al., 2000, Poole et al., 2000, Schneider et al., 2006).
More detailed studies suggest that patients with schizophrenia specifically experience problems with recognising negative emotions, including fear, sadness, anger and disgust (Edwards et al., 2001, Kohler, 2003, Brüne, 2005, Hall et al., 2008). Imaging studies on healthy subjects show that amygdala activation has been associated with the recognition of negative emotions, especially fear (Fusar-Poli et al., 2009, Phan et al., 2002). Structural abnormalities found in the amygdala (Wright et al., 2000) as well as functional abnormalities in relation to emotion recognition (Gur, 2002, Holt et al., 2006) in patients with schizophrenia suggest a pathogenetic role of the amygdala in schizophrenia. These findings are in line with the hypothesis of a distinctive impairment in the recognition of (negative) emotions in patients with schizophrenia (Aleman and Kahn, 2005, Amminger et al., 2012).
In the current study we aimed to further explore the relation between facial emotion recognition, facial identity recognition and neurocognitive functioning. We therefore compared patients with schizophrenia and matched controls on recognition of facial emotions and face identity, specifically contrasting results with those on (non-social) abstract pattern recognition. For that purpose we have used the Amsterdam Neuropsychological Tasks (ANT; de Sonneville, 2014), which allows for directly contrasting facial emotion recognition with identification of more basic social patterns (face identity) and non-social patterns (abstract figures), as the paradigms used in these tasks are similar and differ only on the degree to which they call on social information processing (high on emotion recognition, intermediate on face recognition and low on abstract pattern recognition).
The ANT examines accuracy as well as the speed of performance on tasks (such as pattern and emotion recognition), which may help to understand the strategy that respondents used in these processes. There are suggestions that patients with schizophrenia require more visual information, and therefore more time, to correctly identify emotional expression in faces, compared to controls (Clark et al., 2013, Lee et al., 2011). Furthermore there are indications that problems with face identity recognition in patients with schizophrenia may be the result of impairments in configural processing and an over-reliance on featural processing (Joshua and Rossell, 2009, Shin et al., 2008). Therefore we hypothesized that differences in emotion recognition between patients with schizophrenia and healthy controls may be more prominent in speed of performance than in accuracy of the emotion recognition task.
2. Method
2.1. Subjects
We performed a cross-sectional study including 42 individuals (mean age 38.4 ± 9.4 years), diagnosed with schizophrenia or schizoaffective disorder according to the Structured Clinical Interview for DSM-IV Disorders (SCID; First et al., 1996) and 42 controls, matched on sex, age and level of education.
Patients were selected from inpatient and outpatient facilities for the treatment of psychotic disorders of three mental healthcare institutions in the greater Amsterdam area. All patients experienced two or more psychotic episodes and had experienced a psychotic relapse or a clinical deterioration in the past year, resulting in hospitalisation and/or a detoriation on the Clinical Global Impression scale, severity of illness (CGI-S; Guy, 1976). At the time of inclusion in the study, antipsychotic treatment was resumed with at least minimal symptomatic improvement, defined as a score of 3 (minimal improved) or better on the Clinical Global Impression scale for Improvement (CGI-I; Guy, 1976). Exclusion criteria were: presence of an organic disease that is known as an etiological factor in psychotic illnesses; intellectual dysfunction (IQ < 70). The control group was recruited among hospital facility crew and firemen; controls and patients were carefully matched on level of education, age and gender.
Exclusion criteria for controls were a psychiatric history and a Symptoms Checklist (SCL-90) score of higher than 170 for male or higher than 204 for female subjects (Arrindell and Ettema, 2003).
All assessments were performed by trained psychologists and psychiatrists. Informed consent was obtained in all cases. The study was approved by the Medical Ethical Committee of the Academic Medical Center, Amsterdam.
2.2. Instruments
2.2.1. Amsterdam Neuropsychological Tasks (ANT)
The ANT is a computerized neuropsychological test battery, which is developed to evaluate basal processes underlying the execution of complex cognitive processes in a standardized and systematic manor (De Sonneville, 1999) and has proved to be a reliable and valid instrument with satisfactory test-retest reliability, construct, criterion, and discriminant validity of the tasks used in the study. (De Sonneville, 2005, De Sonneville, 2014, Günther et al., 2005, Rowbotham et al., 2009). The ANT consists of 38 tasks investigating functions of attention, memory, executive functioning and social cognition. Tasks will be briefly described, for detailed descriptions including examples of signals and timing between signals, see e.g. De Sonneville et al. (2002). Four tasks were administered for the purpose of this study, namely Identification of Facial Emotions (IFE), Face Recognition (FR), Feature Identification (FI) and Baseline Speed (BS).
3. Identification of Facial Emotions (IFE)
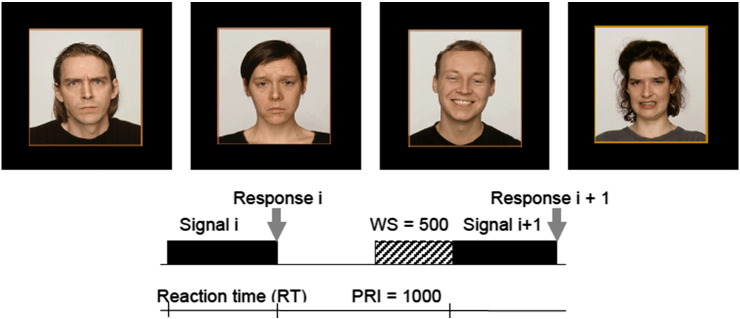
This task examines the ability to identify facial emotions, by asking the subject to judge whether a face on a picture shows a specific (target) expression (‘yes'-key) or a different expression (‘no'-key). The signal consists of one photo of a face that may show any of the following eight expressions: happiness, sadness, anger, fear, disgust, surprise, shame, and contempt. The total stimulus set consists of 32 pictures from four different persons, each showing the eight emotions. The original task consists of eight parts of 40 trials. In this study five parts are administered with the target emotions happiness, sadness, anger, fear and disgust, respectively (see Fig. 1).
Fig. 1.
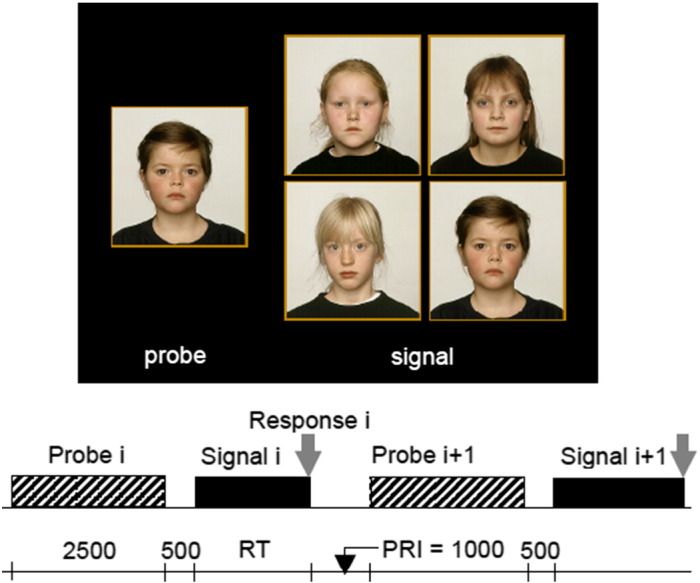
Example of the (target) signal in the Face Recognition (FR) task and timing between signals in ms. First the probe (left) is shown, followed by the signal (right), consisting of four photos, which in half of the cases contain the target signal. PRI = post response Interval, RT = reaction time.
3.1. Face Recognition (FR)
This task examines speed and accuracy in recognising unfamiliar faces. The signal consists of four photos of human faces ‘en face', with a neutral expression, taken from a set of 40 pictures of boys, girls, adult men and women. Preceding each signal, a probe (the to-be-recognised face) is presented for 2.5 seconds. Gender and age category of probe and signal always match, i.e. when the probe is a girl's face, the signal contains the pictures of four girls of the same age, and so forth. The subject should press the ‘yes'-key when the probe is present in the signal, and the ‘no'-key when this is not the case. In half the signals the probe is present (target condition) and in the other half it is not present (non-target condition) (See Fig. 2).
Fig. 2.
Example of signals used in the Identification of Facial Emotions (IFE) task and timing between signals in ms. A picture of a single face with a specific emotion is shown as a probe, after which a series of signals is presented containing a single face, half of them expressing the target emotion. WS = warning signal, PRI = post response interval.
4. Feature Identification (FI)
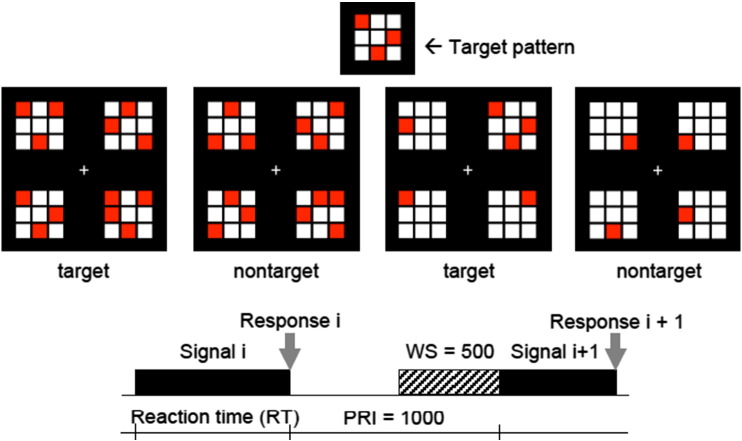
Feature Identification is a pattern recognition task designed to examine speed and accuracy of processing non-social abstract visuospatial patterns processing. The signal consists of four visuospatial patterns, each pattern being a 3x3 matrix of red and white coloured squares. The subject is asked to decide whether a specific pattern is present in the signal. The task consists of a random mix of 40 target trials and 40 non-target trials with two possible modes of signal presentation. In the similar condition, the target signal contains the target pattern and three distracters that look relatively similar to the target pattern, and the non-target signal contains four similar looking distracters. In the dissimilar condition the distracters are relatively different from the target signal. In the similar condition, pattern recognition depends on detailed (featural) processing (controlled information processing), in the dissimilar condition the target pattern can be identified as a ‘gestalt’ against the background of dissimilar distracters, which enables the use of a configural processing strategy. (See Fig. 3)
Fig. 3.
Example of the four signal types in Feature Identification (FI) task and timing between signals in ms. Preceding the very first trial, the target pattern is shown during the instruction phase, which is followed by the presentation of the signals in random order. WS = warning signal, PRI = post response interval.
5. Baseline speed (BS)
This task measures basic processing speed. The participant is required to press a button as quickly as possible when a fixation cross changes into a white square. Immediately after the response, the fixation cross reappears. The post-response interval until the next signal varies randomly between 500–2500 ms to prevent anticipation strategies. There are 32 trials for each hand. For all tasks the main outcome parameters are mean reaction time of correct responses and percentage of errors (except for task BS).
6. Statistical analyses
To compare performance on facial emotion recognition between patients and controls, mixed between-within subjects analyses of variance were conducted with happiness, sadness, anger, fear and disgust as levels of the within subject (WS) factor ‘Emotion’, and reaction time of correct responses and error percentage as dependent variables, respectively. Differences in performance on face recognition were evaluated using multivariate analyses of variance with reaction time and error percentage as dependent variables. Differences in pattern recognition between patients and controls were investigated using mixed between-within subject analyses of variance with similar and dissimilar task conditions as levels of the WS factor ‘Signal’.
For the comparison of speed between tasks, the speed in each task was transformed to a Process Time per element (PT), using the formula PT = (RTRESPONSE – RTBS)/n, with RTBS = the mean reaction time of baseline speed and n = the number of elements in the stimulus, as these differ across tasks (see De Sonneville et al., 2002). For comparison of accuracy no transformation was needed. The FI task is a combined identification and discrimination test; in these comparisons only the non-target items were included, because in the target signals not all the elements in the signal need to be examined to give an accurate response.
The comparisons were executed using mixed between-within subject analyses of variance with performance on tasks IFE, FR, FI similar condition, and FI dissimilar condition as levels of the WS factor ‘Task’, with process time and error percentage as dependent variable, respectively. To control for the influence of face recognition (FR) and pattern recognition (FI) on emotion recognition (IFE), analyses of covariance were carried out with performance on IFE as the dependent variable and performance on FR and FI as covariates. Furthermore, multiple regression analyses were carried out for each group with performance on IFE as the dependent variable and performance on FI and FR as predictors.
The data were analysed with SPSS, version 20.0 (SPSS Inc., USA). For each analysis, the F-statistic with its degrees of freedom, p-value and effect size (ηp2) are reported (weak effect: ηp2 > 0.03; moderate: ηp2 > 0.06; large: ηp2 ≥ 0.14)(Stevens, 1986).
7. Results
7.1. Sample
Eighty-four participants were included, 42 patients and 42 controls. Both groups consisted of 30 male and 12 female participants, with a mean age of 38.4 (SD = 9.7) in the patient group and 41.2 (SD = 11.8) in the control group. There was a significant difference in ethnic background. In the patient group 42.9 % of the participants was white European, compared to 90.5 % in the control group (χ2 = 38.9, p < 0.001). The other ethnic origins included Asian, Turkish, Moroccan, Surinam, Antillean and African participants. In the patient group, 15 participants (36%) were hospitalized. There were no significant differences between inpatients and outpatients on demographic and illness-related variables. Sample characteristics are displayed in Table 1.
Table 1.
Demographic and illness related characteristics of the sample.
| Patients | Controls | ||
|---|---|---|---|
| N | 42 | 42 | |
| Sex, % male | 28.6 | 28.6 | |
| Age, mean (SD) | 38.4 ± 9.4 | 41.2 ± 3.9 | ns |
| Education, highest, % | ns | ||
| • Elementary school | 11.9 | 4.8 | |
| • Intermediate Vocational education | 69 | 69 | |
| • College/University | 16.7 | 26.2 | |
| Ethnicity, % white European | 42.9 | 90.5 | p < 0.001 |
| Diagnosis | N/A | ||
| • Schizophrenia, % | 64.3 | ||
| • Schizoaffective disorder, % | 35.7 | ||
| Antipsychotic Medication type, % | |||
| • FGA | 38.5 | 0 | |
| • SGA | 61.5 | 0 | |
| Anticholinergic use, % | 16.7 | 0 | |
| Illness duration in years (SD) | 9,5 (± 7.0) | N/A | |
| Previous hospitalisations, N (SD) | 4.6 (± 6.0) | N/A | |
| PANSS scores, mean (SD) | |||
| • Positive symptoms | 16.2 (± 6.1) | ||
| • Negative symptoms | 17.4 (± 6.2) | N/A | |
| • General symptoms | 36.6 (± 9.4) | ||
| • Total score | 71.5 (± 19.2) |
Note: FGA, First Generation Antipsychotic; SGA, Second Generation Antipsychotic; PANSS, Positive and Negative Syndrome Scale.
7.2. Identification of Facial Emotions
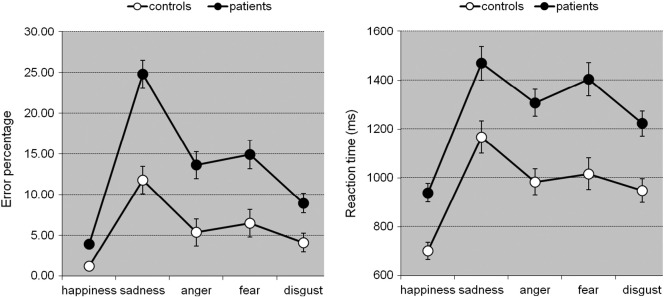
Significant effects were found for Group [F(1,79) = 34.310, p < 0.0001, ηp2 = 0.298], Emotion [F(4,316) = 43.887, p < 0.0001, ηp2 = 0.351], and the Emotion x Group interaction [F(4,316) = 4.949, p = 0.001, ηp2 = 0.058]. These results indicate that patients made more errors than controls, processing of negative emotions was less accurate, and differences between groups were largest for the recognition of negative emotions (see Fig. 4, left).
Fig. 4.
Mean ± SE of accuracy (left) and speed (right) of facial emotion recognition of controls and patients.
With regard to speed, significant effects were found for Group [F(1,79) = 20.892; p < 0.0001, ηp2 = 0.209] and Emotion [F(4,316) = 57.283, p < 0.0001, ηp2 = 0.420], but not for the Emotion x Group interaction (p = 0.276). These results indicate that patients were slower than controls and negative emotions were slower processed than positive emotions, but differences in speed between patients and controls did not depend on type of emotion (see Fig. 4, right).
7.3. Face recognition
The multivariate analysis with face recognition as dependent variable was significant [F(2,81) = 10.833, p < 0.0001, ηp2 = 0.211]. The univariate analyses demonstrated that patients made more errors than controls (13.2 ± 10.3 % vs. 6.5 ± 5.6 %) [F(1,82) = 13.901, p < 0.0004, ηp2 = 0.145] and were also slower than controls (1985 ± 527 ms vs. 1628 ± 326 ms) [F(1,82) = 13.958, p < 0.0004, ηp2 = 0.145].
7.4. Feature identification
With regard to accuracy, there was a significant effect of Signal [F(1,80) = 101.013, p < 0.0001, ηp2 = 0.558], reflecting that more errors were made when processing ‘similar’ patterns (14.5 ± 11.2 %) compared to ‘dissimilar’ patterns (5.4 ± 8.1 %). There was no significant main effect for Group (p = 0.153), nor an interaction of Group x Signal (p = 0.929).
With regard to speed, there were significant effects for Signal [F(1,80) = 441.178, p < 0.0001, ηp2 = 0.817] and Group [F(1,80) = 10.937, p = 0.001, ηp2 = 0.120], but there was no interaction of Group x Signal (p = 0.239). These results indicate that ‘similar’ patterns (2098 ± 601 ms) were slower processed than ‘dissimilar’ patterns (1233 ± 362 ms) and patients (1828 ± 519 ms) were slower than controls (1511 ± 389 ms), but group differences did not depend on signal type.
7.5. Comparison between tasks
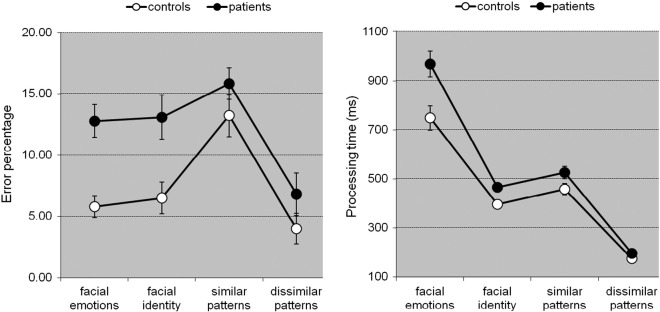
Comparing the accuracy of processing, significant effects were found for Group [F(1,79) = 12.024, p = 0.001, ηp2 = 0.132], and Task [F(3,237) = 24.339, p < 0.0001, ηp2 = 0.235], and a trend significant interaction of Group x Task [F(3,237) = 2.407, p = 0.068, ηp2 = 0.030]. These results indicate that accuracy differed between tasks, that patients made more errors than controls, and suggest that differences in accuracy depended on type of task (see Fig. 5, left). Contrast analyses revealed (trend) significant interactions when contrasting emotion recognition with ‘similar’ pattern recognition [F(1,79) = 3.142, p = 0.08, ηp2 = 0.038] and with ‘dissimilar’ pattern recognition [F(1,79) = 4.326, p = 0.041, ηp2 = 0.052], respectively, reflecting larger differences in accuracy between groups on emotion recognition compared to pattern recognition (see Fig. 5, left).
Fig. 5.
Mean ± SE of accuracy (left) and speed (right) of the recognition of facial emotions, facial identity, and patterns of controls and patients.
With regard to speed of processing, a significant main effect for Group was found [F(1,79) = 10.73, p = 0.002, ηp2 = 0.121], as well as a significant interaction effect between Group and Task [F(3,237) = 5.816, p = 0.001, ηp2 = 0.069]. Contrast analyses revealed significant interactions when contrasting emotion recognition with face recognition [F(1,78) = 5.136, p = 0.026, ηp2 = 0.062], with ‘similar’ pattern recognition [F(1,78) = 5.305, p = 0.024, ηp2 = 0.064] and with ‘dissimilar’ pattern recognition. [F(1,79) = 9.250, p = 0.003, ηp2 = 0.106] (see Fig. 2, right), respectively, reflecting larger differences in processing time on emotion recognition compared to both face recognition and pattern recognition (see Fig. 5, right).
Controlling for the influence of face recognition (FR) and pattern recognition (FI) on emotion recognition (IFE), significant effects were found for the speed of processing on IFE [F(1,78) = 13.711, p < 0.001, ηp2 = 0.150], as well for accuracy on IFE [F(1,78) = 5.151, p = 0.026, ηp2 = 0.062].
In patients, 25 % of the variance of accuracy on IFE was explained by accuracy on FR (β = 0.519, p = 0.002) and FI [(β = − 0.046, ns) [F(2,37) = 6.237, p = 0.005], compared to 33% of the variance of accuracy on IFE in controls that was explained by accuracy on FR (β = 0.509, p = 0.004) and FI (β = 0.356, p = 0.010), respectively [F(2,39) = 9.811, p < 0.001].
For the speed of processing on IFE, 24 % of the variance in patients was explained by speed on FR (β = 0.321, p = 0.071) and FI [(β = 0.237, ns), respectively [F(2,37) = 5.969, p = 0.006], compared to 56% of the variance of speed on IFE in controls that was explained by speed on FR (β = 0.539, p < 0.001) and FI (β = 0.338, p = 0.006), respectively [F(2,39) = 24.610, p < 0.001],
8. Discussion
The aim of the present study was to explore whether facial emotion recognition in patients with schizophrenia represents a specific deficit or should be regarded as part of a more general cognitive dysfunction.
We found that patients were slower and less accurate than healthy controls in recognising facial emotions as well as face identity. These differences between patients and controls on facial information processing are in line with results of previous studies (Kohler et al., 2010, Marwick and Hall, 2008). Although patients were also slower in the recognition of non-social abstract patterns, no differences in accuracy were found on this domain. This suggests that patients experience more problems with processing facial information compared to non-social abstract information.
To further explore this, we directly contrasted differences in task performance between groups across tasks and found more pronounced differences between patients and controls in facial emotion recognition compared to non-social pattern recognition, both for speed and accuracy. This confirms that patients showed more difficulties in information processing of this type of social information compared to the processing of non-social information. Furthermore, the differences between groups on emotion recognition remained significant after controlling for the influence of face recognition and abstract pattern recognition skills. It was also found that in controls more variance in emotion recognition was explained by face recognition and abstract pattern recognition than in patients. These differences were more substantial for speed (56% vs. 24%) than for accuracy (33% vs. 25%)
Within the narrower domain of facial information processing we found differences between patients and controls to be more substantial for facial emotion recognition compared to face identity recognition. However, this was only the case for processing speed and not for accuracy.
This lends support to the idea that deficits in facial emotion recognition in patients with schizophrenia cannot fully be accounted for by processes involved in face identity recognition, which is in line with the findings of several other studies (Hall et al., 2004, Kosmidis et al., 2007, Kucharska-Pietura et al., 2005, Penn et al., 2000, Van Rijn et al., 2011). Others did not find evidence for the specificity of emotion recognition compared to face identity recognition (Kerr and Neale, 1993, Salem et al., 1996, Addington and Addington, 1998, Sachs et al., 2004). In these latter studies however, only the accuracy of responses was assessed, which failed to provide evidence for the specificity of emotion recognition in our study too. The majority of studies have not assessed speed of facial emotion recognition. Among those who did, Silver et al. (2009) found a similar differential deficit between emotion recognition and face identity recognition. Yet another study that found a comparable result (Pomarol-Clotet et al., 2010) interpreted this specific difference in speed as a general slowing of reaction time in patients with schizophrenia and therefore insignificant in this respect. There is indeed evidence that processing speed in itself accounts for 25 % in general ability, which in turn explains 50% of the variation in all cognitive measures (Deary, 2012, Joyce, 2013). In the present study however, the significant interactions between Group and Task on processing time, in which differences in baseline speed are controlled for, demonstrate a distinctive impairment in facial emotion recognition, which cannot be attributed to general slowing. Furthermore, our results suggest that differences in speed between patients and controls become more prominent when the amount of social information in the task increases (emotion recognition > face recognition > non-social pattern recognition), underscoring the specific limitations in the social cognitive domain in patients with schizophrenia.
The clear difference between groups in speed of processing facial emotions is of importance, as the consequences of a slower processing of emotional state of others can unfavourably affect social functioning. When analysing components of the processing of facial emotions, patients were slower on both negative and positive emotions, compared to controls. They were also less accurate on negative emotions, but not on positive emotions. This valence difference between patients and controls is largely consistent with previous studies (Schneider et al., 1995, Bryson et al., 1997, Silver et al., 2002, Kohler, 2003) and may indicate that patients with schizophrenia have specific difficulties in recognising negative emotions.
It may be argued that the recognition of positive emotions (happiness) in the facial emotion recognition task is relatively easy. Applying a configural processing strategy – i.e. the ability to recognise a face as a “gestalt” - the salient characteristic of a smiling mouth with teeth will not easily be missed. This may explain that patients were equally accurate as controls on positive emotions. Nevertheless, patients needed more time to be as accurate as controls, so this still suggests problematic processing of this type of information.
8.1. Strengths and limitations
Strength of the present study was that, unlike most previous studies, we have used an instrument that allowed for observation of both accuracy as well as speed of performance and the evaluation of different levels of social cognition (facial emotion recognition and face identity recognition) and non-social cognition (dissimilar and similar abstract pattern recognition), using within and across task contrasts when comparing groups. Another strength was that our study included patients with a relapsing and chronic course of the disorder who recently showed medication nonadherence, which constituted a clinical representative sample of patients with severe schizophrenia. Moreover we included a healthy control sample that matched the patient sample concerning level of education. Furthermore our sample size was relatively large.
However, several limitations regarding this study should be acknowledged. Firstly, the ethnic background differs between groups. In the control group, 90.5% was white-European, versus 42.9% in the patient group. It has been shown that patients with schizophrenia as well as healthy controls have more difficulties in recognising facial expressions in members of a different ethnic group (Dailey et al., 2010, Pinkham et al., 2008). As all photos in the ANT-tasks show Caucasian faces, we therefore compared our results within the patient group between the white-European subjects and those from other ethnic backgrounds. The results revealed that there were no significant differences between the ethnic patient groups on emotion recognition, neither for the recognition of negative emotions nor for positive emotions. There were also no significant ethnicity differences within the patient group on accuracy (p = 0.07) as well as on speed (p > 0.10) of face recognition. Likewise, there were no significant differences for abstract pattern recognition between the two ethnic patient groups.
Secondly, the patient sample consisted of relatively chronic patients, as the mean illness duration was 9.5 years. We do not know whether the results of our study are generalizable to patients in earlier stages of illness or with a less severe disorder. However, facial emotion recognition deficits are observed in first-episode patients as well (Bediou et al., 2007, Edwards et al., 2001) and there is evidence that social cognitive deficits are relatively stable across phases of illness (Comparelli et al., 2013, Green et al., 2012, Vohs et al., 2014, Yalcin-Siedentopf et al., 2014).
In conclusion, this study shows that facial emotion recognition is impaired in patients with schizophrenia compared to healthy controls and can be distinguished from impairments in both face recognition and non-social pattern recognition. Especially recognition of negative emotions appears to be affected in patients. These results support the hypothesis that social cognitive impairments in patients with schizophrenia cannot (fully) be explained by impairments in general cognitive abilities. Based on our results one could tentatively argue that cognitive performance in patients with schizophrenia is worse when the amount of social information increases.
Moreover we showed that speed of processing is of importance and should be measured in tests of (non) social cognition.
Role of funding source
This study was supported by a non-restricted educational grant from the Dr. Paul Janssen Foundation.
Contributors
All authors contributed to and have approved the final manuscript. EB and CM designed the study, collected and analysed data. EB wrote the first draft of the manuscript. LdS analysed data and supported the statistical analyses. LdH supervised the study. LdS, CM and LdH revised the manuscript.
Conflicting interests
The authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank Mrs. Sara Maaskant for assistance in the data collection and data analyses.
References
- Addington J., Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr. Res. 1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- Addington J., Saeedi H., Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr. Res. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Aleman A., Kahn R.S. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog. Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Amminger G.P., Schäfer M.R., Papageorgiou K. Emotion recognition in individuals at clinical high-risk for schizophrenia. Schizophr. Bull. 2012;38:1030–1039. doi: 10.1093/schbul/sbr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrindell W.A., Ettema J.H.M. Swets Test Publishers; Lisse, The Netherlands: 2003. SCL-90; Handleiding bij een multidimensionele psychopathologie-indicator. [Google Scholar]
- Barbato M., Liu L., Penn D.L. Social cognition as a mediator between neurocognition and functional outcome in individuals at clinical high risk for psychosis. Schizophr. Res. 2013;150:542–546. doi: 10.1016/j.schres.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bediou B., Asri F., Brunelin J. Emotion recognition and genetic vulnerability to schizophrenia. Br. J. Psychiatry. 2007;191:126–130. doi: 10.1192/bjp.bp.106.028829. [DOI] [PubMed] [Google Scholar]
- Brüne M. Emotion recognition, “theory of mind”, and social behavior in schizophrenia. Psychiatry Res. 2005;133:135–147. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Bryson G., Bell M., Lysaker P. Affect recognition in schizophrenia: a function of global impairment or a specific cognitive deficit. Psychiatry Res. 1997;71:105–113. doi: 10.1016/s0165-1781(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Clark C.M., Gosselin F., Goghari V.M. Aberrant patterns of visual facial information usage in schizophrenia. J. Abnorm. Psychol. 2013;122:513–519. doi: 10.1037/a0031944. [DOI] [PubMed] [Google Scholar]
- Comparelli A., Corigliano V., De Carolis A. Emotion recognition impairment is present early and is stable throughout the course of schizophrenia. Schizophr. Res. 2013;143:65–69. doi: 10.1016/j.schres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Dailey M.N., Joyce C., Lyons M.J. Evidence and a computational explanation of cultural differences in facial expression recognition. Emotion. 2010;10:874–893. doi: 10.1037/a0020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sonneville L. Amsterdam neuropsychological tasks: a computer-aided assessment program. In: Beek P., Brand A., Den Brinker B., Maarse F., LJM M., editors. Cognitive Ergonomics, Clinical Assessment and Computer-Assisted Learning: Computers in Psychology. Swets; Lisse, The Netherlands: 1999. pp. 187–203. [Google Scholar]
- De Sonneville L.M.J. Amsterdam neuropsychological tasks: Scientific and clinical applications. Tijdschr. Neuropsychol. 2005:25–40. (Dutch) [Google Scholar]
- De Sonneville L.M.J. Boom Testuitgevers; Amsterdam, The Netherlands: 2014. Handboek Amsterdam Neuropsychological Tasks. [Google Scholar]
- De Sonneville L.M.J., Verschoor C.A., Njiokiktjien C., Op het Veld V., Toorenaar N., Vranken M. Facial identity and facial emotions: speed, accuracy, and processing strategies in children and adults. J. Clin. Exp. Neuropsychol. 2002;24:200–213. doi: 10.1076/jcen.24.2.200.989. [DOI] [PubMed] [Google Scholar]
- Deary I.J. Intelligence. Annu. Rev. Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- Edwards J., Pattison P.E., Jackson H.J., Wales R.J. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr. Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- Fett A.-K.J., Viechtbauer W., Dominguez M.-G., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., W.J. Biometrics Research Department, New York State Psychiatric Institute; New York (NY): 1996. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Olivier B., Crawley J.N., Penn D.L., Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr. Bull. 2005;31:882–887. doi: 10.1093/schbul/sbi049. [DOI] [PubMed] [Google Scholar]
- Green M.F., Bearden C.E., Cannon T.D. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr. Bull. 2012;38:854–864. doi: 10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther T., Herpertz-Dahlmann B., Konrad K. Reliability of attention and verbal memory tests with normal children and adolescents–clinical implications] Z. Kinder. Jugendpsychiatr. Psychother. 2005;33:169–179. doi: 10.1024/1422-4917.33.3.169. [DOI] [PubMed] [Google Scholar]
- Gur R.E. An fMRI Study of Facial Emotion Processing in Patients With Schizophrenia. Am. J. Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Guy W. US Department of Health, Education and Welfare Publication (ADM) National Institute of Mental Health; Rockville, MD: 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- Hall J., Harris J.M., Sprengelmeyer R. Social cognition and face processing in schizophrenia. Br. J. Psychiatry. 2004;185:169–170. doi: 10.1192/bjp.185.2.169. [DOI] [PubMed] [Google Scholar]
- Hall J., Whalley H.C., McKirdy J.W. Overactivation of fear systems to neutral faces in schizophrenia. Biol. Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Holt D.J., Kunkel L., Weiss A.P. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr. Res. 2006;82:153–162. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Joshua N., Rossell S. Configural face processing in schizophrenia. Schizophr. Res. 2009;112:99–103. doi: 10.1016/j.schres.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Joyce E.M. Cognitive function in schizophrenia: insights from intelligence research. Br. J. Psychiatry. 2013;203:161–162. doi: 10.1192/bjp.bp.112.109553. [DOI] [PubMed] [Google Scholar]
- Kerr S.L., Neale J.M. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J. Abnorm. Psychol. 1993;102:312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- Kohler C.G. Facial Emotion Recognition in Schizophrenia: Intensity Effects and Error Pattern. Am. J. Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Kohler C.G., Bilker W., Hagendoorn M., Gur R.E., Gur R.C. Emotion recognition deficit in schizophrenia: association with symptomatology and cognition. Biol. Psychiatry. 2000;48:127–136. doi: 10.1016/s0006-3223(00)00847-7. [DOI] [PubMed] [Google Scholar]
- Kohler C.G., Walker J.B., Martin E.A., Healey K.M., Moberg P.J. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmidis M.H., Bozikas V.P., Giannakou M., Anezoulaki D., Fantie B.D., Karavatos A. Impaired emotion perception in schizophrenia: a differential deficit. Psychiatry Res. 2007;149:279–284. doi: 10.1016/j.psychres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K., David A.S., Masiak M., Phillips M.L. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br. J. Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- Lee J., Gosselin F., Wynn J.K., Green M.F. How do schizophrenia patients use visual information to decode facial emotion? Schizophr. Bull. 2011;37:1001–1008. doi: 10.1093/schbul/sbq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick K., Hall J. Social cognition in schizophrenia: a review of face processing. Br. Med. Bull. 2008;88:43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- Mehta U.M., Thirthalli J., Subbakrishna D.K., Gangadhar B.N., Eack S.M., Keshavan M.S. Social and neuro-cognition as distinct cognitive factors in schizophrenia: A systematic review. Schizophr. Res. 2013;148:3–11. doi: 10.1016/j.schres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Oerlemans A.M., Droste K., van Steijn D.J., de Sonneville L.M.J., Buitelaar J.K., Rommelse N.N.J. Co-segregation of social cognition, executive function and local processing style in children with ASD, their siblings and normal controls. J. Autism Dev. Disord. 2013;43:2764–2778. doi: 10.1007/s10803-013-1807-x. [DOI] [PubMed] [Google Scholar]
- Penn D.L., Combs D.R., Ritchie M. Emotion recognition in schizophrenia: further investigation of generalized versus specific deficit models. J. Abnorm. Psychol. 2000;109:512–516. [PubMed] [Google Scholar]
- Penn D.L., Sanna L.J., Roberts D.L. Social Cognition in Schizophrenia: An Overview. Schizophr. Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E. Implications for the Neural Basis of Social Cognition for the Study of Schizophrenia. Am. J. Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E., Sasson N.J., Calkins M.E. The other-race effect in face processing among African American and Caucasian individuals with schizophrenia. Am. J. Psychiatry. 2008;165:639–645. doi: 10.1176/appi.ajp.2007.07101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E., Hynes F., Ashwin C., Bullmore E.T., McKenna P.J., Laws K.R. Facial emotion processing in schizophrenia: a non-specific neuropsychological deficit? Psychol. Med. 2010;40:911–919. doi: 10.1017/S0033291709991309. [DOI] [PubMed] [Google Scholar]
- Poole J.H., Tobias F.C., Vinogradov S. The functional relevance of affect recognition errors in schizophrenia. J. Int. Neuropsychol. Soc. 2000;6:649–658. doi: 10.1017/s135561770066602x. [DOI] [PubMed] [Google Scholar]
- Rowbotham I., Pit-ten Cate I.M., Sonuga-Barke E.J.S., Huijbregts S.C.J. Cognitive control in adolescents with neurofibromatosis type 1. Neuropsychology. 2009;23:50–60. doi: 10.1037/a0013927. [DOI] [PubMed] [Google Scholar]
- Sachs G., Steger-Wuchse D., Kryspin-Exner I., Gur R.C., Katschnig H. Facial recognition deficits and cognition in schizophrenia. Schizophr. Res. 2004;68:27–35. doi: 10.1016/S0920-9964(03)00131-2. [DOI] [PubMed] [Google Scholar]
- Salem J.E., Kring A.M., Kerr S.L. More evidence for generalized poor performance in facial emotion perception in schizophrenia. J. Abnorm. Psychol. 1996;105:480–483. doi: 10.1037//0021-843x.105.3.480. [DOI] [PubMed] [Google Scholar]
- Schneider F., Gur R.C., Gur R.E., Shtasel D.L. Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr. Res. 1995;17:67–75. doi: 10.1016/0920-9964(95)00031-g. [DOI] [PubMed] [Google Scholar]
- Schneider F., Gur R.C., Koch K. Impairment in the specificity of emotion processing in schizophrenia. Am. J. Psychiatry. 2006;163:442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- Sergi M.J., Rassovsky Y., Widmark C. Social cognition in schizophrenia: Relationships with neurocognition and negative symptoms. Schizophr. Res. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Shin Y.-W., Na M.H., Ha T.H., Kang D.-H., Yoo S.-Y., Kwon J.S. Dysfunction in configural face processing in patients with schizophrenia. Schizophr. Bull. 2008;34:538–543. doi: 10.1093/schbul/sbm118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver H., Shlomo N., Turner T., Gur R.C. Perception of happy and sad facial expressions in chronic schizophrenia: Evidence for two evaluative systems. Schizophr. Res. 2002;55:171–177. doi: 10.1016/s0920-9964(01)00208-0. [DOI] [PubMed] [Google Scholar]
- Silver H., Bilker W., Goodman C. Impaired recognition of happy, sad and neutral expressions in schizophrenia is emotion, but not valence, specific and context dependent. Psychiatry Res. 2009;169:101–106. doi: 10.1016/j.psychres.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Stevens J. Lawrence Erlbaum Associates Ltd.; Hillsdale, NJ, USA: 1986. Applied multivariate statistics for the social sciences. [Google Scholar]
- Turetsky B.I., Kohler C.G., Indersmitten T., Bhati M.T., Charbonnier D., Gur R.C. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr. Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Wout M., Aleman A., Kessels R.P.C., Cahn W., de Haan E.H.F., Kahn R.S. Exploring the nature of facial affect processing deficits in schizophrenia. Psychiatry Res. 2007;150:227–235. doi: 10.1016/j.psychres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Van Hooren S., Versmissen D., Janssen I. Social cognition and neurocognition as independent domains in psychosis. Schizophr. Res. 2008;103:257–265. doi: 10.1016/j.schres.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Van Rijn S., Aleman A., de Sonneville L. Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol. Med. 2011;41:499–508. doi: 10.1017/S0033291710000929. [DOI] [PubMed] [Google Scholar]
- Vohs J.L., Lysaker P.H., Francis M.M. Metacognition, social cognition, and symptoms in patients with first episode and prolonged psychoses. Schizophr. Res. 2014;153(1–3):54–59. doi: 10.1016/j.schres.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Weniger G., Lange C., Rüther E., Irle E. Differential impairments of facial affect recognition in schizophrenia subtypes and major depression. Psychiatry Res. 2004;128:135–146. doi: 10.1016/j.psychres.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Wright I.C., Rabe-Hesketh S., Woodruff P.W.R., David A.S., Murray R.M., Bullmore E.T. Meta-Analysis of Regional Brain Volumes in Schizophrenia. Am. J. Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yalcin-Siedentopf N., Hoertnagl C.M., Biedermann F. Facial affect recognition in symptomatically remitted patients with schizophrenia and bipolar disorder. Schizophr. Res. 2014;152:440–445. doi: 10.1016/j.schres.2013.11.024. [DOI] [PubMed] [Google Scholar]