Abstract
Patients with delusions exhibit an increased tendency to arrive at decisions based on very limited evidence (jumping-to-conclusions; JTC), making this reasoning bias relevant for the treatment of delusions. Neurocognitive deficits contribute to JTC, but it is not known whether this has any bearing on the clinical syndrome of delusions. We addressed this question by reanalyzing data from an efficacy study of non-pharmacological interventions as adjunctive treatments in schizophrenia. We investigated the longitudinal associations of cognitive functioning, JTC and delusions in patients with psychotic disorders receiving either a metacognitive intervention addressing reasoning biases (n = 59), or cognitive remediation (n = 58). Both interventions improved JTC; in the cognitive remediation group, tentative evidence suggested that better neurocognitive performance contributed to this improvement. However, JTC gains were associated with delusion improvement only in the metacognitive intervention group, suggesting a content-specific mechanism of action.
Keywords: Schizophrenia, Delusions, Cognitive biases, Metacognitive training, Cognitive remediation
1. Introduction
Cognitive theories of delusions postulate that these result from disruptions in the normal mechanisms involved in belief generation and evaluation (Garety et al., 2005, Langdon et al., 2010), collectively referred to as reasoning biases. The best studied among these so far is the jumping-to-conclusions bias, which is characterized by hasty evidence gathering leading to an increased tendency for people with delusions to arrive at decisions based on very limited evidence (Garety and Freeman, 2013).
The jumping-to-conclusions bias has been reliably replicated across several clinical populations with delusions (Fine et al., 2007), as well as in high-risk subjects and relatives of patients (Broome et al., 2007, Menon et al., 2013, Zawadzki et al., 2012). Although it is not affected by antipsychotics (Andreou et al., 2013, Menon et al., 2008), it has been shown to predict antipsychotic medication response (Menon et al., 2008, So et al., 2014). Critically, jumping-to-conclusions and other reasoning biases are amenable to specific metacognitive interventions, and converging evidence suggests that such interventions lead to improvement of delusional symptoms (Garety et al., 2014, Moritz et al., 2014). Thus, the association of the jumping-to-conclusions bias with delusions and its relevance for their treatment have received much support.
Cognitive and neuroimaging studies suggest that evidence gathering is dependent on cognitive resources such as attention, working memory and/or executive functions (Broome et al., 2007, Esslinger et al., 2013, Fine et al., 2007, Furl and Averbeck, 2011, Woodward et al., 2009a). Indeed, patients who displayed a jumping-to-conclusions bias were shown to have a lower working memory capacity than those who did not (Garety et al., 2013). Moreover, a recent study reported that patients with higher baseline working memory scores responded better to a brief intervention targeting reasoning biases (i.e., they showed greater improvement in reasoning biases) (Garety et al., 2014). Accordingly, it has been suggested that interventions directed at improving reasoning biases would benefit from incorporating cognition-enhancing tasks (Garety et al., 2013).
On the other hand however, neurocognitive deficits have been very consistently reported to be a relatively stable trait of psychosis, unrelated to positive psychotic symptoms and their fluctuations (Dominguez Mde et al., 2009, Kravariti et al., 2012). Moreover, although neurocognitive deficits are significantly and durably improved by cognitive remediation interventions, the effects of such interventions on symptoms are small and short-lived (Wykes et al., 2011). Thus, a direct effect of cognitive remediation training on delusions is at best questionable. Of course, this does not exclude the possibility that cognitive remediation training might act to promote the efficacy of metacognitive interventions, when used in combination with the latter. However, this assumption holds only if it can be shown that (a) changes in neurocognitive capacity, rather than baseline cognitive status, are relevant for improvement of reasoning biases, and that (b) in turn, improvement of reasoning biases can lead to a decrease in delusions.
In view of the above considerations, the present study aimed to clarify the associations between neurocognitive functioning, reasoning biases, and delusions, by investigating their longitudinal associations in patients with schizophrenia spectrum disorders undergoing either metacognitive intervention or cognitive remediation training. Because jumping-to-conclusions is the best established delusion-related reasoning bias, often used as a primary outcome in efficacy studies of metacognitive interventions, in the present study we focused on hasty evidence gathering as a representative reasoning bias. We hypothesized that (a) more cautious evidence gathering would be associated with neurocognitive gains following cognitive remediation, but not following metacognitive training; and (b) that more cautious evidence gathering would be associated with reductions in delusion severity in both treatment groups.
2. Materials and methods
The present study used data collected in the context of a two-center, randomized controlled trial that compared two non-pharmacological interventions as adjunctive treatments in patients with schizophrenia spectrum disorders — a metacognitive intervention addressing reasoning biases (Metacognitive Training, MCT) (Moritz et al., 2005), and cognitive remediation training focusing on more elementary neurocognitive functions such as attention and memory (CogPack®)(Marker, 2003). The study was approved by the local ethics committees (Hamburg and Heidelberg), and patients were required to provide their written informed consent prior to participation. Details on the design and results of the original randomized controlled trial can be found in Moritz et al., 2013. In brief, 150 patients with schizophrenia spectrum disorders and a history of at least one delusional episode were recruited for the study (allocated to MCT n = 76, CogPack n = 74). Inclusion criteria were age 18–64 years, ability to provide written informed consent and sufficient capacity to participate in therapy groups, IQ greater than 70, no current substance dependence, and no history of severe brain damage.
Both interventions were administered in a twice-weekly format over 4 weeks (8 sessions), with the possibility of attending a second 4-week course of the same intervention (parallel version) immediately thereafter. Assessments of psychopathology, cognitive biases and neurocognitive functioning were conducted at baseline, following completion of the core intervention phase (4 weeks) and 6 months thereafter.
Using data from the baseline assessment and the 6-month follow-up, we investigated how changes in evidence gathering, neurocognitive functioning and delusions were associated to each other, and whether they were differentially affected by the two different interventions. The Fish task, a computerized probabilistic reasoning task similar to the Beads Task (Moritz and Woodward, 2005), was used to assess evidence gathering; measure of interest was the number of draws to decision, i.e. the number of fish the participant saw before reaching a decision. The Trail Making Test, part B (Reitan and Wolfson, 1985) was used as a broad measure of neurocognitive capacity, because this task depends on a variety of cognitive functions (e.g., visual scanning, attention, processing speed, abstract conceptual processing, cognitive flexibility) (Palmer and Heaton, 2000). Delusion severity was assessed with the Positive and Negative Syndrome Scale (Kay et al., 1987); a "core delusion score" corresponding to the sum of items P1 (delusions), P5 (grandiosity), P6 (suspiciousness) and G9 (unusual thought content) was the variable of interest.
The present analysis considered the per-protocol sample of the original clinical trial by Moritz et al. (2013). In contrast to the original controlled trial, which focused on clinical outcomes, neurocognitive performance was of primary importance for the current analysis. Therefore, we excluded two patients (both from the CogPack group) with conditions that might have affected neuropsychological test performance (uncorrected visual impairment and epilepsy).
Difference scores were calculated for all measures of interest (baseline minus follow-up score). Because there was significant improvement in delusions, evidence gathering and TMT-B performance in both groups (see Moritz et al., 2013), we conducted moderation analyses to investigate whether (a) gains in neurocognitive performance were associated with gains in evidence gathering in a similar manner in the two groups, and whether (b) improved evidence gathering was associated with a decrease in delusions in a similar manner in the two intervention groups. It is noted here that 'improved' evidence gathering in this context was defined as an increase in the number of draws to decision. This definition was based on the consistent association of delusions with hasty evidence gathering (see Introduction) and should not be taken to suggest that more cautious evidence gathering is necessarily more optimal in a Bayesian probabilistic reasoning framework (cf. Speechley et al., 2010).
All analyses were conducted using SPSS 20.0; the PROCESS macro (Hayes, 2012) was used for moderation analyses, as it includes corrections for heteroscedasticity as well as bootstrapping procedures that provide robust estimates of standard errors and significance levels. Because the two intervention groups were matched at baseline on all sociodemographic variables except age (Moritz et al., 2013), age was included as a covariate in all analyses.
3. Results
As shown in Table 1, significant improvement in all measures of interest was noted from baseline to follow-up. As detailed in Moritz et al. (2013) (per-protocol analyses), patients in the MCT group showed significantly greater decline in delusions (p = 0.047) and trend-wise greater improvement in probabilistic reasoning (p = 0.10) than patients in the CogPack group.
Table 1.
Mean scores and standard deviations (SD) for variables of interest at baseline and at the 6-month follow-up for each group separately.
| MCT (n = 59) |
CogPack® (n = 58) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Follow up |
Baseline |
Follow-up |
|||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| PANSS delusion subscore | 8.05 | (3.9) | 6.08 | (3.3)⁎⁎⁎ | 7.78 | (3.6) | 6.83 | (3.5)⁎⁎ |
| Fish Task (number of draws to decision) | 2.68 | (2.0) | 3.83 | (2.2)⁎⁎ | 2.86 | (2.4) | 3.66 | (2.6)⁎ |
| Trail-Making Test B (s) | 82.78 | (38.3) | 73.20 | (36.1)⁎⁎ | 68.26 | (29.1) | 58.91 | (25.4)⁎⁎⁎ |
Note: In contrast to Table 2 in Moritz et al. (2013), scores are only presented for patients for whom both baseline and follow-up scores were available for all measures of interest (missing data in n = 4 patients).
RBMT: Rivermead Behavioural Learning Test; PANSS: Positive and Negative Syndrome Scale.
p < 0.05 compared to baseline.
p < 0.01 compared to baseline.
p < 0.001 compared to baseline.
Gains in neurocognitive functioning over time were not a significant predictor of improved evidence gathering [F(4,113) = 1.424, p = 0.23]. Group (MCT vs. CogPack) did not significantly moderate the relationship between changes in neurocognitive functioning and changes in evidence gathering [interaction term t = 1.354, p = 0.18]. However, there was some evidence indicating differences between the two interventions regarding the effects of neurocognitive capacity on evidence gathering: In the CogPack group, improvements in TMT-B were significantly associated with more cautious evidence gathering (t = 2.124, p = 0.04), whereas this effect was not significant in the MCT group (t = 0.506, p = 0.61) (Fig. 1).
Fig. 1.
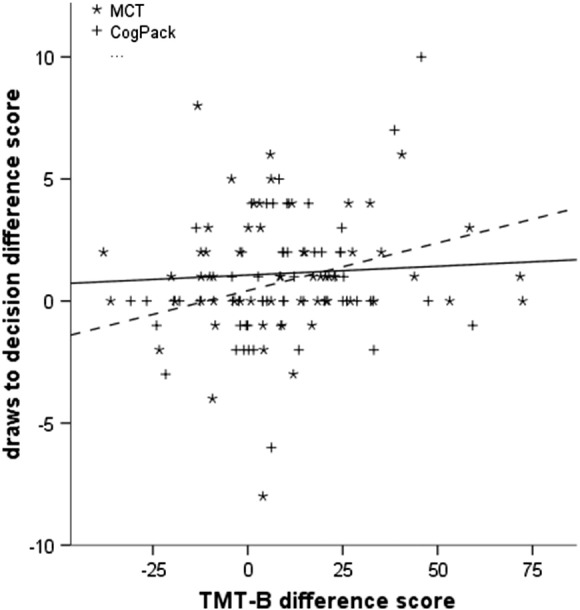
Correlation between change in performance on the Trail Making Test, part B (TMT-B) and changes in evidence gathering from baseline to the 6-month follow-up in the two treatment groups. Continuous regression line: MCT group; dotted regression line: CogPack group.
Overall, the effects of changes in evidence gathering on delusion improvement were significant [F(4,116) = 4.665, p = 0.002], and were moderated by group allocation at trend level (interaction term t = 1.902, p = 0.06). As visualized in Fig. 2, improvement in evidence gathering was significantly associated with delusion decline in the MCT group (t = 3.006, p = 0.003), while this was not the case in the CogPack group (t = 0.630, p = 0.53).
Fig. 2.
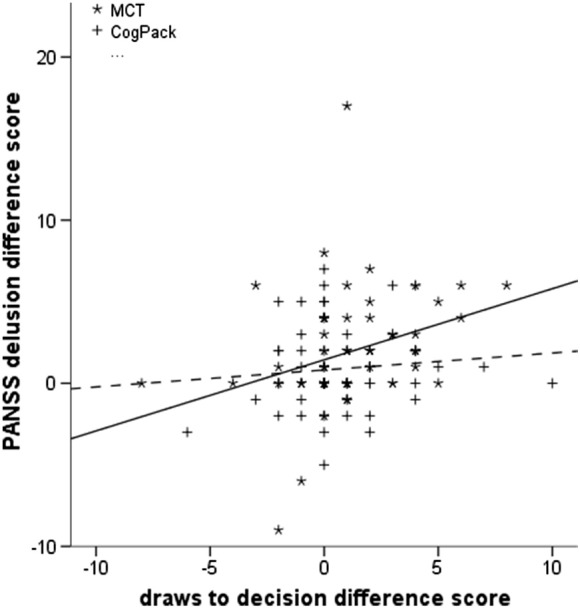
Relationship between change in evidence gathering performance and change in delusions from baseline to the 6-month follow-up in the two treatment groups. Continuous regression line: MCT group; dotted regression line: CogPack® group.
3.1. Subsidiary analyses
In the parent controlled trial, participants could flexibly attend up to 16 sessions of the intervention they were allocated to. Patients allocated to the MCT group attended more sessions than those in the CogPack group; moreover, in the MCT group, the number of attended sessions significantly correlated with change in PANSS positive score (Moritz et al., 2013). Therefore, we repeated moderation analyses including the number of attended sessions as an additional covariate in the models. However, the effect of this variable was not significant (both p > 0.32), and it did not affect the significance level of moderation effects. Moreover, we used the PROCESS macro to investigate whether the number of attended sessions contributed to the observed significant associations (a) between TMT-B score improvement and Fish Task performance changes in the CogPack group, and (b) between changes in evidence gathering and delusion improvement in the MCT group. The results were not significant in either case (Sobel test p > 0.60 in both analyses).
4. Discussion
The present study investigated longitudinal associations between neurocognitive functioning, evidence gathering and delusions in patients with schizophrenia spectrum disorders, with a special focus on how these associations are affected by two different types of intervention: a metacognitive intervention (which explicitly addresses reasoning biases) and cognitive remediation training (designed to improve neurocognitive abilities such as attention, working memory and executive functioning). By including intervention type as a moderator, we focused on intervention-specific effects, ruling out association patterns that might result from non-specific factors common in both interventions (e.g. practice effects).
When the patient sample was regarded as a whole, changes in neurocognitive functioning were not associated with changes in evidence gathering. However, when the two intervention groups were considered separately, improved cognitive performance did lead to significantly less hasty evidence gathering in the group receiving cognitive remediation training. Although this effect was small, this finding indirectly confirms previous reports associating jumping-to-conclusions in patients with schizophrenia with working memory and executive functioning (Broome et al., 2007, Fine et al., 2007, Garety et al., 2013, Garety et al., 2014, Moritz and Woodward, 2005, Woodward et al., 2009a). It also possibly explains why there was no clear superiority of MCT over CogPack® in terms of evidence gathering performance in the original randomized controlled trial (Moritz et al., 2013). However, this finding should be interpreted with caution, given the small effect size. It may be that the beneficial effect of cognitive remediation on reasoning only applies to patients with marked neurocognitive impairments; further studies are warranted to investigate this possibility.
A very interesting finding was that, although both groups showed improved evidence gathering over time, the latter was associated with a reduction in delusion severity only in the metacognitive training group. Thus, even if improved neurocognitive performance led to more cautious evidence gathering in the CogPack group, this effect did not translate into a decline in delusion severity. A possible explanation for this finding is that neurocognitive deficits are only one of several factors assumed to contribute to the jumping-to-conclusions bias (Fine et al., 2007), and do not necessarily represent the decisive aspect that links jumping-to-conclusions with delusions. In line with this hypothesis, a recent functional neuroimaging study (Furl and Averbeck, 2011) stressed the ability to estimate the value of continuing to seek evidence (relative to reaching a decision) as a central aspect of evidence gathering performance. The explicit emphasis placed by metacognitive interventions on the advantages of seeking more evidence before making a decision (Moritz et al., 2005) might provide a more appropriate means to modulate this process. This conclusion is consistent with two previous studies that noted a parallel decline of jumping-to-conclusions and delusions in patients receiving group cognitive–behavioral therapy (Sanford et al., 2013, Woodward et al., 2009b), which also places emphasis on appropriate weighing of available evidence. Thus, conscious awareness of metacognitive processes may be the key for improved reasoning to be translated into a decrease in clinical symptoms (delusions).
The present results should not be taken to suggest that cognitive remediation is redundant as an adjunct to metacognitive interventions, as the present study was not designed to directly address this issue. Rather, our findings suggest that changes in reasoning biases following cognitive remediation alone are not sufficient to induce symptom changes, i.e., that an explicit metacognitive element is indispensable for treatment effects to occur. However, this does not exclude the possibility that combination of the two approaches might produce superior treatment effects compared to metacognitive interventions alone. In fact, our findings tentatively suggest that cognitive remediation can lead to more cautious evidence gathering. It is conceivable that this effect may provide an additional advantage in the treatment of delusions, if it can be put into the appropriate (i.e., metacognitive) context.
In summary, two non-pharmacological interventions addressing different aspects of cognition led to differential patterns of association between improvements in neurocognitive functioning, evidence gathering and delusions. Although both interventions reduced the jumping-to-conclusions bias, an association between more cautious evidence gathering and delusion decline could only be observed for MCT, speaking for a content-specific mechanism of action. We propose that explicit imparting of information regarding the consequences of hasty decision-making might be the key to the beneficial effects of metacognitive interventions on delusions.
Role of the Funding Source
The funding source was not involved in the methodology design, in the collection and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Contributors
S.M. and D.R.E. designed the study and wrote the protocol. B.C.S. and D.L. managed literature searches and analyses. C.A. and S.M. conducted statistical analyses. C.A. and R.B. interpreted findings. C.A. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
None.
Acknowledgements
Data used in the current manuscript were collected in the context of a trial supported by a grant from the clinical trial program of the German Research Foundation (Deutsche Forschungsgemeinschaft; Mo 969/6-1).
References
- Andreou C., Moritz S., Veith K., Veckenstedt R., Naber D. Dopaminergic modulation of probabilistic reasoning and overconfidence in errors: a double-blind study. Schizophr. Bull. 2014;40(3):558–565. doi: 10.1093/schbul/sbt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome M.R., Johns L.C., Valli I., Woolley J.B., Tabraham P., Brett C., Valmaggia L., Peters E., Garety P.A., McGuire P.K. Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br. J. Psychiatry Suppl. 2007;51:s38–s42. doi: 10.1192/bjp.191.51.s38. [DOI] [PubMed] [Google Scholar]
- Dominguez Mde G., Viechtbauer W., Simons C.J., van Os J., Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol. Bull. 2009;135(1):157–171. doi: 10.1037/a0014415. [DOI] [PubMed] [Google Scholar]
- Esslinger C., Braun U., Schirmbeck F., Santos A., Meyer-Lindenberg A., Zink M., Kirsch P. Activation of midbrain and ventral striatal regions implicates salience processing during a modified beads task. PLoS One. 2013;8(3):e58536. doi: 10.1371/journal.pone.0058536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine C., Gardner M., Craigie J., Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn. Neuropsychiatry. 2007;12(1):46–77. doi: 10.1080/13546800600750597. [DOI] [PubMed] [Google Scholar]
- Furl N., Averbeck B.B. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J. Neurosci. 2011;31(48):17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garety P.A., Freeman D. The past and future of delusions research: from the inexplicable to the treatable. Br. J. Psychiatry. 2013;203(5):327–333. doi: 10.1192/bjp.bp.113.126953. [DOI] [PubMed] [Google Scholar]
- Garety P.A., Freeman D., Jolley S., Dunn G., Bebbington P.E., Fowler D.G., Kuipers E., Dudley R. Reasoning, emotions, and delusional conviction in psychosis. J. Abnorm. Psychol. 2005;114(3):373–384. doi: 10.1037/0021-843X.114.3.373. [DOI] [PubMed] [Google Scholar]
- Garety P., Joyce E., Jolley S., Emsley R., Waller H., Kuipers E., Bebbington P., Fowler D., Dunn G., Freeman D. Neuropsychological functioning and jumping to conclusions in delusions. Schizophr. Res. 2013;150(2–3):570–574. doi: 10.1016/j.schres.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garety P., Waller H., Emsley R., Jolley S., Kuipers E., Bebbington P., Dunn G., Fowler D., Hardy A., Freeman D. Cognitive mechanisms of change in delusions: an experimental investigation targeting reasoning to effect change in paranoia. Schizophr. Bull. 2015;41(2):400–410. doi: 10.1093/schbul/sbu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. PROCESS: a versatile computational tool for observed variable mediation, moderation, and conditional process modeling [white paper] 2012. http://www.afhayes.com/public/process2012.pdf Retrieved from.
- Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kravariti E., Russo M., Vassos E., Morgan K., Fearon P., Zanelli J.W., Demjaha A., Lappin J.M., Tsakanikos E., Dazzan P., Morgan C., Doody G.A., Harrison G., Jones P.B., Murray R.M., Reichenberg A. Linear and non-linear associations of symptom dimensions and cognitive function in first-onset psychosis. Schizophr. Res. 2012;140(1–3):221–231. doi: 10.1016/j.schres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Langdon R., Ward P.B., Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr. Bull. 2010;36(2):321–330. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker K. Marker Software; Ladenburg: 2003. COGPACK manual version 5.9. [Google Scholar]
- Menon M., Mizrahi R., Kapur S. 'Jumping to conclusions' and delusions in psychosis: relationship and response to treatment. Schizophr. Res. 2008;98(1–3):225–231. doi: 10.1016/j.schres.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Menon M., Quilty L.C., Zawadzki J.A., Woodward T.S., Sokolowski H.M., Boon H.S., Wong A.H. The role of cognitive biases and personality variables in subclinical delusional ideation. Cogn. Neuropsychiatry. 2013;18(3):208–218. doi: 10.1080/13546805.2012.692873. [DOI] [PubMed] [Google Scholar]
- Moritz S., Woodward T.S. Jumping to conclusions in delusional and non-delusional schizophrenic patients. Br. J. Clin. Psychol. 2005;44(Pt 2):193–207. doi: 10.1348/014466505X35678. [DOI] [PubMed] [Google Scholar]
- Moritz S., Woodward T.S., Burlon M. VanHam Campus Verlag; Hamburg: 2005. Metacognitive skill training for patients with schizophrenia (MCT). Manual. [Google Scholar]
- Moritz S., Veckenstedt R., Bohn F., Hottenrott B., Scheu F., Randjbar S., Aghotor J., Kother U., Woodward T.S., Treszl A., Andreou C., Pfueller U., Roesch-Ely D. Complementary group metacognitive training (MCT) reduces delusional ideation in schizophrenia. Schizophr. Res. 2013;151(1–3):61–69. doi: 10.1016/j.schres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Moritz S., Andreou C., Schneider B.C., Wittekind C.E., Menon M., Balzan R.P., Woodward T.S. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin. Psychol. Rev. 2014;34(4):358–366. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Palmer B.W., Heaton R.K. Executive dysfunction in schizophrenia. In: Sharma T., Harvey P., editors. Cognition in schizophrenia: impairments, importance, and treatment strategies. Oxford University Press; New York: 2000. [Google Scholar]
- Reitan R., Wolfson D. Neuropsychological Press; Tucson, AZ: 1985. The Halstead–Reitan Neuropsychological Test Battery: therapy and clinical interpretation. [Google Scholar]
- Sanford N., Woodward T.S., Lecomte T., Leclerc C., Wykes T. Change in jumping to conclusions linked to change in delusions in early psychosis. Schizophr. Res. 2013;147(1):207–208. doi: 10.1016/j.schres.2013.02.042. [DOI] [PubMed] [Google Scholar]
- So S.H., Peters E.R., Swendsen J., Garety P.A., Kapur S. Changes in delusions in the early phase of antipsychotic treatment — an experience sampling study. Psychiatry Res. 2014;215(3):568–573. doi: 10.1016/j.psychres.2013.12.033. [DOI] [PubMed] [Google Scholar]
- Speechley W.J., Whitman J.C., Woodward T.S. The contribution of hypersalience to the "jumping to conclusions" bias associated with delusions in schizophrenia. J. Psychiatry Neurosci. 2010;35(1):7–17. doi: 10.1503/jpn.090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward T.S., Mizrahi R., Menon M., Christensen B.K. Correspondences between theory of mind, jumping to conclusions, neuropsychological measures and the symptoms of schizophrenia. Psychiatry Res. 2009;170(2–3):119–123. doi: 10.1016/j.psychres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Woodward T.S., Munz M., LeClerc C., Lecomte T. Change in delusions is associated with change in "jumping to conclusions". Psychiatry Res. 2009;170(2–3):124–127. doi: 10.1016/j.psychres.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Wykes T., Huddy V., Cellard C., McGurk S.R., Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zawadzki J.A., Woodward T.S., Sokolowski H.M., Boon H.S., Wong A.H., Menon M. Cognitive factors associated with subclinical delusional ideation in the general population. Psychiatry Res. 2012;197(3):345–349. doi: 10.1016/j.psychres.2012.01.004. [DOI] [PubMed] [Google Scholar]


