Abstract
The Helicoverpa/Heliothis complex can cause serious damage to agricultural crops. Phenotypic similarity makes it difficult to discriminate between closely related Helicoverpa species. Currently, morphology of the male genitalia complemented with molecular techniques constitutes the best approach for species identification. In this work, a broad microscopic examination of adult Helicoverpa zea (Boddie) males (n = 200) captured in central Argentina was carried out in order to provide a detailed description of the valvae and the phallus. A considerable degree of variability was recorded. Both rounded and sharp valve apices were observed and valvae were not always parallel-sided. Most evident differences were detected concerning the number of cornuti on the phallus. A range of 15–21 cornuti per phallus was recorded, the mode being 18. A significant minority of the samples (3.5%) displayed an abnormal genital condition showing a constricted phallus lacking cornuti, and pointed valvae. This form was initially attributed to a distinct species, Heliothis stombleri, and later proposed as a synonym of H. zea based on additional morphological observations and molecular studies. Here, a phylogenetic analysis combining mitochondrial (cytochrome oxidase subunit I) and nuclear (elongation factor –1 alpha) genes was performed on these and other Helicoverpa specimens collected in the same geographical region, in order to further verify the taxonomic status of H. stombleri. The tree topology clearly grouped H. stombleri with H. zea, supporting the assumption that the former represents, in fact, an anomalous form of the latter. Further experiments are needed to clarify the etiology of this anomaly and its persistence over time.
Keywords: corn earworm, identification, morphology, molecular marker
Resumen
El complejo Helicoverpa/Heliothis produce graves daños a la agricultura. Las especies del género Helicoverpa son difíciles de diferenciar porque poseen un fenotipo similar. Actualmente, su identificación se basa en características morfológicas de la genitalia masculina y en el uso de marcadores moleculares. En este trabajo, se examinaron por microscopía machos adultos de Helicoverpa zea (Boddie; n = 200) capturados en la zona central de Argentina, a fin de detallar la forma y variabilidad de valvas y falo. Las valvas no siempre presentaron lados paralelos, y mostraron ápices agudos o redondeados. Las diferencias más evidentes se refirieron al número de cornuti del falo (15 a 21, moda = 18). Una minoría significativa de las muestras (3,5%) exhibió una fuerte anomalía en el aspecto del aparato reproductor: falo sin cornuti, estrechado en su extremo, y valvas terminadas en punta. Esta variante, que había sido atribuida inicialmente a una especie distinta, Heliothis stombleri, fue luego considerada como sinónimo de H. zea mediante estudios morfológicos y moleculares. Para verificar esta conclusión, se llevó a cabo un análisis filogenético que combinó genes mitocondriales (COI) y nucleares (EF-1α) a partir de especímenes de H. stombleri y otras Helicoverpa spp. provenientes de la misma región. La topología de los árboles agrupó claramente a H. stombleri con H. zea, permitiendo suponer que, en efecto, ambas formas constituyen la misma especie. Experimentos adicionales son necesarios para clarificar la etiología de esta anomalía, así como su persistencia en el tiempo.
The corn earworm, Helicoverpa zea (Boddie), is an economically important agricultural pest distributed across the Americas (Hardwick 1965). This noctuid is considered a major threat to corn, and occasionally it attacks other field and horticultural crops, including alfalfa, cotton, tobacco, peanuts, sorghum, and sunflower. In Argentina, H. zea completes five generations per year in the north and three to four generations in the central region (Margheritis and Rizzo 1965). Adults begin to emerge from wintering pupae between October and November. The moths lay their eggs on the stigmas of carpellate (female) corn flowers (“corn silk”), where the newly-hatched larvae start feeding. As crop phenology progresses, larvae feed on the grains throughout kernel filling (Navarro et al. 2009). The extent of damaged ears can reach up to 100% in late sowing, even in transgenic hybrids expressing toxins derived from Bacillus thuringiensis (Balbi and Flores 2015).
H. zea partially overlaps its geographical range with other pest species in the Helicoverpa/Heliothis complex: Heliothis virescens (Fabricius; found from USA to central Argentina) and Helicoverpa gelotopoeon (Dyar; restricted to southern South-America). One of the most devastating members of the complex, the exotic Helicoverpa armigera (Hübner), has recently become established in Brazil and Argentina (Murúa et al. 2016). The regular monitoring of Helicoverpa spp. has thus become imperative in the highly productive South-American temperate and subtropical agricultural lands. These polyphagous species sometimes co-occur on the same host plants, and due to their similar external morphology, they are difficult to differentiate from each other. Several molecular-based techniques have been developed for species identification within Heliothinae (Orui et al. 2000, Behere et al. 2008, Arneodo et al. 2015, Gilligan et al. 2015, Perera et al. 2015, Nagoshi et al. 2016). However, the microscopic observation of adult genitalia continues to be the most widely used method. The most obvious characters for the separation of related species are found in the genitalia of males. The shape, width and length of the valvae, the presence and number of cornuti in the vesica, the shape and number of coils of the inflated vesica, and the presence or absence of lobes at the base of the vesica constitute the key features for species discrimination (Hardwick 1965, Pogue 2004). A thorough knowledge of these morphological and morphometric traits is essential to avoid misidentification, especially in the case of the closely related H. zea and H. armigera.
During a survey of Heliothinae pests in California, Okumura and Bauer (1969) recorded the presence of a lepidopteran species resembling H. zea in external appearance, but possessing particular genital characters. They described it as a new species, which they named Heliothis stombleri. Male H. stombleri specimens were reported to have narrower valvae than H. zea, with their apical end sharpened. The phallus was apically constricted and lacked cornuti. A subsequent microscopic analysis by Hardwick (1970) concluded that, in fact, H. stombleri was an aberrant form of H. zea. Moreover, he assumed that those individuals probably would not succeed in mating. In a more recent study, Pogue (2004) re-checked the same above mentioned morphological attributes, and proposed that H. stombleri and H. zea should be treated as synonyms. In agreement with this, a later work using molecular markers revealed high similarity between H. stombleri and H. zea haplotypes (Nagoshi et al. 2016).
As for other insect species, the current classification of the Heliothinae is being supported by genetic studies involving molecular markers. Sequencing of conserved regions of the insect genome, combined with classical morphological studies, has proven a powerful tool for resolving taxonomic controversies. The present work provides additional details on the morphology of the H. zea male genitalia (both the “normal” and the “H. stombleri forms”), which should be useful for routine diagnosis. Furthermore, a phylogenetic analysis comprising nuclear and mitochondrial DNA sequences was performed in order to present supplementary genetic information to corroborate the synonymy between H. zea and H. stombleri.
Materials and Methods
Insect Sampling
Moth catches were carried out using mercury vapor light traps in January and February 2016 in Marcos Juárez (central Argentina), near corn and soybean crops. In total, 202 male specimens, which had wing markings resembling H. zea, were separated and conserved at −20 °C for posterior morphological and genetic analyses.
Microscopic Examinations
The final portion of the abdomen of all individuals was dissected, placed in KOH solution and heated in a water bath for 15 min at increasing temperature below boiling point. Then, the genitalia were removed and placed in alcohol for 24 h. After cleaning with a fine-bristled brush, genitalia were examined under a Zeiss Stemi DV4 stereomicroscope (Carl Zeiss GMBH, Hamburg, Germany) at 16× magnification. Photographs were taken at 5× zoom with a digital camera (Canon G10, Canon Inc., Tokyo, Japan). The shape of the valvae was described, and their size assessed using the program Image Pro Plus (Media Cybernetics, Silver Spring, MD). The presence and number of cornuti in the phallus were determined. The vesica was inflated by introducing 70% ethanol, using a syringe with a 0.3 mm diameter needle, in order to observe the lobes at its base.
Molecular Characterization
Five H. stombleri specimens were subjected to DNA extraction, amplification and sequencing. DNA was extracted by the CTAB method (Doyle and Doyle 1990). Amplification of an 812-bp region of the mitochondrial cytochrome oxidase subunit I gene (COI) was performed with primers H3Fw/H3Rv according to Arneodo et al. (2015). On the basis of available Heliothinae sequences, a new primer pair was designed to amplify a 587- bp fragment of the nuclear elongation factor – 1 alpha gene (EF-1α): HelicoEFaFw6 (5′-CGTCAACCAAAATGCCCTGG-3′) and HelicoEFaRv6 (5′-GGCGTCACCAGACTTGATGG-3′). For the latter, the following PCR conditions were used: 95 °C for 5 min, 40 cycles of 95 °C for 30 s, 53 °C for 30 s, and 72 °C for 45 s; and a final step of 72 °C for 5 min. All PCR products were purified using ADN Puri-Prep-GP kit (INBIO, Tandil, Argentina) and both strands sequenced in an automated genetic analyzer ABI PRISM 3500 XL (Applied Biosystems, Foster City, CA) at CICVyA-INTA (Hurlingham, Argentina). Unlike COI, EF-1α sequences from local specimens of “normal” H. zea and the two other Heliothinae reported in the study area were not available at GenBank. DNAs extracted from H. zea, H. armigera and H. gelotopoeon during a recent survey in central Argentina (Arneodo et al. 2015) were used as templates to amplify this gene. A phylogenetic analysis of COI and EF-1α sequences was conducted on newly-obtained and reference sequences retrieved from public databases. The noctuid Spodoptera exigua served as outgroup. Maximum likelihood trees were constructed with 1,000 bootstrap replicates using MEGA7 software (Kumar et al. 2016).
Results
Morphological Features
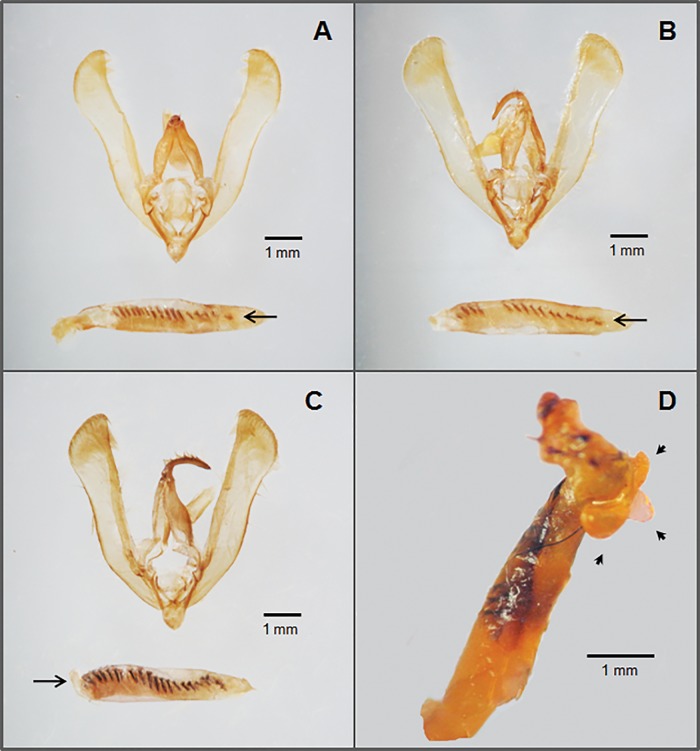
One hundred ninety three out of the 202 male moths dissected were “normal” H. zea specimens, whereas seven individuals corresponded to the “H. stombleri form”. The remaining two were identified as H. armigera. In H. zea, the length of valvae ranged from 4.7 to 5.5 mm. A certain degree of variability was observed in the shape of the valve apex. Some samples had a rounded apex and others had a sharper one. In some moths, valvae were not parallel-sided and the internal side was more curved (Fig. 1A). Notorious differences were observed concerning the number of cornuti on the vesica. Most of the samples (>70%) exhibited 18 cornuti, but this value fluctuated from 15 to 21 (Fig. 1A–C). When the vesica was inflated, three lobes or diverticula were observed at the base, with the central smallest (Fig. 1D).
Fig. 1.
Helicoverpa zea male genitalia. (A) Valvae and phallus with 17 cornuti. (B) Valvae and phallus with 15 cornuti. (C) Valvae and phallus with 21 cornuti. (D) Vesica inflated. Arrows and arrowheads show the cornuti and the lobes at the base of the vesica, respectively.
On the other hand, H. stombleri specimens displayed a distinct genital morphology. Valvae were similar in length to H. zea but with a tapering apex (Fig. 2A). The phallus presented a more or less clear constriction near the apical end and had no cornuti (Fig. 2B and C). The inflated vesica did not evert. However, three small lobes appeared close to the constriction (Fig. 2C).
Fig. 2.
Heliothis stombleri male genitalia. (A) Valvae. (B) Vesica uninflated. (C) Vesica inflated. Arrows and arrowhead show the apical constriction and lobes, respectively.
Genetic Analyses
PCR amplifications of partial COI and EF-1α genes yielded fragments of the expected sizes, as observed by agarose gel electrophoresis (data not shown). The resulting sequences were edited and aligned (Clustal W). Both the mitochondrial and nuclear sequences obtained from the five H. stombleri specimens analyzed were identical among them. Representative H. stombleri COI and EF-1α sequences are available at GenBank under accession numbers KY623667 and KY623663, respectively. EF-1α sequences corresponding to local male specimens of “normal” H. zea (KY623664), H. armigera (KY623665) and H. gelotopoeon (KY623666) were also submitted.
Overall, and regardless of the gene considered, sequence analyses clearly clustered together H. stombleri and H. zea, whereas the other Helicoverpa spp. formed separate clades. Indeed, the mitochondrial COI phylogeny revealed three well-supported groups enclosing the different species, one of which grouped H. zea and H. stombleri with a bootstrap support of 100 (Fig. 3). Phylogenetic analysis of nuclear EF-1α sequences showed the same pattern. Again, all nodes had high bootstrap values (>70%; Fig. 4).
Fig. 3.
Maximum likelihood phylogenetic tree based on COI sequences of H. stombleri (highlighted with a black circle) and related Heliothinae occurring in central Argentina. Numbers at the nodes indicate bootstrap support (1,000 replicates). Spodoptera exigua was chosen as outgroup. GenBank accession numbers are provided next to the species names. The scale bar shows the number of nucleotide substitutions per site.
Fig. 4.
Maximum likelihood phylogenetic tree based on EF-1α sequences of H. stombleri (highlighted with a black circle) and related Heliothinae. Newly reported sequences obtained in this work are marked with a white circle. Numbers at the nodes indicate bootstrap support (1,000 replicates). Spodoptera exigua was chosen as outgroup. GenBank accession numbers are provided next to the species names. The scale bar shows the number of nucleotide substitutions per site.
Discussion
In his pioneer work, Hardwick (1965) described and illustrated the genitalia of Heliothinae from different continents. The article included a series of figures focusing on the most useful characters to discriminate one species from another. This study still serves as an identification guide for world-wide researchers dealing with Heliothinae pests. However, variability is found in some cases, and species determination should be based on many characters considered as a whole.
The present paper analyzed and documented the variability of male genitalia in H. zea individuals captured in central Argentina. Emphasis was placed on the description of the valvae and on the number of cornuti in the phallus. With respect to the latter, a non-negligible proportion of moths have been shown to possess more or fewer (15–21) cornuti than the mode (18). This must be kept in mind when trying to differentiate H. zea from H. armigera, which has a lower number of cornuti (Pogue 2004). Nevertheless, the supplementary diagnostic character of three lobes at the base of the inflated vesica (vs. only one in the case of H. armigera) was observed in all H. zea samples. This fact reinforces the necessity of evaluating the morphological traits in a comprehensive manner.
Some moths displayed the unusual genitalia originally attributed to a different species, H. stombleri (Okumura and Bauer 1969). On the basis of morphology, Hardwick (1970) and then Pogue (2004) concluded that H. stombleri was an abnormal form of H. zea. This question was taken up again by Nagoshi et al. (2016), who partially sequenced COI and Z-linked triosephosphate isomerase (Tpi) genes from “normal” H. zea males and externally similar Helicoverpa specimens with aberrant genital structures, collected in Florida (USA). Their results were in line with the previous morphological deductions. The evidence presented here from additional genomic regions and different insect populations, also supports the affirmation that H. stombleri and H. zea constitute the same species. In fact, phylogenies of mitochondrial (maternal lineage) and nuclear (inherited from all ancestors) markers were congruent in placing H. stombleri within the H. zea cluster. Crosses between Heliothinae spp. have been reported (Laster et al. 1988), and these can yield individuals with genital malformations (Zhao et al. 2005). According to the nucleotide sequences provided in this paper, however, the possibility that H. stombleri could be the result of hybridization between species seems unlikely.
Hardwick (1970) reported an incidence of 3.6%, 5.6% and 15.2% of the “H. stombleri form” among H. zea captures from Argentina, Hawaii (USA) and Brazil, respectively. The percentage detected during a sampling in Texas (USA) was 9.1% (Pogue 2004). The ratio of H. stombleri to the total H. zea obtained in this study (7 out of 200, i.e., 3.5%) was similar to that obtained more than four decades ago in the Argentinian sample (Hardwick 1970). It has to be noted that, at this time, only 28 specimens were analyzed. H. stombleri individuals were considered by Hardwick (1970) as “mutant phenotypes”, doubtfully fertile in any degree and not even capable of copulating. He suggested that the abnormal genitalia would be subjected to genetic control, and not to physiological or environmental factors. The accumulated data, including those from the present work, lead to the inference that the regular and persistent occurrence of the “H. stombleri form” may represent a polymorphism. What is the origin of such anomaly, and how these forms are maintained at a relatively stable frequency is worthy of future research.
Acknowledgments
We are grateful to the JIS reviewers who contributed valuable remarks that improved our manuscript. This work was funded by Instituto Nacional de Tecnología Agropecuaria (Argentina).
References Cited
- Arneodo J. D., Balbi E. I., Flores F. M., Sciocco-Cap A. 2015. Molecular identification of Helicoverpa armigera (Lepidoptera: Noctuidae: Heliothinae) in Argentina and development of a novel PCR-RFLP method for its rapid differentiation from H. zea and H. gelotopoeon. J. Econ. Entomol. 108: 2505–2510. [DOI] [PubMed] [Google Scholar]
- Balbi E. I., Flores F. M. 2015. Evaluación del daño causado por el “Cogollero de maíz” (Spodoptera frugiperda) y la presencia de la “Isoca de la espiga” (Helicoverpa zea) en diferentes híbridos de maíz transgénico. Maíz Actualización 2015. INTA Ediciones. ISSN 1851-9245. Informe de actualización Técnica N°34: 21–26.
- Behere G. T., Tay W. T., Russell D. A., Batterham P. 2008. Molecular markers to discriminate among four pest species of Helicoverpa (Lepidoptera: Noctuidae). Bull. Entomol. Res. 98: 599–603. [DOI] [PubMed] [Google Scholar]
- Doyle J. J., Doyle J. L. 1990. Isolation of plant DNA from fresh tissue. Focus. 12: 13–15. [Google Scholar]
- Gilligan T. M., Tembrock L. R., Farris R. E., Barr N. B., Van Der Straten M. J., Van De Vossenberg B.T.L.H., Metz-Verschure E. 2015. A multiplex real-time PCR assay to diagnose and separate Helicoverpa armigera and H. zea (Lepidoptera: Noctuidae) in the New World. PLoS ONE. 10(11): e0142912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick D. F. 1965. The corn earworm complex. Mem. Entomol. Soc. Can. 97: 5–247. [Google Scholar]
- Hardwick D. F. 1970. The biological status of “Heliothis stombleri”. Can. Entomol. 102: 339–341. [Google Scholar]
- Kumar S., Stecher G., Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster M. L., King E. G., Furr E. 1988. Interspecific Hybridization of Heliothis subflexa and H. virescens (Lepidoptera: Noctuidae) from Argentina. Environ. Entomol. 17: 1016–1018. [Google Scholar]
- Margheritis A. E., Rizzo H.F.E. 1965. Lepidópteros de interés agrícola. Orugas, isocas y otras larvas que dañan a los cultivos. Editorial Sudamericana, S.A. Buenos Aires. 197 p.
- Murúa M. G., Cazado L. E., Casmuz A., Herrero M. I., Villagrán M. E., Vera A., Sosa-Gómez D. R., Gastaminza G. 2016. Species from the Heliothinae complex (Lepidoptera: Noctuidae) in Tucumán, Argentina, an update of geographical distribution of Helicoverpa armigera. J. Insect Sci. 16: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi R. N., Gilligan T. M., Brambila J. 2016. Combining Tpi and CO1 genetic markers to discriminate invasive Helicoverpa armigera from local Helicoverpa zea (Lepidoptera: Noctuidae) populations in the Southeastern United States. J. Econ. Entomol. 109: 2115–2124. [DOI] [PubMed] [Google Scholar]
- Navarro F. R., Saini E. D., Leiva P. D. 2009. Clave pictórica de polillas de interés agrícola, agrupadas por relación de semejanza. Primera Edición. Instituto Nacional de Tecnología Agropecuaria, INTA – Estación Experimental Agropecuaria Pergamino e IMyZA-CNIA Castelar/Facultad de Ciencias Naturales e Instituto “Miguel Lillo”, Universidad Nacional de Tucumán. Buenos Aires, Argentina. 100 p.
- Okumura G. T., Bauer W. R. 1969. A new species of Heliothis resembling Heliothis zea (Boddie), corn earworm (Lepidoptera: Noctuidae). Occasional Papers N°18, Bureau of Entomology, California Department of Agriculture, Sacramento, California. 8 pp.
- Orui Y., Matsuzawa H., Koike Y., Yoshimatsu S. 2000. Discrimination of Helicoverpa armigera (Hübner) and H. assulta (Guenée) (Lepidoptera: Noctuidae) by PCR-RFLP analysis, and application to surveying occurrence of H. armigera in tobacco fields of Japan. Jpn. J. Appl. Entomol. Zool. 44: 73–79. [Google Scholar]
- Perera O. P., Allen K. C., Jain D., Purcell M., Little N. S., Luttrell R. G. 2015. Rapid Identification of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) using ribosomal RNA internal transcribed spacer 1. J. Insect Sci. 15: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue M. G. 2004. A new synonym of Helicoverpa zea (Boddie) and differentiation of adult males of H. zea and H. armigera (Hübner) (Lepidoptera: Noctuidae: Heliothinae). Ann. Entomol. Soc. Am. 97: 1222–1226. [Google Scholar]
- Zhao X.-C., Dong J.-F., Tang Q.-B., Yan Y.-H., Gelbic I., Van Loon J.J.A., Wang C.-Z. 2005. Hybridization between Helicoverpa armigera and Helicoverpa assulta (Lepidoptera: Noctuidae): development and morphological characterization of F1 hybrids. Bull. Entomol. Res. 95: 409–416. [DOI] [PubMed] [Google Scholar]