Abstract
Glioblastoma (GBM) is a devastating brain tumor with poor prognosis despite advances in surgery, radiation, and chemotherapy. Survival of patients with glioblastoma remains poor, with only 1 in 4 patients alive at 2 years, and a 5-year survival rate of about 5%. Recurrence is nearly universal and, after recurrence, prognosis is poor with very short progression-free survival and overall survival (OS). Various salvage chemotherapy strategies have been applied with limited success. Tumor Treating Fields (TTFields) are a novel treatment modality approved for treatment of either newly diagnosed or recurrent GBM. TTFields therapy involves a medical device and transducer arrays to provide targeted delivery of low intensity, intermediate frequency, alternating electric fields to produce antimitotic effects selective for rapidly dividing tumor cells with limited toxicity. In the phase 3 EF-14 trial, TTFields plus temozolomide provided significantly longer progression-free survival and OS compared with temozolomide alone in patients with newly diagnosed GBM after initial chemoradiotherapy. The addition of TTFields to standard therapy improved median OS from 15.6 to 20.5 months (P=0.04). In the phase 3 EF-11 trial, for recurrent GBM, TTFields provided comparable efficacy as investigator’s choice systemic therapy, with improved patient-reported quality of life and a lower incidence of serious adverse events. Primary toxicity associated with TTFields is skin irritation generally managed with array relocation and topical treatments including antibiotics and steroids. TTFields therapy has demonstrated proven efficacy in management of GBM, including improvement in OS for patients with newly diagnosed GBM, and is under current investigation in other brain and extracranial tumors.
Key Words: tumor treating fields, TTFields, glioblastoma, alternating electric fields, Optune
Despite the advances in surgical techniques, radiation therapy, chemotherapy, targeted agents, and immune modulators, the survival of patients with glioblastomas (GBM) remains poor, with only 1 in 4 patients alive at 2 years, and a 5-year survival rate of about 5%.1,2 There is also an increasing incidence of primary malignancies of the brain, although the etiology for this change is unclear.3 Current standard of care for GBM includes maximal safe resection and conformal radiation therapy with concurrent and then adjuvant temozolomide (TMZ).2 Several large randomized trials have investigated the role of dose-intensified TMZ (RTOG 0525)4 or concomitant bevacizumab (AVAglio5 and Radiation Therapy Oncology Group 08256) in the initial management of GBM and reported no significant benefit in overall survival (OS).
Almost all GBMs recur, and the prognosis after recurrence is poor, with very short progression-free survival (PFS) and OS.7–9 Various salvage chemotherapy strategies have been applied in this setting with limited success.7–10 A novel treatment utilizing alternating electric fields, Tumor treating Fields (TTFields), has been developed as an innovative mechanism of tumor cell injury and has now been used in the management of both newly diagnosed and recurrent GBM. In this manuscript, we will review this novel technology, including its mechanism of action, evolving clinical data, current indications, and potential future applications.
WHAT ARE TTFIELDS?
TTFields therapy utilizes low intensity, intermediate frequency, alternating electric fields whose overall effects are interference with and prolongation of cell division, and disruption of cytokinesis in rapidly dividing cells, resulting in apoptosis.11 TTFields take advantage of the electrical polarity, geometric shape, and rapid replication rate of cancer cells, and especially macromolecules within these cells to produce selective anticancer effects (Fig. 1). The optimal electrical frequency for the most effective cell kill varies by tumor type.11,12 For recurrent GBM, TTFields are delivered at an intensity of 1 to 3 V/cm and frequency of 200 kHz. The effect of TTFields on normal cells is limited, enabling a potentially high therapeutic index to be achieved in the treatment of GBM and other malignancies.
FIGURE 1.
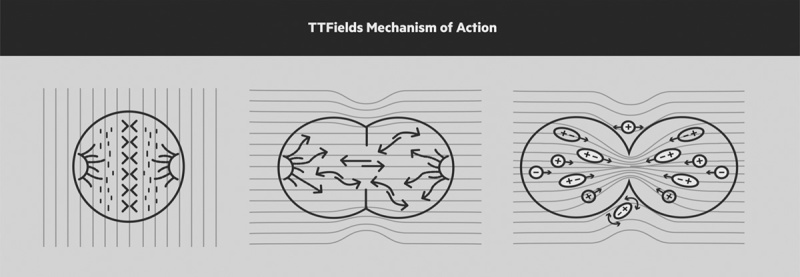
TTFields mechanism of action. The alternating electric fields interfere with mitosis leading to apoptosis and cell death. The alternating electric fields effects are interference and prolongation of cell division, and disruption of cytokinesis in rapidly dividing cells, resulting in apoptosis. Copyright Novocure, 2015. Copyright [Novocure], [Portsmouth, NH]. All permission requests for this image should be made to the copyright holder.
PRECLINICAL STUDIES IN VITRO AND IN VIVO ANIMAL MODELS
The concept for TTFields as a therapeutic option for malignancy was evaluated in preclinical studies in the early 2000s. TTFields therapy was initially shown to effectively inhibit cancer cell growth in various cell lines in vitro.13 The efficacy of TTFields depends on the intensity, frequency, and direction of the applied electric fields.11,13 Antimitotic effects were shown to be dose-dependent in the range of 1 to 3 V/cm for rat glioma, with the strongest inhibition of cell division at 200 kHz.11 This study demonstrated that the antimitotic effect was enhanced by applying electrical fields in >1 direction. As the tumor cells are not necessarily oriented in the same direction, maximal antimitotic effects are achieved when the electrical fields are parallel to the axis of cell division.11 The antitumor effects of TTFields were confirmed in an in vivo intracranial rat glioma model, where tumor volume reductions of 42.6% (bidirectional TTFields) and 53.4% (tridirectional TTFields) were observed, compared with untreated tumors.11 The inhibitory effect associated with unidirectional TTFields delivery was modest, whereas statistically significant tumor growth inhibition was observed with 2 or 3 directional TTFields, consistent with the in vitro results. Additional studies reported an additive antitumor effect of TTFields plus chemotherapy and radiation therapy in both in vitro and in vivo models.14–16
Further study has been performed on the electric field distribution and its dependence on tissue dielectric properties and anatomy utilizing a realistic head model. The researchers found that the average field strength values were about 10% higher in the tumor when incorporating anisotropy. They also concluded that the electric field in the tumor, in their realistic head model, exceeds 1 V/cm which is in the previously studied antimitotic range.17
TTFIELDS PILOT STUDIES
The encouraging in vitro and in vivo results led to preliminary evaluation of TTFields in patients with GBM. The initial trial examined TTFields as monotherapy in 10 patients with recurrent, TMZ-refractory GBM, comparing time to PFS and OS with historical controls.11 The patients treated with TTFields had a median time to radiographic progression of 26.1 weeks, compared with 9.5 weeks for historical controls, and a median OS of 62.2 weeks, compared with 29.3 weeks for historical controls. Of note, 67.5% of the TTFields-treated patients with recurrent high-grade gliomas were still alive 1 year after beginning therapy.11
A second pilot trial tested TTFields plus TMZ in 20 concurrent newly diagnosed GBM patients who received initial therapy with standard radiotherapy and TMZ.14 The median time to tumor progression with TTFields plus TMZ was 155 weeks versus 31 weeks with TMZ alone in the concurrent historical control group (P=0.0002). Median OS was >39 months in the patients treated with adjuvant TTFields plus TMZ versus approximately 14.7 months in a matched historical control group treated with adjuvant TMZ alone (P=0.0018). All patients treated with the TTFields had grade I-II dermatitis. There were no reported grade 3 or higher toxicities.
PROSPECTIVE RANDOMIZED TRIAL IN THE MANAGEMENT OF RECURRENT GBM
The prospective, randomized, international, phase 3 EF-11 trial compared TTFields monotherapy with investigator’s choice of systemic therapy in patients with recurrent GBM.18 I total, 117 patients were randomly assigned to TTFields monotherapy and 120 patients to investigator’s choice systemic therapy.18 The primary endpoint was OS. Secondary endpoints included PFS, PFS at 6 months, overall response rate, 1-year survival, safety, and quality of life (QoL).
Study patients treated with TTFields were instructed to wear the device ≥18 hours a day, with the exception of short treatment breaks of 1 hour twice a day for personal care needs. The treatment arms were well balanced, with a median age of 54 years and median KPS of 80. The vast majority (90%) of patients were at second or subsequent recurrence, with 20% bevacizumab failures before entering the trial.18,19
The median survival of 6.6 months in the TTFields arm and 6.0 months in the investigators’-choice chemotherapy arm was not statistically significant different. Patients treated with TTFields alone had comparable OS to that of patients who received investigator’s-choice chemotherapy with various agents as monotherapy, or in combination including bevacizumab (31%), irinotecan (31%), nitrosurea (25%), carboplatin (13%), or TMZ (11%). There was no statistically significant difference in radiographic response rates, 14% versus 9.6% (P=0.19) between the 2 arms, that is, TTF versus control. PFS was also not different, 2.2 months for the TTFields group and 2.1 months for the investigators’-choice group (P=0.16).
Patients randomized to the TTFields arm self-reported a higher QoL, including improved cognitive and emotional functioning. Patients in the chemotherapy arm had statistically higher incidence of gastrointestinal, hematologic, and infectious adverse events. Severe adverse events also occurred less frequently in the TTFields-treated group compared with the chemotherapy-treated patients (6% vs. 16%, P=0.022). The most common device-related events experienced with TTFields therapy were mild to moderate scalp irritation (16%) beneath the arrays. These were generally managed with topical ointments and periodic relocation of the arrays.
In a post-hoc analysis, the most significant predictor of response in the TTFields arm was treatment compliance.20–24 Other post-hoc analyses showed that OS was significantly longer in patients whose time on therapy was 18 hours/day or greater (>75% compliance rate) than in those with a <75% compliance rate (7.7 vs. 4.5 mo, P=0.042). Given its mechanism of action, the antitumor effects of TTFields are immediately removed once TTFields therapy is stopped, explaining the need for continuous application. This might explain the superior survival in the patients with a more continuous utilization of the device. Post-hoc analyses also pointed to significantly higher median OS with TTFields versus investigator’s choice chemotherapy for patients with Karnofsky performance status ≥80, tumor size ≥18 cm2, prior low-grade glioma, and (perhaps most interesting) those who had previously failed bevacizumab therapy. These findings warrant further examination in suitably designed studies powered to better evaluate the impact of the variables on OS in recurrent GBM patients treated with TTFields.24
In 2011, the US Food and Drug Administration (FDA) approved TTFields therapy for recurrent GBM, based largely on the results from the EF-11 trial showing equivalent survival with TTFields therapy compared with a broad range of investigator’s choice systemic therapy, together with improved patient-reported QoL and a lower incidence of serious adverse events with TTFields.
POSTAPPROVAL REGISTRY: TTFIELDS IN REAL WORLD SETTING
The impact of TTFields therapy on outcomes in patients with recurrent GBM treated outside of clinical trials has been examined using data from the Patient Registry Data set (PRiDe).25 PRiDe is a postmarketing registry of all recurrent GBM (presumed on the basis of locally reported diagnosis) patients treated with TTFields in a real-world, clinical practice setting in the United States between 2011 and 2013. The PRiDe data set analyzed 457 patients in 91 US centers, and demonstrated a median OS of 9.6 months, significantly longer than the 6.6 months reported in the EF-11 trial (P=0.0003).25 One- and 2-year OS rates were more than double for TTFields therapy patients in PRiDe than in the EF-11 trial (1-year: 44% vs. 20%; 2-year: 30% vs. 9%). Favorable prognostic factors were improved survival were first/second versus third or subsequent recurrences, higher Karnofsky performance status, and no prior bevacizumab use. There were no unexpected adverse events or safety issues. Similar to the results of prior TTFields studies, the most common adverse event was skin toxicity, which was reported in 24.3% of the patients.
PROSPECTIVE RANDOMIZED TRIAL IN THE MANAGEMENT OF NEWLY DIAGNOSED GLIOBLASTOMA
A prospective randomized phase-3 trial (EF-14) evaluated the use of TTFields in the initial management of newly diagnosed GBM, and the findings from the study led to the FDA approving TTFields in combination with TMZ for the treatment of newly diagnosed GBM in October 2015.26 In the international EF-14 trial, 695 patients who had completed chemoradiotherapy were randomized in a 2:1 ratio to receive maintenance treatment with either TTFields plus TMZ or TMZ alone (standard adjuvant therapy). No placebo or sham device was utilized. The arms were well balanced in regards to age, performance status, resection, and MGMT promoter methylation. A prespecified interim analysis performed after the first 315 patients reached a minimum follow-up of 18 months demonstrated efficacy with acceptable tolerability and safety and led to early mandatory stoppage of the trial, as per the independent Data Safety Monitoring Committee’s recommendations.
The primary endpoint of EF-14 was PFS in the intent-to-treat population.26 OS in the per-protocol (as-treated) population was a key secondary endpoint. The prespecified interim analysis demonstrated a significantly longer median PFS in the TTFields arm versus control arm after a median follow-up of 38 months (7.1 vs. 4.0 mo; P=0.001; Fig. 2). The median OS in the per-protocol population was 20.5 months in the experimental arm versus 15.6 months in the control arm (P=0.04). The median OS in the per-protocol population was significantly longer in the TTFields versus control arm (20.5 vs. 15.6 mo; P=0.004). The trial was stopped before the planned accrual of 700 patients (randomized 695 patients) at the recommendation of independent data monitoring committee.26 The results for all 695 enrolled patients with a mature minimum follow-up of 18 months and median follow-up of 36 months confirmed the results of the interim analysis that resulted in early stopping and continued to show that the addition of TTFields to TMZ confers greater benefit in PFS and OS than TMZ alone.27
FIGURE 2.
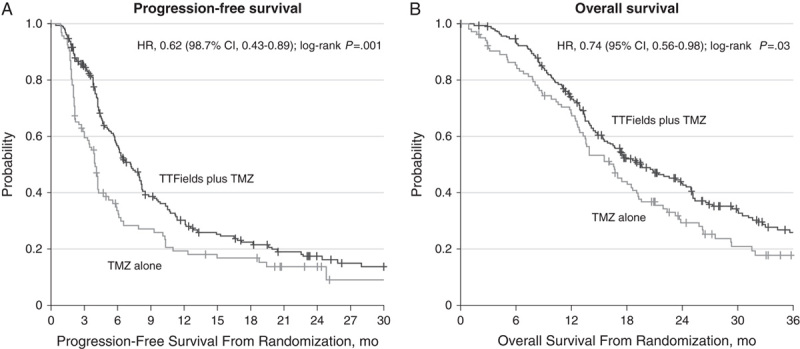
Survival curves for patients included in the interim analysis in the intent-to-treat population of EF-14. Kaplan-Meier curves for patients with GBM in the EF-14 trial, treated with TTFields/TMZ versus TMZ alone. (A) PFS (ITT) (B) OS.26 Figure adapted with permission from Stupp et al.26 GBM indicates glioblastoma; OS, overall survival; PFS, progression-free survival; TMZ, temozolomide; TTFields, tumor treating fields. Copyright © 2017 The Author(s).
Per protocol, OS was analyzed in the as-treated population that excluded all patients in both arms who (1) never started TMZ, (2) had a major protocol violation, (3) crossed over to the other treatment group, or (4) received TTFields outside the protocol setting. It is important to note that the randomization was not performed until after completion of the initial radiation and TMZ (chemoradiotherapy), which means patients were enrolled in the trial approximately 4 months after initial diagnosis. Historically, trials of initial management of newly diagnosed GBM measure survival from randomization before chemoradiotherapy. The randomization approach used in EF-14 was intended to minimize the effect of pseudoprogression with a time to progression endpoint, but also has the effect of excluding some of the most unfavorable patients (from both arms)—which, in turn, should be considered when comparing the OS results in EF-14 to other trials in the literature.
Three-quarters of the patients in the TTFields arm of EF-14 were considered adherent, wearing the device >18 hours per day on average during the first 3 months of therapy.26 Two thirds of the patients randomized to the TTFields continued treatment with the device after first progression. The most common adverse event related to the device was skin irritation, occurring in 43% of patients (2% grade 3 or higher). Patients treated with TTFields were also more likely to report grade 1 or 2 mild anxiety, confusion, insomnia, and headaches, most commonly at the initiation of therapy.
TTFIELDS DELIVERY
TTFields therapy is the delivery of low intensity, intermediate frequency, alternating electric fields by 2 orthogonal pairs of transducer arrays placed on the shaved scalp of GBM patients. The device is generally worn at all times and requires an electrical power source, either direct AC plug or portable battery. Compliance can be a concern—particularly in patients with poor Karnofsky performance status. The older, first generation version of the device plus battery weighed >5 pounds which was difficult for some patients. A second generation device is now approved in the United States and Europe, weighing only 2.7 pounds including battery (Fig. 3). It is thought that the reduced weight of the second-generation device may improve patient compliance. Regular shaving of the scalp is also an essential requirement and can also cause a certain degree of noncompliance.
FIGURE 3.

Optune with battery, charger, arrays, and carrying case. The Optune system includes electric field generator, color-coordinated arrays, charger with spare batteries, carrying case, and power outlet adapter. This image features the second-generation Optune system, currently approved for use in Europe and in the United States. Copyright Novocure, 2016. Copyright [Novocure], [Portsmouth, NH]. All permission requests for this image should be made to the copyright holder.
MANAGEMENT OF PRIMARY TOXICITY
The primary toxicity associated with TTFields is skin irritation (Fig. 4), as reported in prior clinical trials and the PRiDe data review. Skin care strategies can help maximize adherence to TTFields while maintaining QoL. Prophylactic strategies include proper shaving, cleansing of the scalp, and frequent array relocation. When skin issues arise, they can generally be managed by array relocation and topical or oral antibiotics, topical corticosteroids, and isolation of affected skin areas from adhesives and pressure.28
FIGURE 4.
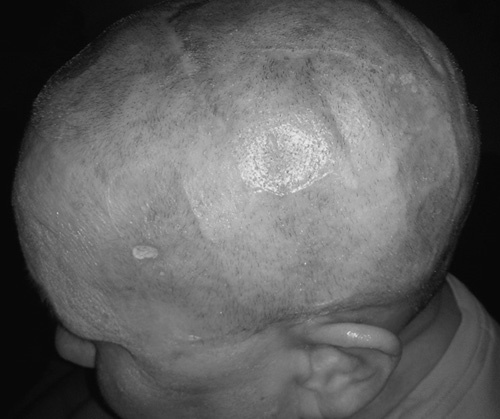
Dermatological toxicity from transducer arrays. Contact dermatitis can occur from long-term use (≥6 mo) of transducer arrays. These dermatitis sequelae may or may not be symptomatic. Most adverse effects could be managed using published skin care guidelines for patients receiving TTFields.28 Reproduced with permission from Lacouture et al.28 TTFields indicates tumor Treating fields. Copyright © 2017 The Author(s).
DURATION OF THERAPY
The antitumor effects of TTFields only occur when the device delivering them is actively in use (turned “on”). Unlike chemotherapy, there is no treatment-related “half-life” that continues after initial administration. Hence, compliance is especially critical for the effectiveness of TTFields therapy.
Of note, studies indicate that approximately 15% of patients with recurrent GBM who ultimately show durable response exhibit initial tumor growth before shrinkage.29 Moreover, many of these patients with slowly emerging responses have been reported to still be alive >7 years after beginning TTFields therapy.29–31 These findings suggest that it is important to be patient and allow time when assessing the effectiveness of TTFields therapy in GBM. TTFields therapy should not be discontinued on the basis of early radiographic changes alone.29–31 In EF-14, the device was generally worn until second progression to account for this potential transient initial enlargement of the tumor; this concept is similar to the pseudoprogression issues encountered with the use of temozolomide.
TTFIELDS ARRAY PLACEMENT
Correct placement of the transducer arrays on the shaved scalp is important for the success of the TTFields therapy. Proprietary software is used with patient magnetic resonance imaging (MRI) data to optimize array placement for maximal effectiveness (Fig. 5). In the United States, NovoTAL software is utilized for treatment mapping and planning.32 It needs to be noted however that neither the EF-11 or EF-14 studies, nor the majority of patients in the PRiDe data set were treated using the NovoTAL array placement software.
FIGURE 5.
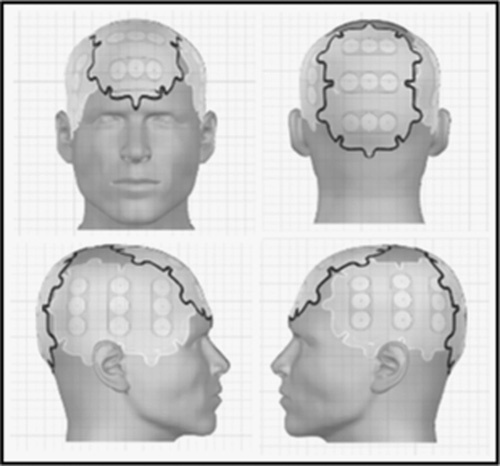
Transducer array placement for treating patients with GBM. An array map used as guidance for optimal placement of transducer arrays on the basis of tumor size and location. The array map is personalized for each patient and generated using NovoTAL System software.32 The customization of the array layout is dependent on the patient’s size and location of the tumor. GBM indicates glioblastoma. Copyright Novocure, 2015. Copyright [Novocure], [Portsmouth, NH]. All permission requests for this image should be made to the copyright holder.
ADOPTION OF THE TECHNOLOGY
Although supported, in the newly diagnosed setting, by prospective phase 3 data, adoption has been relatively slow in the management of patients with glioblastoma. In the fourth quarter of 2015, there were 499 new prescriptions for Optune in the United States and this had increased to 544 for the fourth quarter of 2016. Around, 55% of the new prescriptions in the fourth quarter of 2016 were for newly diagnosed patients. This was a 9% increase year over year, but represents a minority of patients with newly diagnosed GBM with only approximately 15% of newly diagnosed patients being treated with TTFields.33
There is limited published available data as to the slow rate of adoption thus far. In the authors’ experiences, there is a variety of reasons for lack of adoption. The main reason is likely the newness of the technology and the need for the medical community to become familiar with the technology, device, and published data. It also is outside the usual 3 approaches to cancer therapy of surgery, radiation, and medication so there remains some skepticism on the utility of the therapy. Some clinical trials do not allow the therapy, which may also limit utilization in some of the most motivated patients. Initially, there was some question of adoption/coverage by insurance companies as well.
In addition, to utilize the device, patients are required to shave their head with no prospect for allowed regrowth, which is a deterrent. The fact that the device is visible during treatment can also reduce patient enthusiasm. Finally, the requirement for the battery pack can make the device difficult to utilize for patients with limited performance status or other physical infirmities. Generally, a committed caregiver is required to effectively manage the device with shaving, application, etc. and not all patients are able to manage the logistics associated.33
The slow growth of uptake may increase with continued publication of data showing efficacy, as well as, increased familiarity for both physicians and patients.
ONGOING CLINICAL QUESTIONS
TTFields are a novel cancer treatment modality for GBM. It is actively being investigated in a number of other cancer types, as well as for different GBM indications, for example, as initial therapy with bevacizumab for unresectable GBM or in combination with reirradiation or with bevacizumab (with or without reirradiation) for recurrent GBM. Ongoing and/or planned trials are exploring TTFields in low-grade gliomas as well as recurrent atypical and anaplastic meningiomas. The METIS trial is a phase 2 open-label randomized study of radiosurgery with or without TTFields for patients with 1 to 10 brain metastases from nonsmall cell lung cancer with a primary endpoint of time to first intracranial failure.34 This trial is designed to address whether TTFields can provide the intracranial control benefit of whole-brain radiotherapy but without its cognitive toxicity, and thus includes neurocognition as a secondary endpoint.
Extracranial applications of TTFields are also being evaluated. There are trials exploring the use of TTFields in the thorax, in advanced nonsmall cell lung carcinoma, in mesothelioma, as well as for intraabdominal indications with pancreatic carcinoma and recurrent ovarian carcinoma. As preclinical data suggest synergistic benefit with radiation therapy and certain chemotherapy agents,14,35 this is likely to be an area of active investigation.
CONCLUSIONS
TTFields, a novel anticancer therapy, has demonstrated efficacy and been approved for use in patients with GBM. The first FDA approval (2011) was for recurrent GBM, on the basis of a phase 3 study that showed TTFields exhibited similar efficacy with improved QoL and reduced rate of serious adverse events compared with investigator’s choice systemic therapy. More recently, the phase 3 EF-14 international trial demonstrated the efficacy of TTFields plus TMZ versus TMZ alone as maintenance therapy following chemoradiotherapy in patients with newly diagnosed GBM. This led to the October 2015 approval of TTFields in combination with TMZ for the treatment of newly diagnosed GBM.
The neurooncology community, including many radiation oncologists, now has several years of experience with TTFields in the clinical setting of GBM. On the basis of the results of the EF-14 trial, it can be reasonably argued that TTFields should be discussed with all patients with newly diagnosed GBM as part of their initial therapy, although further studies would be useful to refine the population most likely to benefit, and more importantly identify subsets where benefit is minuscule or not present.
Treatment with TTFields can be inconvenient for patients as a result of the required application of transducer arrays directly to the shaved scalp of GBM patients for >18 hours a day, and also because of the requirement of a power supply for the unit. This lack of convenience may be at least partially compensated by the general lack of other toxicities that are associated with other focal or systemic therapies.
Although there is opportunity for further investigation of TTFields in the management of GBM, TTFields are also being actively explored as a treatment approach for patients with other brain or extracranial tumor types.
Footnotes
S.H.B. reports receiving personal fees from Novocure. M.P.M. reports grants and personal fees from Novocure and Novelos, as well as personal fees from Abbott, BMS, Cavion, Celldex, Elekta, Monteris, Novartis, Pharmacyclics, Phillips, and Roche. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26:825–853. [DOI] [PubMed] [Google Scholar]
- 8.Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015;22:e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro Oncol. 2013;15:4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta M, Brem S. Recent updates in the treatment of glioblastoma: introduction. Semin Oncol. 2014;41(suppl 6):S1–S3. [DOI] [PubMed] [Google Scholar]
- 11.Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novocure Inc Optune (NovoTTF-100A System Portsmouth, NH). Instructions for Use. Issue date, October 2015:1–26.
- 13.Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. [DOI] [PubMed] [Google Scholar]
- 14.Kirson ED, Schneiderman RS, Dbaly V, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karanam NK, Srinivasan K, Sishc B, et al. Tumor treatment fields can enhance the radio- and chemosensitivity of non-small cell lung cancer cell lines. Int J Radiat Oncol Biol Phys. 2016;96(2S):E578. [Google Scholar]
- 16.Kim EH, Kim YJ, Song HS, et al. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. 2016;7:62267–62279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenger C, Salvador R, Basser PJ, et al. The electric field distribution in the brain during TTFields therapy and its dependence on tissue dielectric properties and anatomy: a computational study. Phys Med Biol. 2015;60:7339–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. [DOI] [PubMed] [Google Scholar]
- 19.Gutin PH, Wong ET. Noninvasive application of alternating electric fields in glioblastoma: a fourth cancer treatment modality. Am Soc Clin Oncol Educ Book. 2012;32:126–131. [DOI] [PubMed] [Google Scholar]
- 20.Kanner AA, Wong ET, Villano JL, et al. EF-11 Investigators. Tumor treating fields (TTFields) in recurrent GBM. An updated subgroup analysis of the phase III data (abstract). Neuro Oncol. 2013;15(suppl):3iii114. [Google Scholar]
- 21.Ram Z, Gutin PH, Stupp R. Subgroup and quality of life analyses of the phase III clinical trial of NovoTTF-100A versus best standard chemotherapy for recurrent glioblastoma. Neuro Oncol. 2010;12:48–49. [Google Scholar]
- 22.Ram Z, Wong ET, Gutin PH. NO-50. Comparing the effect of NovoTTF to bevacizumab in recurrent GBM: a post-hoc sub-analysis of the phase III trial data (abstract). Neuro Oncol. 2011;13(suppl):3iii52.21149252 [Google Scholar]
- 23.Wong ET, Elzinga G, Chung A, et al. Objective response in recurrent glioblastoma from adjuvant NovoTTF-100A and TCCC after temozolomide and bevacizumab failure (abstract). Neuro Oncol. 2013;15(suppl):3iii134. [Google Scholar]
- 24.Kanner AA, Wong ET, Villano JL, et al. Investigators EF. Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A system versus best physician's choice chemotherapy. Semin Oncol. 2014;41(suppl 6):S25–S34. [DOI] [PubMed] [Google Scholar]
- 25.Mrugala MM, Engelhard HH, Dinh Tran D, et al. Clinical practice experience with NovoTTF-100A system for glioblastoma: the Patient Registry Dataset (PRiDe). Semin Oncol. 2014;41(suppl 6):S4–S13. [DOI] [PubMed] [Google Scholar]
- 26.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–2543. [DOI] [PubMed] [Google Scholar]
- 27.Stupp R, Idaih A, Steinberg DM, et al. LTBK-01: prospective, multi-center phase III trial of tumor treating fields together with temozolomide compared to temozolomide alone in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18(suppl 6):i1.26705298 [Google Scholar]
- 28.Lacouture ME, Davis ME, Elzinga G, et al. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014;41(suppl 4):S1–S14. [DOI] [PubMed] [Google Scholar]
- 29.Vymazal J, Wong ET. Response patterns of recurrent glioblastomas treated with tumor-treating fields. Semin Oncol. 2014;41(suppl 6):S14–S24. [DOI] [PubMed] [Google Scholar]
- 30.Rulseh AM, Keller J, Klener J, et al. Long-term survival of patients suffering from glioblastoma multiforme treated with tumor-treating fields. World J Surg Oncol. 2012;10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villano JL, Williams LE, Watson KS, et al. Delayed response and survival from NovoTTF-100A in recurrent GBM. Med Oncol. 2013;30:338. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry A, Benson L, Varshaver M, et al. NovoTTF™-100A system (tumor treating fields) transducer array layout planning for glioblastoma: a NovoTAL™ system user study. World J Surg Oncol. 2015;13:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novocure reports fourth quarter and full year 2016 financial results and provides company update. Available at: https://www.novocure.com/novocure-reports-fourth-quarter-and-full-year-2016-financial-results-and-provides-company-update/. Accessed March 30, 2017.
- 34.Weinberg U, Farber O, Bomzon Z. A phase III study of radiosurgery with TTFields for 1-10 brain metastases from NSCLC. J Thoracic Oncol. 2016;11:4SLS143–S146. [Google Scholar]
- 35.Schneiderman RS, Shmueli E, Kirson ED, et al. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]


