Abstract
Uterine fibroids can cause abnormal uterine bleeding and their removal is beneficial in the treatment of heavy menstrual bleeding associated with fibroids for women who would like to preserve their uterus and fertility. Endoscopic (hysteroscopic and laparoscopic) approaches are the preferred methods of fibroid removal when appropriate. In the presence of submucosal fibroids, hysteroscopic resection is a simple, safe and effective treatment for heavy menstrual bleeding and reduces the need for more major surgery, such as hysterectomy. When abdominal myomectomy is required, laparoscopic myomectomy is the preferred choice in selected cases due to its advantages over open myomectomy.
Keywords: abdominal myomectomy, fibroids, hysteroscopic morcellator, hysteroscopic myomectomy, laparoscopic myomectomy, leiomyoma, morcellator, myomectomy, open myomectomy, resectoscope
Uterine fibroids or leiomyomas can be identified by ultrasound in about 70–80% of women by the time of the menopause [1]. While a large proportion of women with fibroids are asymptomatic, some experience significant symptoms such as abnormal uterine bleeding (AUB), pressure symptoms, pain and infertility. Uterine fibroids can cause heavy menstrual bleeding (HMB), intermenstrual bleeding or completely erratic and prolonged episodes of bleeding which may all lead to excessive blood loss and anemia. In general, submucosal fibroids are more likely to cause AUB. However, large intramural, and to some extent subserosal fibroids, that enlarge or distort the uterine cavity can cause bleeding problems. Surgery is quite often required in these situations as medical interventions may be less effective. Hysterectomy is still the most common operation carried out for fibroids [2] but is not an acceptable option for women who want to preserve their fertility or uterus. For this reason many women choose to seek removal of their fibroids. Transabdominal surgical approaches are necessary to remove intramural and subserosal fibroids, whereas a less invasive transcervical approach can be utilized to remove submucosal fibroids.
Removal of fibroids or myomectomy has long been an established treatment for conservative treatment of these symptoms. Myomectomy at laparotomy (open myomectomy) was described as early as 1840 when Amussat removed a tumor thought to be an ovary because it had a pedicle [3]. Hysteroscopic resection of submucosal fibroids was first introduced in 1976 [4] and laparoscopic myomectomy (LM) was first performed the following year [5].
Classification of fibroids
A widely used classification of submucosal fibroids was developed by the European Society of Hysteroscopy, which was later renamed as the European Society for Gynaecological Endoscopy [6]. The classification was shown to have a significant predictive value in determining the successful removal following hysteroscopic resection of fibroids. In 2011, FIGO proposed a classification system for all leiomyomas, which incorporated the European Society for Gynaecological Endoscopy classification of submucosal fibroids (Table 1) [7]. A potential drawback of the new FIGO system is that it does not take the number and size of fibroids into consideration. These two parameters may impact on the feasibility and outcome of surgery. A potentially significant contribution of the new FIGO classification is the description of a ‘type 3 fibroid’; a location of fibroid that may affect the endometrial function in terms of reproduction and abnormal bleeding, although it does not protrude into the cavity. The clinical utility of the FIGO system remains to be seen.
Table 1.
FIGO classification of fibroids.
| Location | Type | Description |
|---|---|---|
| Submucosal | 0 | Completely in the cavity |
| 1 | >50% in the cavity | |
| 2 | ≤50% in the cavity | |
| Other | 3 | Intramural but touches endometrium |
| 4 | Intramural, no contact with endometrium | |
| 5 | Subserosal, ≥50% intramyometrial | |
| 6 | Subserosal, <50% intramyometrial | |
| 7 | Pedunculated subserosal | |
Hysteroscopic myomectomy
Submucous fibroids protrude into the uterine cavity and can usually be removed hysteroscopically. This is a less invasive, simpler and safer approach compared with abdominal myomectomy. In fact, submucosal fibroids may be difficult to locate during abdominal operations and they may be left behind such that AUB symptoms persist. Urological resectoscopes were adapted to produce hysteroscopic resection systems capable of providing visualization and cutting of fibroid material within the uterine cavity. Miniaturization of endoscopic equipment has allowed the development of smaller diameter continuous flow hysteroscopes. These contain integrated operating channels through which small diameter electrodes or morcellators can be passed to perform myomectomy [8,9]. The latter equipment is suitable for treating smaller submucous fibroids because they avoid the need for wide dilatation of the cervix such that hysteroscopic myomectomy may be feasible in the outpatient setting in some instances.
Hysteroscopic myomectomy using resectoscopes
Equipment & set-up
Resectoscopes consist of a rigid hysteroscope, a cutting loop, a working element which moves the cutting loop forward and back and inner and outer sheaths for continuous inflow and outflow of the distension media. The hysteroscope usually employs a 12° or 30° offset distal lens so that the cutting loop can be kept in view. The working element includes two rings, one of which is movable with the thumb to advance the cutting loop forward, and also a spring which moves the loop back slowly when the ring is released under control. Cutting loops can be monopolar or bipolar; the older systems used monopolar loops whereas the new generation resectoscopes are usually developed with bipolar systems. Resectoscope sheaths are commonly 8.7–9.0 mm (26–27 Fr) in outer diameter, but there are smaller resectoscopes in the market.
Hysteroscopic resection of fibroids requires a fluid medium to distend the uterine cavity, to provide a clear visibility by continuous irrigation and drainage and to allow the diathermy system to work. For monopolar systems the medium needs to be a nonconductive nonelectrolyte solution such as 1.5% glycine or 3% sorbitol. In monopolar systems the electric current flows from the cutting loop to the diathermy pad, using the patient as part of its circuit, before returning to the diathermy generator. Bipolar systems use electrolyte media such as normal saline or lactated Ringer's solution. In bipolar systems, the electric current flows between the active and return electrodes via the conductive distension medium and return to the diathermy generator, without using the patient as part of its circuit. Hence the bipolar systems avoid the risk of diathermy burns.
The distension fluid may enter into the patient's circulation via open blood vessels and myometrium which are cut during operative hysteroscopy or through fallopian tubes and peritoneal cavity. Excessive absorption of this fluid may cause fluid overload leading to cardiovascular, pulmonary and neurological complications. In particular nonelectrolyte solutions can cause hyponatremia which may lead to brain oedema. Electrolyte solutions which are used for bipolar surgery are less likely to cause electrolyte imbalance, but can still cause pulmonary edema and heart failure. For these reasons, it is essential to monitor the fluid balance throughout the procedure and when fluid deficit exceeds a certain limit the procedure should be terminated. In general for hypotonic nonelectrolyte solutions a maximum fluid deficit of 1 l is recommended and 1.5–2.5 l for isotonic electrolyte solutions [10]. Effective monitoring of the fluid balance requires constant calculation of fluid input and output which should include the drainage through the outflow of the resectoscope as well as the backflow through the cervix. Fluid draining through the cervix can be collected using pouches sealed against the perineum or placed under the patient.
Technique
Before resection of submucosal fibroids, the endocervical canal and uterine cavity should be assessed and the position of the tubal ostia should be determined to aid orientation. The number, location and type of submucosal fibroids should be evaluated. Resection of fibroids is achieved by positioning the extended cutting loop at the upper part of the fibroid and activating the diathermy while the electrode is retracted. The diathermy should never be activated while the cutting loop is extended to avoid uterine perforation. In addition to the retraction of the electrode, the resectoscope may also be retracted when larger fibroids are treated, to remove longer ‘chips’ of the fibroid. Resection of the overlying and surrounding endometrium should be kept to minimum in patients who plan to get pregnant in the future. Resection of the intramural portion of a type 1 and particularly type 2 submucosal fibroids is more challenging and requires more advanced skills in hysteroscopic surgery. Identifying the plane between the fibroid and myometrium is important and mechanical dissection of this plane can be achieved with the aid of distension fluid and inactivated cutting loop. Resection then proceeds while remaining within the pseudocapsule of the fibroid to minimize the risk of inadvertent uterine perforation. Another strategy to facilitate resection is to reduce or cease uterine distension, by turning the inflow off or removing the resectoscope. This can induce myometrial contractions which help push the intramural part of the fibroid into the cavity (Figures 1 & 2).
Figure 1.
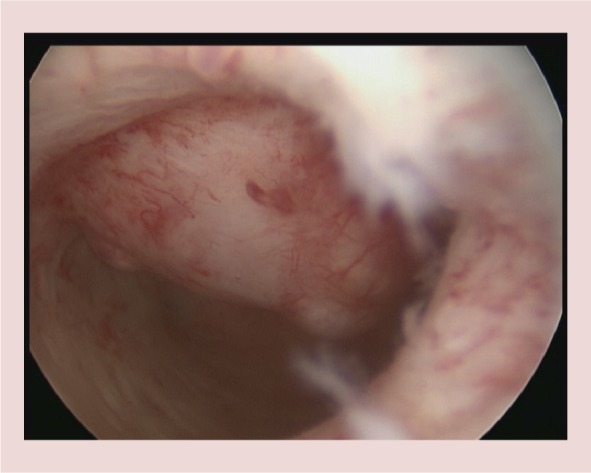
A type 2 anterior wall fibroid measuring 2.5 cm is seen, ∼1/3 of the fibroid is intracavitary.
Figure 2.
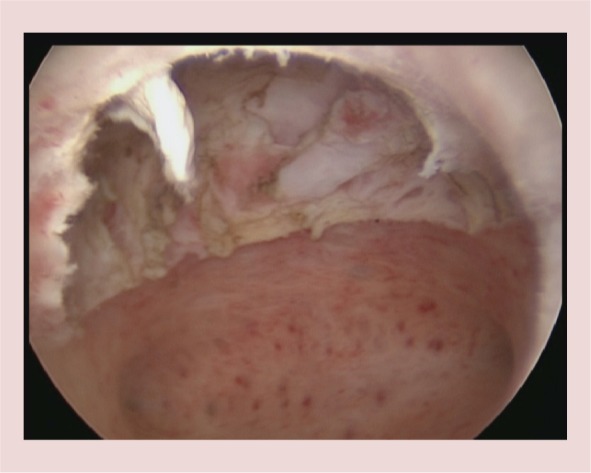
The fibroid seen in Figure 1 has been completely resected, including its intramural portion.
Outcome
It is usually possible to resect the majority of type 0 and type 1 submucosal fibroids completely as a one stage procedure. Larger type 2 fibroids may require a second procedure for complete resection. Some clinicians use medical treatment to reduce the size of the fibroid with gonadotrophin releasing hormone analogues (GnRHa). Pretreatment in this way may also reduce the risk of fluid overload by shortening the duration of the procedure and decreasing the size of blood vessels around the fibroid bed. Potential disadvantages include symptoms arising from induced oestrogen deficiency, difficult cervical dilatation and trouble identifying the plane between the fibroid and underlying myometrium. Some or all of these potential disadvantages may be overcome by the use of ulipristal acetate (UPA) for 3 months prior to hysteroscopic myomectomy [9,11–12]. Reported complete resection rates are as high as 96% for type 0 fibroids, 85.7% for type 1 and 70% for type 2 fibroids [6]. Long-term follow-up studies showed 15–20% of the patients required repeat surgery and less than 10% had a hysterectomy [13,14].
Complications
Complications of hysteroscopic myomectomy include infection, uterine perforation, cervical laceration, hemorrhage, fluid overload leading to pulmonary edema and electrolyte disturbances, air embolism and intrauterine adhesions [15]. Fluid overload is more likely with prolonged operations, larger fibroids and type II fibroids [16]. A recent working party of the American Association of Gynecologic Laparoscopists suggests discontinuing the procedure when the fluid deficit reaches 1 l with noncrystalloid solutions and up to 2.5 l ml with crystalloid solutions for healthy women [10]. For elderly women and others with comorbidity, including those with cardiovascular compromise, the American Association of Gynecologic Laparoscopists guidelines suggest a maximum deficit of 750 ml. Coagulation of overlying endometrial blood vessels may help minimize intraoperative blood loss. Where postoperative bleeding is encountered, a Foley's catheter with a 30 ml balloon can be passed into the uterine cavity to provide tamponade to exposed intrauterine bleeding vessels.
A possible long-term complication of hysteroscopic myomectomy is intrauterine adhesion formation. The risk of this complication was reported to be ∼10% [17] but more recent data suggest this may be as high as 31% following resection of single fibroids and up to 45% for multiple fibroids when using monopolar resectoscopes [18]. A rate of 7.5% has been reported with bipolar resectoscopes [19], but more data are needed to confirm whether bipolar systems are associated with a lower incidence of adhesion formation. This problem is probably more likely when two fibroids on opposite walls are resected at the same time or where large raw areas on the lateral walls or fundus are left which extend on to both anterior and posterior uterine walls after resection. To reduce the risk of adhesion formation some hysteroscopists insert an intrauterine contraceptive device, balloon intrauterine stents or hyalobarrier gel into the uterine cavity at the end of the procedure, however, there are no controlled trials to demonstrate a benefit.
Hysteroscopic myomectomy using mechanical, electrosurgical or morcellation instruments
Small, submucosal fibroids may be removed using smaller diameter hysteroscopy systems, with an outer sheath diameter ≤6.5 mm. These continuous flow systems incorporate an operating channel which can accommodate 5–7 Fr instruments such as bipolar electrodes, scissors and grasping forceps [9]. The fibroid is usually separated from its bed by using a bipolar electrode or scissors. A major challenge with this approach is retrieval of the fibroid. This can be achieved by slicing the fibroid into smaller pieces using the electrode. Alternatively the fibroid can be left in the uterine cavity and is usually liquefied and expelled with uterine contractions over the following few weeks.
The recent development of hysteroscopic morcellators has added a new technique to facilitate removal of both small and larger sized submucosal fibroids. Hysteroscopic morcellation uses specially designed 0° hysteroscopes incorporating an offset proximal eyepiece to allow passage of a rigid, disposable, mechanical morcellating instrument down a straight working channel. The morcellator is connected to a bespoke electrical generator and standard suction apparatus. The submucosal fibroid is then removed by the morcellator, which both cuts and aspirates small pieces off the fibroid at the same time [8]. Avoidance of fibroid ‘chips’ may enhance intraoperative visualization and the simultaneous removal of fibroid tissue avoids the need for blind instrumentation to retrieve the specimen from the uterine cavity. To date, data comparing the safety, feasibility and efficacy of hysteroscopic morcellation with conventional electrosurgery for fibroids are lacking.
Open myomectomy
Myomectomy at laparotomy or ‘open myomectomy’ has been the traditional form of conservative surgery for large uterine fibroids causing HMB and indeed was the only conservative surgical option for the treatment of fibroids until the development of laparoscopic and hysteroscopic techniques. Even today, it is a frequently performed operation in the presence of fibroids which are not suitable for endoscopic approaches. When expertise for LM is available, open myomectomy is usually reserved for women who have multiple or extremely large fibroids.
Pretreatment preparation
Preoperative assessment and preparation should include a detailed imaging for fibroid mapping and checking hemoglobin level. Many women with symptomatic fibroids have HMB and may be anemic. Preoperative anemia is known to increase morbidity of surgery and should be corrected [20]. Strategies to correct anemia include use of oral or intravenous iron and inducing amenorrhea by using GnRHa [21] or UPA [11–12,21], medications that also have the advantage of reducing the size of the fibroids preoperatively. Although GnRHa's have been reported to decrease intraoperative blood loss, probably by reducing uterine vascularity [21], they have the potential disadvantage of obscuring tissue planes between the fibroids and myometrium. This sometimes makes enucleation of fibroids more difficult to achieve. A further problem is missing small fibroids which become too small to palpate during surgery and this can potentially increase recurrence rates due to regrowth of these fibroids. The impact of the selective progesterone receptor modulator UPA on intraoperative blood loss and delineation of tissue planes is not known yet. In cases of mild-to-moderate HMB, additional medical treatment options such as tranexamic acid and progestins may also be helpful in correcting anemia.
Preoperative fibroid mapping is helpful in planning surgery and locating the fibroids during the operation. When preoperative imaging shows submucosal fibroids or if the uterine cavity is difficult to assess with imaging, a hysteroscopy should be performed at the beginning of procedure so that submucosal fibroids are not left behind. These fibroids are naturally located deep in the uterus and may be difficult to palpate during surgery. Sometimes, submucosal fibroids are easier to resect hysteroscopically, instead of trying to locate and remove them during the laparotomy stage of surgery.
Technique
Abdominal entry is usually via a low transverse incision even for large uteri, but when the uterus is very large, such as those which reach the epigastrium, or access is anticipated to be restricted, for example, due to obesity or pelvic adhesions, a midline incision may be more sensible. After entering the abdomen, the pelvic and abdominal structures should be assessed and the location of significant fibroids determined. The ovaries and fallopian tubes should be inspected, although they may only be fully visible later during the course of surgery, after removal of some fibroids.
Interventions to reduce blood loss during myomectomy include intramyometrial injection of vasopressin, bupivacaine with adrenaline, gelatine-fibrin matrix, intravenous tranexamic acid and the use of pericervical tourniquets [22,23]. We frequently use diluted vasopressin (20 units in 20 ml of normal saline) which is injected into both broad ligaments and myometrium overlying the fibroids. This may cause bradycardia and rarely cardiovascular collapse [24] and, for this reason, its use is not permitted in some countries. Cell-saver technology allows the collection and filtering of blood lost during surgery and this can be given back to the patient intravenously to reduce the need for blood transfusion and its associated risks.
The choice of uterine incisions depends on the location of fibroids. Some authors recommend transverse incisions to avoid cutting through arcuate vessels thereby reducing blood loss and allowing for better myometrial healing [25]. However, the uterine vasculature often becomes very distorted in the presence of multiple fibroids [26] such that a transverse incision is not considered by other authors to be the best choice. In general, uterine incision sites are chosen to minimize the final number of incisions through which the maximum number of fibroids are removed. Care should be taken to avoid the fallopian tubes, uterine and ovarian vessels and the bladder. Fibroids are enucleated by dissecting the fibroid–myometrium plane and applying traction and countertraction on the fibroid and myometrium. Fibroids do not have a single vascular pedicle and the surrounding pseudocapsule houses the ‘vascular layer’. Hence remaining within the pseudocapsule may reduce the blood loss during enucleation. Smaller fibroids are identified by meticulous palpation of the entire uterus/myometrium and removed using an appropriate incision to reduce the risk of ‘residual fibroids’. ‘Myomectomy screws’ and specially designed clamps/forceps are useful for traction and enucleation. Broad ligament and cervical fibroids may be in close proximity to the ureters or may have altered their course. For this reason, the ureter should be carefully identified and dissected laterally away from the fibroid to be removed.
Targeted use of diathermy for hemostasis may be used, but excessive application of diathermy should be avoided for better healing of the myometrium. Hemostasis is usually achieved by effective closure of the myometrial defect. Closure should ensure obliteration of all potential ‘dead space’ in the fibroid bed, but passing sutures through the endometrial cavity should be avoided to reduce the risk of intrauterine adhesions. The myometrium may need to be repaired in multiple layers, the number depending on the depth of incision. The serosa is then closed to achieve good approximation of the edges, covering exposed myometrium and giving additional hemostasis. These measures may potentially reduce the adhesion formation around the uterus postoperatively. After removal of all fibroids and closure of incisions, a peritoneal lavage is carried out to remove any remaining blood which may precipitate adhesion formation. Additional adhesion barriers such as oxidized regenerated cellulose (Interceed) or sodium hyaluronate and carboxymethylcellulose combination (Seprafilm) may be used at the end of the procedure [27].
Outcome
Compared with abdominal hysterectomy, open myomectomy appears to be associated with similar overall morbidity, but a lower prevalence of hemorrhage. However, open myomectomy usually takes longer compared with hysterectomy [28]. Open myomectomy also carries the risk of fibroid recurrence as well as persistent or recurrent HMB. It is estimated that the risk of recurrent fibroids is between 4–30% and 10% of women require further intervention after myomectomy [29]. A cohort study compared open myomectomy with uterine artery embolization (UAE) and found that women were significantly less likely to require further invasive therapy after myomectomy compared with UAE (3 vs 29%) after 3–5-year follow-up [30]. Symptomatic improvement rates were similar in women who did not require further intervention; 90% after myomectomy and 92% after UAE. A randomized controlled trial (the FEMME study) comparing UAE with myomectomy is currently underway.
Complications
Intraoperative complications include bleeding, transfusion, intra-abdominal damage to visceral organs or structures and hysterectomy. The latter is rare and is more likely to occur in the presence of multiple large fibroids or in cases of repeat myomectomy [31]. Febrile morbidity is quite common after myomectomy with high temperatures encountered on the first 1 or 2 days after myomectomy. This is usually due to pyrogenic substances being released from the myometrium and does not require antibiotic treatment. However, the possibility of infection should be considered, particularly when the temperature remains high beyond the second day. Other postoperative risks include pelvic abscess formation, development of a paralytic ileus, bowel obstruction and thrombosis. Adhesion formation in the long term is a common occurrence after myomectomy.
Laparoscopic myomectomy
Since its first description in 1977, LM has now become a popular alternative to open or abdominal myomectomy and is performed by a large number of clinicians worldwide. It however remains a challenging procedure and requires advanced laparoscopic surgery skills.
While there is still some scepticism over its place in clinical use amongst certain quarters, there are a significant number of publications, including randomized controlled trials comparing LM to open myomectomy, which demonstrate feasibility, safety and superiority of LM [32,33]. These studies show that LM is associated with reduced blood loss, a lower drop in hemoglobin, less postoperative pain, shorter hospital stay and recovery period and a lower risk of complications [33]. Although the likelihood of missing small fibroids may be higher due to the lack of tactile sensation during laparoscopy, this does not seem to be of any clinical consequence [34]. Thus, it appears that the laparoscopic approach to myomectomy is a better choice than the open approach in appropriately selected patients.
Preoperative patient selection
The number and size of fibroids that can be removed laparoscopically depend on the surgeon's experience and expertise. Preoperative fibroid mapping prior to LM becomes a more important step to determine the number, size and location of fibroids, compared with open myomectomy. This is an essential requirement as there is no possibility of palpating the uterus intraoperatively. Mapping not only helps determine the feasibility of LM versus an open approach, but also guides in planning the uterine incision(s) and reduces the risk of residual fibroids. In experienced hands, transvaginal ultrasound examination using high-quality machines is usually adequate for evaluating fibroids preoperatively. When ultrasound findings are equivocal, MRI may help to confirm the diagnosis and to differentiate fibroids from adenomyosis. This is an important distinction to be made because adenomyosis presents an added challenge during laparoscopy owing to the lack of a clearly defined dissection plane.
Saline sonohysterography and hysteroscopy may also assist in identifying submucosal fibroids and may be particularly useful when there is significant uterine distortion from several other fibroids. The final decision as to whether LM is feasible may only be possible at the time of surgery. It is therefore imperative that the woman is warned of the possibility of an open approach and that this is reflected in the preoperative consultation and consent.
Pretreatment preparation
Preoperative reduction in the size of fibroids and the uterus using GnRHa [21] may be particularly beneficial for LM by creating more space to work around the uterus with the relatively small laparoscopic instruments and help minimize blood loss. A smaller fibroid is easier to handle laparoscopically and reduces morcellation time. The use of GnRHa may, however, obscure the plane between the fibroid and myometrium and this may make fibroid enucleation more difficult. In addition, smaller fibroids may be easier to miss during the operation, increasing the risk of fibroid recurrence postoperatively. UPA can also be used to shrink the fibroids preoperatively, without the menopausal side effects. Its impact on the tissue planes is not known. Both GnRHa and UPA are helpful in correcting anemia preoperatively.
Technique
At surgery, port placement should be planned to create enough space between the laparoscope and the uterus. An umbilical port may be appropriate for the laparoscope when the uterine size is up to 12–14 weeks gestation size. However, using an epigastric port may be more useful for larger uteri. The other ports should be positioned according to the location of fibroid(s), type of incision(s) that would be made and the suturing technique of the surgeon.
Prior to incising the serosa, a dilute solution of synthetic vasopressin is injected into the myometrium, and possibly into the broad ligaments (Figures 3 & 4). Vasopressin causes vasoconstriction and reduces the blood loss significantly [22,33]. It may cause untoward cardiovascular complications, making it important to forewarn the anesthetist prior to its instillation [24]. Due to this complication, the use of a synthetic vasopressin for myomectomies is not permitted in some countries.
Figure 3.
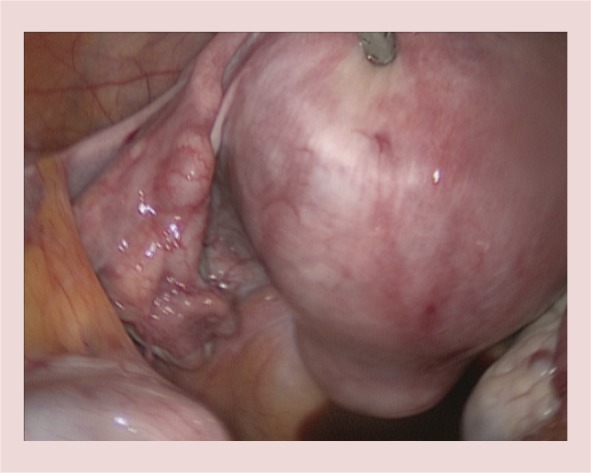
An enlarged uterus due to a large deep intramural fibroid in the left posterolateral wall. An additional small subserosal posterior wall fibroid is also visible.
Figure 4.
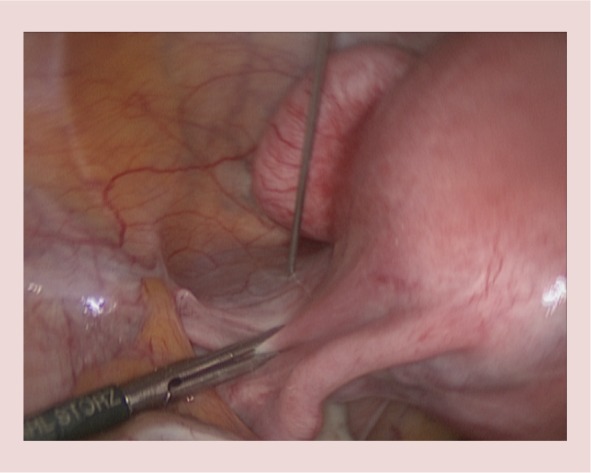
Diluted vasopressin is injected into the left broad ligament. A small pedunculated anterior wall fibroid is also visible.
The location and direction of uterine incision(s) will depend on the location of fibroid(s) and the surgeon's preference; those who use ipsilateral suturing may prefer transverse incisions while the contralateral operators would find longitudinal or oblique incisions easier to suture (Figure 5). The location of the Fallopian tubes and ovarian ligaments in relation to the fibroids should also be taken into account [35].
Figure 5.
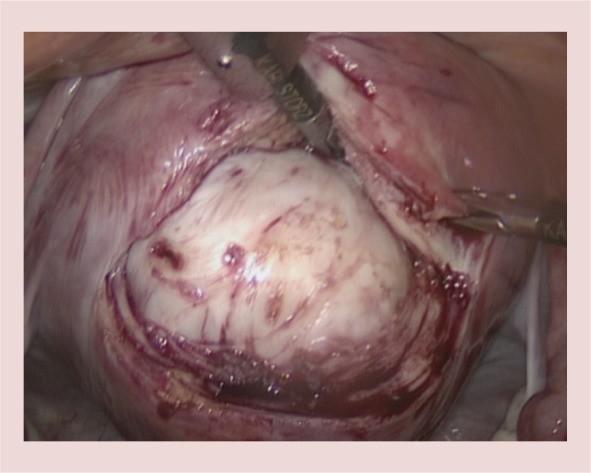
A posterior wall transverse incision is made over the fibroid to expose the fibroid and the plane between the fibroid and myometrium.
Energy modalities including the ultrasonic scalpel, monopolar diathermy attached to a hook or scissors and laser may be used to incise the overlying serosa and myometrium. It is helpful to cut deep into the fibroid so that the actual plane between the fibroid and the myometrium is easier to identify (Figure 5). Once the plane is determined, the incision is then enlarged to the required size and a combination of instruments such as myomectomy screws, claw forceps and tooth graspers is used to enucleate the fibroids. Traction and counter traction using these instruments, as well as the counter traction applied by a second assistant via a volsellum on the cervix, together with division of the myometrial and fibrous bands, utilizing the energy source used for the initial incision, would eventually result in enucleation of the fibroid (Figure 6). Care should be taken to avoid pushing laparoscopic instruments into the uterine cavity if possible, and similarly, the second assistant should refrain from using excessive force on the uterine manipulator to prevent uterine perforation.
Figure 6.
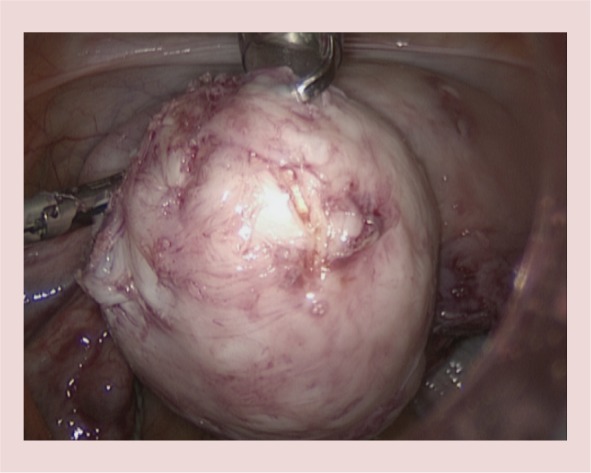
Intramural fibroid after enucleation.
Once removed, the smaller fibroids should be placed in the pouch of Douglas to avoid difficulty in locating them later in the upper abdomen among the loops of bowel and the omentum. Larger fibroids may be placed in the upper abdomen as they would be difficult to fit into the pouch of Douglas and would be easy to locate. Hemostasis is usually achieved by targeted electrocoagulation and suturing. Indiscriminate and excessive diathermy should be avoided as this is probably one of the main factors contributing to uterine rupture in future pregnancies, along with inadequate suturing [36].
The repair of the myometrial defect should be similar to the repair at open myomectomy. If the uterine cavity is breached, separate repair of the endometrium may be beneficial in preventing the rare future cases of myometrial pregnancy. The myometrium should be repaired using large curved needles to avoid leaving significant ‘dead-spaces’ within the defect. The suture material should either be delayed absorbable conventional products or so-called ‘barbed sutures’ which reduce the operating time as they do not require knot-tying. The serosa should be closed with similar materials, however, if barbed sutures are used it is probably useful to cover the incision with an antiadhesion method to reduce risk of bowel adherence which may result in postoperative mechanical ileus (Figures 7 & 8).
Figure 7.
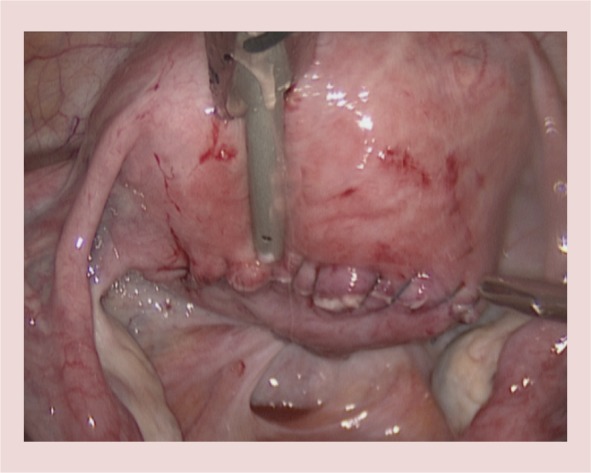
Closed serosal incision using barbed suture.
Figure 8.
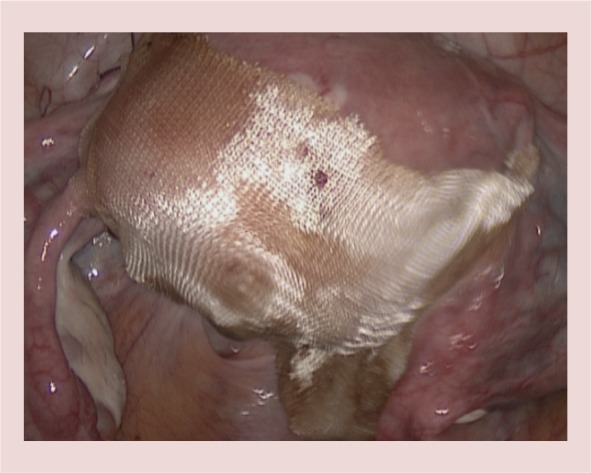
The incision is covered with a barrier antiadhesion agent.
The fibroid is usually removed using a single-use or reusable electromechanical morcellator. It is important to have a complete view of the morcellator when it is activated, taking extreme care that its tip is free of the abdominal wall, bowel and any other adjacent structure. After removing the fibroid, complete inspection of the abdominopelvic cavity is necessary to remove any fibroid fragments, as morcellated fragments of fibroid can cause disseminated peritoneal leiomyomatosis [37].
After thorough irrigation and confirmation of hemostasis, an antiadhesion method may be applied onto the incision(s). At the end of the procedure it is important to repair the rectus sheath defects of larger ports, particularly the one used for the morcellator to avoid later herniation.
Complications
Complications of LM are similar to open myomectomy, but LM also carries the inherent risks of laparoscopic surgery such as visceral injury and conversion to laparotomy. The overall complication rate was reported to be ∼11% and major complication rate was 2% in one of the largest case series of LM [33].
The risk factors associated with higher risk of complications were fibroids larger than 5 cm, presence of three or more fibroids, nonuse of vasopressin and preoperative anemia.
In order to reduce complications the surgeon should ensure appropriate patient selection. This requires detailed preoperative mapping of fibroids to reduce the likelihood of embarking on operations which may be too challenging for the level of experience of the surgeon. Surgeons should follow the principles of appropriate training before attempting the procedure and receive mentoring during the initial stages of their learning curve. Preoperatively consideration should be given to the preoperative use of GnRHa or UPA to reduce the size of larger fibroids and correct anemia. The benefits should be weighed against the cost, side effects, possibilities of difficult fibroid enucleation and increased risk of recurrence in the presence of multiple small fibroids. Intraoperatively, the use of vasoconstrictive agents should be considered because they reduce excessive blood loss. Hemostasis should be targeted and excessive and indiscriminate use of diathermy should be avoided. A good repair technique which resembles the reconstitution of myometrium at open surgery would also help with hemostasis and reduce the possibility of uterine rupture in future pregnancies.
Morcellation & risk of undiagnosed leiomyosarcoma
Risk of undiagnosed malignancy is uterine fibroids is thought to be extremely low, hence expectant management, nonsurgical treatment and uterine preserving surgery are all acceptable treatment options for HMB secondary to uterine fibroids. The US FDA released a statement on 17 April 2014 discouraging use of power morcellators during LM or hysterectomy. This was based on an assessment that the prevalence of unsuspected uterine sarcoma and leiomyosarcoma (LMS) was 2.8 and 2 per 1000 persons, respectively (1:352 for uterine sarcoma and 1:498 for LMS) [38]. However, professional organisations around the world released reports and statements disagreeing with the FDA statement and recommended that all current tissue extraction methods should be available, as there was no single method that can protect all patients [39–41]. These reports concluded that that exact prevalence of LMS was unknown but that the FDA estimate was probably an overstatement. The current consensus is that patients should be informed of risk of undiagnosed LMS prior to LM procedures and that further research should be conducted on the prevalence of sarcomas and safer methods of morcellation.
Conclusion
Removal of fibroids is beneficial in the treatment of HMB associated with fibroids for women who would like to preserve their uterus and fertility. Endoscopic approaches are the preferred methods of fibroid removal when appropriate. In the presence of submucosal fibroids, hysteroscopic resection is a simple, safe and effective treatment for HMB and reduces the need for more major surgery, such as hysterectomy. When abdominal myomectomy is required, LM is the preferred choice in selected cases due to its advantages over open myomectomy.
Future perspective
Currently surgery for fibroids is an important component of treatment options for HMB. New developments in both hysteroscopic and laparoscopic surgery are expected and these are likely to make these procedures easier and faster, and increase their efficacy. Some examples of these include further developments in hysteroscopic morcellators and laparoscopic suturing and tissue retrieval systems. Surgical treatment of fibroids may, however, be replaced altogether in the future with medical treatment alternatives.
Executive summary
Removal of fibroids is beneficial in treatment of heavy menstrual bleeding for women.
Hysteroscopic resection of fibroids is a minimally invasive, safe and effective treatment for submucosal fibroids.
Laparoscopic myomectomy is the preferred choice in selected cases when abdominal removal of fibroids is required.
Open myomectomy is a well established and effective treatment for heavy menstrual bleeding secondary to fibroids, when laparoscopic myomectomy is not possible or available.
Financial & competing interests disclosure
E Saridogan received consultancy fees and honoraria from Ethicon and G Richter for provision of training to healthcare professionals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am. J. Obstet. Gynecol. 188(1), 100–107 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Wechter ME, Stewart EA, Myers ER, Kho RM, Wu JM. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am. J. Obstet. Gynecol. 205(5), 492–495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Dowd MJ, Philipp EE. The History Of Obstetrics And Gynecology. Parthenon, New York, NY, USA, 411 (1994). [Google Scholar]
- 4.Neuwirth RS, Amin HK. Excision of submucus fibroids with hysteroscopic control. Am. J. Obstet. Gynecol. 126(1), 95–99 (1976). [DOI] [PubMed] [Google Scholar]
- 5.Semm K. Pelviscopic surgery in gynecology. Geburtshilfe Frauenheilkd 37(11), 909–920 (1977). [PubMed] [Google Scholar]
- 6.Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet. Gynecol. 82(5), 736–740 (1993). [PubMed] [Google Scholar]
- 7.Munro MG, Critchley HO, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynaecol. Obstet. 113(1), 3–13 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J. Minim. Invasive Gynecol. 12(1), 62–66 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Bettocchi S, Ceci O, Di VR, et al. Advanced operative office hysteroscopy without anaesthesia: analysis of 501 cases treated with a 5 Fr. bipolar electrode. Hum. Reprod. 17(9), 2435–2438 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Munro MG, Storz K, Abbott JA, et al. AAGL Practice Report: Practice Guidelines for the Management of Hysteroscopic Distending Media: (Replaces Hysteroscopic Fluid Monitoring Guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167–168.). J. Minim. Invasive Gynecol. 20(2), 137–148 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 366(5), 409–420 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N. Engl. J. Med. 366(5), 421–432 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Hart R, Molnar BG, Magos A. Long term follow up of hysteroscopic myomectomy assessed by survival analysis. Br. J. Obstet. Gynaecol. 106(7), 700–705 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet. Gynecol. 93(5 Pt 1), 743–748 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr. Opin. Obstet. Gynecol. 25(4), 332–338 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil. Steril. 68(5), 881–886 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Hallez JP. Single-stage total hysteroscopic myomectomies: indications, techniques, and results. Fertil. Steril. 63(4), 703–708 (1995). [PubMed] [Google Scholar]
- 18.Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J. Am. Assoc. Gynecol. Laparosc. 7(3), 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Touboul C, Fernandez H, Deffieux X, Berry R, Frydman R, Gervaise A. Uterine synechiae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertil. Steril. 92(5), 1690–1693 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 378(9800), 1396–1407 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst. Rev. (2), CD000547, (2001). [DOI] [PubMed] [Google Scholar]
- 22.Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst. Rev. (11), CD005355, (2011). [DOI] [PubMed] [Google Scholar]
- 23.Taylor A, Magos A. Reducing blood loss at open myomectomy using triple tourniquet: a randomised controlled trial. BJOG 113(5), 618–619 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Deschamps A, Krishnamurthy S. Absence of pulse and blood pressure following vasopressin injection for myomectomy. Can. J. Anaesth. 52(5), 552–553 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Koh C, Janik G. Laparoscopic myomectomy: the current status. Curr. Opin. Obstet. Gynecol. 15(4), 295–301 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Falcone T, Parker WH. Surgical management of leiomyomas for fertility or uterine preservation. Obstet. Gynecol. 121(4), 856–868 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Ahmad G, Duffy JM, Farquhar C, et al. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst. Rev. (2), CD000475 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am. J. Obstet. Gynecol. 183(6), 1448–1455 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Buttram VC, Jr., Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil. Steril. 36(4), 433–445 (1981). [DOI] [PubMed] [Google Scholar]
- 30.Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstet. Gynecol. 100(5 Pt 1), 864–868 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Pundir J, Krishnan N, Siozos A, et al. Peri-operative morbidity associated with abdominal myomectomy for very large fibroid uteri. Eur. J. Obstet. Gynecol. Reprod. Biol. 167(2), 219–224 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy – a meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 145(1), 14–21 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J. Minim. Invasive Gynecol. 14(4), 453–462 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long-term results of laparoscopic myomectomy: recurrence rate in comparison with abdominal myomectomy. Hum. Reprod. 16(4), 770–774 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Saridogan E, Cutner A. Endoscopic management of uterine fibroids. Hum. Fertil. (Camb.) 9(4), 201–208 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Seracchioli R, Manuzzi L, Vianello F, et al. Obstetric and delivery outcome of pregnancies achieved after laparoscopic myomectomy. Fertil. Steril. 86(1), 159–165 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J. Minim. Invasive Gynecol. 14(6), 770–775 (2007). [DOI] [PubMed] [Google Scholar]
- 38.US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. www.fda.gov
- 39.American Association of Gynecologic Laparoscopists. Morcellation during uterine tissue extraction. www.aagl.org
- 40.British Society for Gynaecological Endoscopy. BSGE Statement on Power Morcellation. http://bsge.org.uk/newsFull.php?id=75&start=0
- 41.Brölmann H, Tanos V, Grimbizis G, et al. Options on fibroid morcellation: a literature review. Gynecol. Surg. 12(1), 3–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]



