Abstract
Older women experience a large share of breast cancer incidence and death. With the projected rise in the number of older cancer patients, adjuvant chemo-, radiation and endocrine therapy management will become a key component of breast cancer treatment in older women. Many factors influence adjuvant treatment decisions including patient preferences, life expectancy and tumor biology. Geriatric assessment predicts important outcomes, identifies key deficits, and can aid in the decision making process. This review utilizes clinical vignettes to illustrate core principles in adjuvant management of breast cancer in older women and suggests an approach incorporating life expectancy and geriatric assessment.
Keywords: adjuvant chemotherapy, breast cancer, elderly, endocrine therapy, geriatric assessment, geriatric oncology, radiation
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Future Medicine Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMAPRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at www.medscape.org/journal/whe; (4) view/print certificate.
RELEASE DATE: 14 January 2016; EXPIRATION DATE: 14 January 2017
LEARNING OBJECTIVES
Upon completion of this activity, participants will be able to:
Describe how patient goals, beliefs, preferences, and quality-of-life considerations affect adjuvant treatment options for older women with breast cancer
Evaluate the fitness and suitability for adjuvant treatment of the older patient with breast cancer, as defined by life expectancy, geriatric assessment, and biologic, nodal, and tumor differences
Determine the role of adjuvant treatment options, including chemotherapy, endocrine therapy, and radiation therapy, and tools to assist in decision making
Two 70-year-old women present to discuss adjuvant treatment for breast cancer.
Mrs A has a history of osteoarthritis. She self-detected a right breast lump during personal care. She lives with her ailing sister and assists with her care. She reports walking 2 miles per day and has no difficulty pulling heavy objects. Her BMI is 28. She has not been hospitalized in the past year and rates her general health as very good.
Mrs B has a history of Alzheimer's dementia. She lives with her daughter who manages her medications and finances. Her daughter noticed a right breast lump while assisting her mother with personal care. Mrs B ambulates with a walker but is unable to walk more than ¼ mile and has difficulty pulling heavy objects. Her BMI is 19. She has been admitted to the hospital twice in the past year, most recently for a fall and rates her general health as fair.
Both women are lifelong nonsmokers and except for the palpable breast lumps have unremarkable physical examination findings. Imaging confirms the right breast masses. Core needle biopsy reveals grade 2, invasive ductal carcinoma. Immunohistochemical stains show 99% of tumor cells expressing estrogen (ER) and progesterone (PR) receptors but <10% (score 0) stains for the human epidermal growth factor receptor 2 (HER-2/neu). A fluorescent in situ hybridization probe confirms that HER-2/neu is not overexpressed. Both women undergo lumpectomy and sentinel lymph node evaluation. Pathologic specimens reveal a 3.1 cm, grade 2 invasive ductal cancer with no sentinel lymph nodes involved by cancer, that is, stage IIA (pT2, N0).
Background
It is estimated that in 2015 breast cancer will be the most frequently diagnosed malignancy among women in the USA, representing approximately 14% (231,840 people) of all new cancer cases and will be the third leading cause of mortality among women, accounting for an estimated 40,290 deaths [1]. The incidence of breast cancer rises dramatically with age, which is the single most important risk factor for developing cancer. As a result, a large proportion (41%) of all new breast cancer diagnoses and the majority of breast cancer deaths (58%) occur in women 65 years and older [1]. In fact, the median age at breast cancer diagnosis and death in the USA is 61 and 68 years, respectively [1]. These statistics are expected to change dramatically in the years ahead as a result of increasing life expectancy and an overall aging of the US population [2]. By 2030, the proportion of older adults (65 years and older) is projected to almost double from 14 to 20% of the total US population – one in every five persons or 70 million people [3]. Providing high level care for an increasing number of older breast cancer patients is of critical importance and will require health care providers and support staff who are trained in evaluating and treating this patient population.
Most breast cancers in older women are identified at an early, treatable stage [4] and the majority of women diagnosed with early stage breast cancer enjoy prolonged disease free survival [5]. Yet, recurrent disease remains a persistent and vexing problem [6]. Those with loco-regional recurrences will require additional surgery and other therapies while those who develop distant metastases are considered incurable with current therapies and most will eventually succumb to their illness. Thus, adjuvant therapies defined as treatments – chemotherapy, radiation or endocrine therapy – usually offered after primary surgery in an effort to decrease the risk of breast cancer recurrence and improve the odds of long-term disease-free survival have become key a component of breast cancer management.
Older breast cancer patients have not enjoyed as much improvement in survival from recent advances in adjuvant treatment as their younger counterparts [7]. In part, this disparity reflects uncertainties about the value of treatments, such as adjuvant chemotherapy, for older women, and its frequent lack of use even when appropriate. Deciding which patients are optimal candidates for adjuvant treatment in light of the potent toxicities as well as potential benefits of adjuvant treatment is challenging [8]. Older women with unfavorable breast cancer subtypes are reasonable candidates for chemotherapy but many will be ineligible because of significant comorbidities. The aging process is characterized by a variable decline in organ function [9,10] and the accumulation of comorbid medical conditions [11] that can vary greatly between older people of the same age. This inherent heterogeneity can result in two similarly aged individuals – like the ones in the vignettes above, having vastly different general health, physical, cognitive and functional status. In addition, older cancer patients may have different and varied values, goals and preferences with respect to the trade-off between longevity and quality of life [12]. The purpose of this review is to illustrate key concepts and principles in the decision process and management of adjuvant therapies for older women with early stage breast cancer. Our aim is to discuss adjuvant treatment options for older women with breast cancer focusing on three areas: patient goals, beliefs, preferences and quality of life considerations; evaluation of the older patient's fitness and suitability as defined by life expectancy, geriatric assessment, biologic, nodal and tumor differences; and to define the role of adjuvant treatment options, including chemotherapy, endocrine and radiation therapy and tools to assist in decision making.
Primary endocrine therapy
Although the main focus of this paper is adjuvant therapies (i.e., after surgery), declining performance status and organ function as well as increasing comorbidity renders many older women with localized breast cancer suboptimal candidates for surgery. In these patients a discussion of primary endocrine therapy is warranted. Treatment with endocrine therapy as an initial approach, without removal of the primary tumor (i.e., primary endocrine therapy), has been evaluated in women who are not surgical candidates or decline operative intervention and is a reasonable option for such patients [13]. The role of primary endocrine therapy for women who are surgical candidates remains controversial with most studies showing inferior local control but no convincingly different overall survival [14].
A 2007 Cochrane review analyzed data from clinical trials enrolling women 70 years and older with operable breast cancer who were fit for surgery comparing mastectomy or wide local excision with or without tamoxifen to tamoxifen alone [15]. This systematic review found no difference in survival when surgery either with (n = 1076; hazard ratio [HR]: 0.86; 95% CI: 0.73–1.00; p = 0.06) or without (n = 495; HR: 0.98; 95% CI: 0.74–1.30; p = 0.9) adjuvant tamoxifen was compared with tamoxifen alone. However, compared with tamoxifen alone there was a significant difference in progression free survival favoring surgery either with (HR: 0.65 [n = 1076; 95% CI: 0.53–0.81; p = 0.0001]) or without (HR: 0.55; 95% CI: 0.39–0.77; p = 0.0006) tamoxifen therapy. It is worth noting that this review included clinical trials of women with unselected hormone receptor status. Tamoxifen is not effective or indicated in HR receptor negative breast cancer and this may have resulted in the underperformance of the primary endocrine therapy arm.
A 2014 meta-analysis documented similar findings with data from nonrandomized studies suggesting a survival advantage favoring surgery possibly resulting from selection of frail or unfit patients for treatment in the primary endocrine therapy group [16]. Recent long term updates of the Phase III (GRETA) trial showed inferior local control rates with tamoxifen alone versus surgery plus adjuvant tamoxifen but reported more favorable distant metastases free survival in women who took tamoxifen alone (48.8 vs 37.9 months; p = 0.009) [17]. The study found no difference between groups in the rate of distant metastases, disease-free survival, breast cancer and overall survival. Taken together these results suggest that primary endocrine therapy is a reasonable option for fit older women with early breast cancer who wish to delay or decline initial surgery. More recently trials of aromatase inhibitors as primary endocrine therapy have found them to be effective in this setting and may be a better initial option for many older patients.
Treatment goals, patient beliefs & preferences
Many factors influence the decision to pursue or forego adjuvant therapy in older cancer patients. In this review, we present the complex trade-off between benefits and risks of adjuvant therapies to be conveyed to and weighed by the individual patient in light of their own particular treatment goals, beliefs about treatment risks and benefits, and preferences regarding near- and long-term outcomes. Although older patients have been shown to be as agreeable to undergo chemotherapy as younger patients, older patients often differ in their willingness to trade increased survival potential for decreased quality of life in survivorship [12,18]. Older patients generally value life without excessive fatigue, heightened anxiety and/or depression over an extended lifespan complicated by these issues [19]. While most older patients are willing to undergo a high treatment burden for improvements in survival, the vast majority would decline therapies that resulted in severe functional (74%) or cognitive (89%) impairments [20]. Thus, regardless of the estimated benefits and risks of a particular treatment, older patients may have different personal preferences when making treatment decisions. Eliciting and respecting patient goals, beliefs and preferences with regard to adjuvant treatment is a critical step in the decision-making process.
Evaluation of older women with breast cancer
In this section, we describe three major considerations in the determination of treatment options for older breast cancer patients: findings from a geriatric assessment, calculation of average life expectancy and specific biologic, tumor and nodal characteristics.
Geriatric assessment
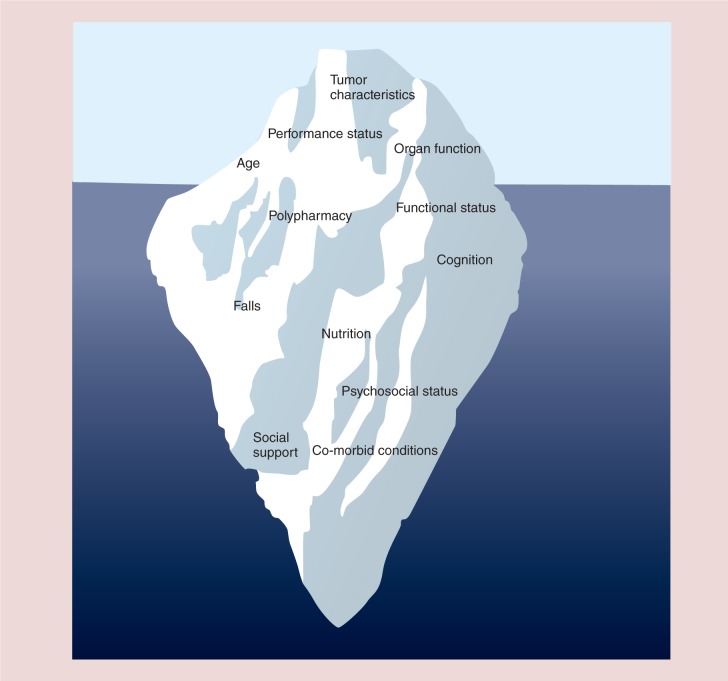
In addition to a standard medical history and physical examination and assessment of tumor characteristics and organ function, oncology providers have traditionally considered chronological age and basic measures of functional status such as the Karnofsky (KPS) [21] or the Eastern Cooperative Oncology Group [22] performance status as the major factors for making adjuvant chemotherapy decisions. For older patients, however, the heterogeneous process of aging can limit the utility of chronologic age and performance status measures that have not been specifically validated in older adults and are not sufficiently sensitive to identify important deficits in function, cognition, psychosocial and other geriatric domains [23]. Figure 1 introduces the concept of the geriatric oncology iceberg illustrating that many factors which may influence adjuvant treatment decisions and impact outcomes in older women with breast cancer are not always evident when considering age, traditional performance status measures, organ function and tumor characteristics. Multidimensional, interdisciplinary geriatric assessment (GA) offers a more thorough approach to evaluating the fitness of older cancer patients for various adjuvant therapies. In fact, the International Society of Geriatric Oncology recently recommended use of GA for all older adults with cancer to identify patient deficits, and better understand a patient's functional age. Geriatric assessment allows for characterization of a patient's function into three groups; fit, vulnerable and frail to assist with clinical decision making [24].
Figure 1.
The Geriatric Oncology Iceberg: Unrecognized deficits in older adults with cancer.
Most GAs include domains of functional status, comorbidity, medication use, nutritional status, social status, cognition and psychological concerns using a variety of reliable and valid measurements. Specifically in older women with breast cancer, the GA has been used to determine frailty, estimate survival [25,26] and predict chemotherapy toxicity [27–30]. The GA can also detect issues that may affect cancer treatment tolerability such as cognitive impairment, depression, nutritional deficits and identification of falls [31,32]. In a recent study of 796 older cancer patients with ‘normal’ interviewer- and self-assessed KPS, GA identified at least one functional, psychosocial or nutritional deficit, comorbid illness or polypharmacy in more than two-thirds (68%) of study participants [33]. Timely identification of such deficits and subsequent intervention with supportive services could decrease disability, institutionalization and cost as well as improve the quality of life in older women with cancer [31,32]. For example, services such as physical and occupational therapy (OT/PT) can be used to address poor endurance, fatigue, strength or decline in activities of daily living and instrumental activities of daily living [34,35]. Early recognition of these functional deficits and timely referrals can decrease long-term disability [36]. Other services, such as nutrition, psychiatry, geriatrics and pharmacy consultation can also be used to remedy potentially modifiable deficits that are often not identified and therefore potentially unaddressed in routine oncology practice. A full GA is time intensive and requires specialized personnel and resources; however, abbreviated GAs have been shown to be feasible in a variety of settings including a co-operative group clinical trial, academic medical center and community oncology practices [37–39].
Life expectancy
In addition to patient perspectives on their treatment options and findings from a brief GA, a further consideration in estimating potential benefits of adjuvant therapy is the patient's estimated life expectancy – independent of their breast cancer, to determine whether they are likely to live long enough to benefit from the treatment. Chronologic age is an integral component of such estimates; however, it is by no means a sufficient consideration. As our two clinical cases illustrate, two 70-year-old breast cancer patients can have very different underlying health, physical activity and social support systems and require a treatment approach that reflects these differences (Figure 1). It can be particularly challenging for physicians to estimate the life expectancy of their patients, especially for patients in advanced stages of cancer [40]. Fortunately, several tools are available to aid in the estimation of life expectancy in a variety of different patient populations (e.g., community dwelling or nursing home) and life expectancy time frame [41]. Many of these tools are available in an online calculator format on the ‘ePrognosis’ website [42]. For example, the Lee–Schonberg index takes into account the patient's age, gender, BMI, comorbidities, cigarette smoking, physical activity, previous hospitalizations and limitations in activities of daily living due to physical, mental, emotional or cognitive problems to estimate the patient's life expectancy [43–46]. None of these tools are perfect, but they provide reasonable estimates and are generally better than a subjective guess. Obtaining an accurate assessment of a patient's life expectancy irrespective of their current cancer provides a broader context in which to frame potential cancer treatment options.
Biologic, tumor & nodal characteristics
Invasive breast cancer can be divided into three biologic subtypes: estrogen and/or progesterone receptor positive (also called hormone receptor/HR positive) and human epidermal growth factor receptor-2 (HER-2) negative; HER-2 positive, and estrogen and progesterone receptor negative and HER-2 negative (so-called ‘triple negative’ breast cancer; see Table 1). This subdivision of breast cancer into biologic subtypes is helpful in providing prognostic information to patients and also aids the clinician in selecting therapeutic options. In addition, biologic subtypes correlate with breast cancer molecular subtypes as defined by molecular based genetic methods, including luminal A, luminal B and basal-like [47]. The early stage tumors commonly identified in older breast cancer patients tend to have more favorable biologic characteristics and are associated with a more favorable prognosis [47,48].
Table 1.
Biologic characteristics and management of breast cancer.
| Type/frequency | Molecular subtypes | Treatment | Comment |
|---|---|---|---|
| Hormone receptor positive (ER and/or PR) and HER-2 negative (about 70% of breast cancer in older women) | Luminal A | Endocrine therapy for most patients, chemotherapy for some patients | New genetic based assays can help select which patients should consider chemotherapy. Most patients relapse >5 years |
| HER-2 positive any ER or PR (about 15%) | Luminal B and HER-2 expressing | Chemotherapy and anti-HER-2 therapy for most patients Endocrine Rx if hormone receptor positive | Major improvements in outcome with anti-HER-2 Rx. Most relapse patients <5 years |
| ER and PR and HER-2 negative ‘triple negative’ (about 15%) | Basal-like | Chemotherapy for most patients | More common in younger patients and African–American patients more chemotherapy is better. Most patients relapse <5 years |
Patients with hormone receptor positive, HER-2 negative breast cancer represent approximately 70% of older patients with early stage breast cancer [48]. Most of these patients have luminal A and luminal B breast cancers, with luminal A cancers having an excellent long-term prognosis especially if they are diagnosed when they are small (≤2 cm in largest diameter) and have no involvement of axillary lymph nodes [5]. The majority of these patients are generally considered for adjuvant endocrine therapy with agents that either block the effect of circulating estrogens (tamoxifen) or lower circulating estrogens (aromatase inhibitors – such as anastrozole, letrozole and exemestane). When added to optimal surgery and radiation therapy (if appropriate), these endocrine agents lower risk of recurrence in both the breast and chest wall as well as in distant metastatic sites (bone, lung, liver or other organs) by about half [49,50].
Some patients at the time of surgery are found to have involvement of adjacent lymph nodes (node-positive). The hazard ratio for relapse (breast cancer recurrence) is much higher in women with node positive disease and typically occurs during the first 5 years after diagnosis. There also remains a small risk of yearly recurrence which persists for as long as 15–20 years after diagnosis and possibly even longer [51]. This may have implications in therapy selection for older patients with shorter life expectancies.
HER-2 positive breast cancers are seen in approximately 15% of older patients. These cancers are biologically more aggressive and tend to recur within the first 5 years of the initial diagnosis and treatment. Patients with hormone receptor positive, HER-2 positive breast cancer have somewhat better long-term prognosis than those with hormone receptor negativity. Patients with hormone receptor negative HER-2 positive breast cancers, if untreated with chemotherapy and HER-2 directed therapy, have the poorest survival. These patients, unless they have extremely short life expectancy, should be considered for cancer treatment, since the majority of recurrences in these patients are in the first several years after diagnosis. Options for chemotherapy and anti-HER-2 directed therapy are discussed below (Table 2).
Table 2.
Common adjuvant therapy regimens doses and schedules.
| Medication/regimen | Dose | Schedule | Ref. |
|---|---|---|---|
| Endocrine therapies | |||
| Tamoxifen | 20 mg | Daily | |
| Aromatase inhibitors: – Anastrozole – Letrozole – Exemestane |
1 mg 2.5 mg 25 mg |
Daily Daily Daily |
|
| Chemotherapy | |||
| Cyclophosphamide Methotrexate 5-fluorouracil (CMF) |
600 mg/m2 40 mg/m2 600 mg/m2 |
Every 21 days for 8 cycles | [52] |
| Docetaxel Cyclophosphamide (TC) |
75 mg/m2 600 mg/m2 |
Every 3 weeks for 4 cycles | [53] |
| Adriamycin Cyclophosphamide (AC) |
60 mg/m2 600 mg/m2 |
Every 3 weeks for 4 cycles | [54] |
| Dose dense Adriamycin Cyclophosphamide Paclitaxel (AC-T) |
60 mg/m2 600 mg/m2 175 mg/m2 |
AC every 2 weeks for 4 cycles and paclitaxel every 2 weeks for 4 cycles with granulocyte growth factor support | [55] |
| Anti HER-2 directed regimens | |||
| Docetaxel Carboplatin Trastuzumab (TCH) |
75 mg/m2 AUC 6 4 mg/kg loading dose then 2 mg/kg/week |
Every 3 weeks for 6 cycles followed by single agent trastuzumab to complete one year | [56] |
| Paclitaxel Trastuzumab (TH) |
80 mg/m2 4 mg/kg loading dose then 2 mg/kg/week |
Every week for 12 cycles followed by single agent trastuzumab to complete 1 year | [57] |
AUC: Area under the plasma drug concentration vs time curve.
Approximately 15% of older patients have triple negative breast cancers. These represent the most aggressive breast cancers with the majority of recurrences occurring within the first several years after treatment. Endocrine therapy has no role in management of triple negative breast cancer and thus adjuvant chemotherapy is a major consideration. However, emerging data suggest that triple negative breast cancer expressing the androgen receptor (AR) may respond to AR inhibition [58]. More aggressive chemotherapy regimens may afford better long-term outcomes but at the risk of greater toxicity. The risk of recurrence and response to therapy for triple negative breast cancers is not affected by age.
Adjuvant chemotherapy
Estimating the benefit of chemotherapy
Not all women with breast cancer derive benefit from adjuvant chemotherapy. In fact, many older women (age >70) with hormone receptor positive early stage breast cancer treated with adjuvant chemotherapy will not accrue a survival advantage despite a reduction in the risk of relapse [8]. At issue is the clinical challenge of identifying patients who are most likely to benefit from adjuvant chemotherapy. This is particularly important in older women where noncancer related causes of mortality can eclipse cancer related causes [59]. To this end, multivariable prognostic indices have been developed which utilize clinical and tumor characteristics to estimate the benefit of adjuvant endocrine and chemotherapy treatment. For example, Adjuvant! Online [60] incorporates age and comorbidities to estimate their impact on treatment benefit and survival, and Predict [61] estimates the added survival benefit of adjuvant trastuzumab therapy in addition to chemotherapy and endocrine therapy. Both tools have been validated in large cohort studies of women with breast cancer and provide general estimates of the benefits of various adjuvant therapies; however, they are limited in their application to any individual patient's circumstances.
To overcome this shortcoming, multigene prognostic assays – such as the 21-gene Oncotype DX® – have been developed and validated in hormone receptor positive, node negative breast cancer patients to predict the risk of recurrence and estimate added value t of adjuvant chemotherapy in addition to endocrine therapy [62]. These assays may influence treatment decisions in older women with early breast cancer who have a high risk of recurrence based on proprietary recurrence scores. If there is a discrepancy between the estimated risks of recurrence based on gene-based assays and clinical characteristics a balanced discussion of the optimal approach should ensue. In clinical practice in the USA and Europe, chemotherapy is considered if there is an estimated 3–5% 10 year survival benefit [63].
Risk of chemotherapy toxicity
Chemotherapy toxicity is a key consideration in adjuvant treatment decisions. Although overall chemotherapy-related mortality is low (∼1% in general), older cancer patients are more susceptible to chemotherapy toxicities as compared with their younger counterparts [64,65]. Further, chemotherapy may exacerbate an older cancer patient's other medical comorbidities, and even low grade toxicities such as diarrhea and neuropathy may be sufficient to cause functional incontinence and decline in patients with pre-existing diabetic neuropathy or limited mobility. In a pivotal study, Hurria et al. investigated factors associated with chemotherapy toxicity. In addition to demographic and standard clinical variables such as age, cancer type, chemotherapy dose and creatinine clearance, their study found that deficits identified by GA such as hearing impairment, limited physical function, falls and hearing impairment predicted risk of grade 3–5 chemotherapy toxicity in older patients whereas the Karnofsky Performance Status measure did not [30]. Similarly, tools such as the Chemotherapy Risk Assessment Scale for High-Age Patient (CRASH) score can provide estimates of a patient's risk of chemotherapy toxicity and help tailor therapy as needed [66].
Selecting adjuvant chemotherapy for older women
Limited data exist to guide optimal adjuvant chemotherapy treatment decisions in women age 70 and older with early stage breast cancer. Nevertheless, prospective trial data suggest that the benefit older women derive from adjuvant chemotherapy is similar to that conferred on their younger counterparts [67]. Table 2 provides an overview of common adjuvant chemotherapy regimens used in the treatment of women with breast cancer. Adjuvant chemotherapy is relatively well tolerated in medically fit older women and the same general principles apply in selecting adjuvant chemotherapy as in younger women. However, the general decline in physiologic reserve and increase in comorbidities predispose older women to increased risk of toxicity which, in turn, may impact overall function, quality of life and survival [65,68]. Of particular concern is the risk of cardiac toxicity and secondary hematologic malignancies with anthracycline-based chemotherapy regimens [69–71]. Selection of therapy for older women should therefore be individualized taking into consideration comorbid illnesses and other factors as recommended by the National Comprehensive Cancer Network [72].
Adjuvant radiation
The role for adjuvant radiation in older women with breast cancer follows the general principles of treatment in the breast conserving and postmastectomy settings. Adjuvant radiation is indicated for node positive breast cancer, which confers a survival advantage. The value of nodal radiation for patients who have achieved a pathologic complete response to neoadjuvant chemotherapy is a topic of ongoing clinical trials. There is a clear-cut local control benefit in the breast conservation setting, but the incremental benefit varies widely depending on tumor size, tumor grade, hormone receptor and HER-2/neu status. For some women with favorable prognostic features (e.g., <2cm node negative and hormone receptor positive tumors), who are appropriate candidates for adjuvant endocrine therapy, adjuvant radiation may be safely omitted. For example, in a seminal study Hughes et al. [73,74] randomized women 70 years and older with breast cancer to lumpectomy and tamoxifen with or without radiation. They found no difference in time to distant metastases, time to mastectomy, breast cancer specific survival, 5-year or 10-year overall survival despite a slightly higher (∼3% at 5 years and 8% at 10 years) rate of locoregional recurrence in the nonirradiated group. Table 3 summarizes the relevant randomized trials and meta-analyses evaluating omission of radiation in older breast cancer patients.
Table 3.
Summary of relevant randomized trials and meta-analyses evaluating omission of radiation in older women with breast cancer.
| Study (year) | Patient population | Treatment | Results | Ref. |
|---|---|---|---|---|
| Fyles et al. (2004) | 769 patients ≥50, path T1 or T2, negative margins, path negative nodes except for ≥65 who were also eligible if clinically node negative | Whole breast in hypofractionated regimen 40 Gy (16 fx) followed by boost of 12.5 Gy (5 fx) to lumpectomy site. All received tamoxifen | 5 year local relapse 0.6% RT + tam 7.7% tam alone. No difference in distant relapse or overall survival | [75] |
| Hughes et al. (2004) and (2010) CALGB | 636 patients ≥70, T1 HR+, clinically and/or path node negative | Whole breast radiation 45 Gy in 25 fractions followed by boost of 14 Gy (7 fx). All received tam | At median follow-up of 10.5 years, the locoregional recurrence was 2% in the RT + tam group, 9% in the tam alone group. No survival difference | [73,74] |
| Darby et al. (2011) EBCTG | Meta-analysis of individual patient data for 10,801 pts with early stage breast cancer who received adjuvant radiation (or not) in RCT | Details of RT varied with the trials | For a threshold of 10% or greater reduction in the 10 year recurrence risk, data support adjuvant RT for T1 grade 3 tumors and T2 grade 2 and 3 tumors for pts ≥70 | [76] |
| Kunkler et al. (2015) Prime II | 1326 patients ≥65, LN negative, T1-T2 up to 3 cm, neg margins; (grade 3 or LVSI permitted, but not both) | Whole breast radiation (40–50 Gy in 15–25 fx). All received endocrine therapy | At median 5 year follow-up, ipsilateral breast recurrence 4.1% in no RT arm vs 1.3% in the RT arm. No survival difference | [77] |
fx: fractions; HR: Hormone receptor; LN: Lymph node; LVSI: Lymphovascular space invasion; RCT: Randomized controlled trials; RT: Radiation therapy; tam: Tamoxifen.
When the risk of locoregional recurrence warrants the use of adjuvant radiation, there are options for shorter courses of radiation that are appropriate for both older and younger women. Several randomized trials have demonstrated excellent local control and equivalent late effects and quality of life with hypofractionated radiation. The Whelan trial (OCOG) randomized 1234 women with node negative, margin negative breast cancer to two fractionation schemes 200 cGy for 25 fractions (the classic NSABP fractionation) versus 266 cGy for 16 fractions. With long term follow-up, there is no difference in local control, survival or late toxicity. This trend has received widespread interest, and is endorsed by the American Society for Radiation Oncology ‘choose wisely’ initiative [78]. Table 4 summarizes the relevant randomized trials evaluating shorter, hypofractionated radiation schedules.
Table 4.
Summary of the relevant randomized trials evaluating hypofractionation of radiation therapy.
| Study (year) | Patient population | Treatment | Results | Ref. |
|---|---|---|---|---|
| Yarnold et al. (2005) Owen et al. (2006) RMH/GOC | 1410 patients (T stage 1–3 with a max of one + node) | Gy/fx#/fx dose/#patients 50/25/2.0 (470) 42.9/13/3.3 (466) 39/13/3.0 (474) | Median fu 9.7 years, 12% local recurrence 10% 15% | [79,80] |
| Whelan et al. (2002), (2010) OCOG | 1234 patients LN negative margin negative breast ca | Gy/fx#/fx dose/#patients 50/25/2.0 (612) 42.5/16/2.66 (622) | 10 year local recurrence 6.7% 6.2% No difference in cosmesis, survival | [81,82] |
| Bentzen et al. (2008) Haviland et al. (2013) START A | 2236 patients with breast cancer (pT1–3a pN0–1) | Gy/fx#/fx dose/#patients 50/25/2.0 (749) 41.6/13/3.2 (750) 39/13/3.0 (737) | Median fu 9.3 years, 7% local recurrence 6% 9%. No difference in late toxicity or survival | [83,84] |
| Bentzen et al. (2008) Haviland et al. (2013) START B | 2215 patients with breast cancer (pT1–3a pN0–1) | Gy/fx#/fx dose/#patients 50/25/2.0 (1105) 40/15/2.67 (1110) | Median fu 9.9 years, 6% local recurrence 4%. No difference in late toxicity or survival | [84–85] |
#: Number; ca: Cancer; fx: Fraction; fu: Follow-up; Gy: Gray; LN: Lymph node.
Adjuvant endocrine therapy
There are two types of endocrine therapies for breast cancer – tamoxifen and aromatase inhibitors. Tamoxifen is a selective estrogen receptor modulator which inhibits estrogen binding to the estrogen receptors in breast tissue. In 2005, the Early Breast Cancer Trialists meta-analysis, which analyzed 194 randomized trials of adjuvant hormone therapy, showed that 5 years of adjuvant tamoxifen reduces the annual breast cancer death rate by 31% regardless of the use of chemotherapy, age, progesterone receptor status and other tumor characteristics [8]. As a result, early recommendations for adjuvant hormonal therapy focused on tamoxifen.
Newer agents, like the aromatase inhibitors (AIs) anastrozole, letrozole and exemestane suppress plasma estrogen levels by inhibiting aromatase, the enzyme that converts peripheral androgens to estrogens [86]. Several large randomized clinical trials have evaluated the benefit of tamoxifen versus AIs, and although there appears to be a small improvement in relapse-free survival, there is no convincing improvement in overall survival [87].
Endocrine therapies have some important side effects. Tamoxifen is generally well tolerated by both younger and older patients, with the most frequent complaint being hot flashes. There is however a small (about 1%) risk of endometrial cancer and venous thrombosis associated with 5 years of use of tamoxifen, and the recommended annual pelvic examination and Papanicolaou (Pap) smear can be a barrier to use for some patients. The approximately 1% excess mortality from endometrial cancer and venous thromboembolism with tamoxifen is overshadowed in most patients by the significant improvement in breast cancer survival [49].
Although Als do not increase the risk of endometrial cancer or venous thrombosis, they frequently cause arthralgias/myalgias that can impede function and reduce treatment compliance. AIs are also associated with accelerated bone loss and an increased risk of fracture, which is of particular concern given that many older women have osteopenia or osteoporosis prior to their breast cancer diagnosis. Older women treated with AIs should be recommended to take daily vitamin D and calcium supplementation and encouraged to engage in routine exercise. For patients with osteoporosis or severe osteopenia, treatment with bone protective agents, such as bisphosphonates or denosumab, should be considered although insurance reimbursement may be an obstacle to obtaining such therapy. Therapeutic decisions should consider the side effect profiles and individual patient characteristics. For example, AIs should be used with caution in women with a history of ischemic heart disease and osteoporosis, while patients taking tamoxifen need to be carefully monitored for the development of venous thromboembolism and endometrial carcinoma [88].
Current ASCO guidelines recommend that postmenopausal women receive either 5 years of an AI, 5 years of tamoxifen followed by an AI or 10 years of tamoxifen [89]. Emerging data have shown benefit with this approach and support continuing endocrine therapy beyond 5 years. The landmark ATLAS trial showed a reduction in recurrence risk and mortality with 10 versus 5 years of tamoxifen [90] while the MA.17 trial comparing letrozole to placebo following 5 years of adjuvant tamoxifen showed a 43% reduction in recurrence in the letrozole arm [91,92]. Ongoing studies are evaluating the benefit of continuing adjuvant AI therapy beyond 5 years.
Conclusion
Identifying the optimal adjuvant treatment strategy for older women with breast cancer is a significant clinical challenge given the marked variability in life expectancy and general health in this patient population. In our review, we suggest an approach based on life expectancy and geriatric assessment (Figure 2). The sequence of administering adjuvant therapies in older women with high risk or unfavorable subtype breast cancer generally follows a similar pattern to that in younger women; chemotherapy first if indicated, followed by radiation and finally endocrine therapy and thus these treatments will be discussed in that order.
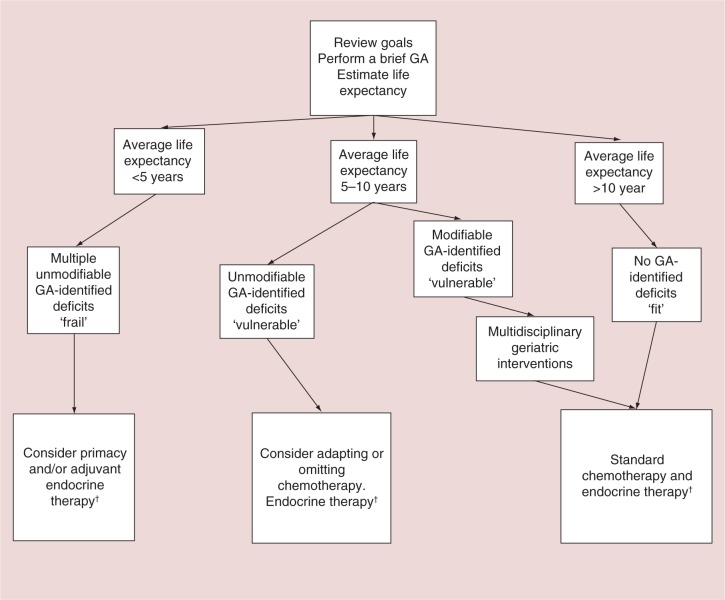
Figure 2.
Suggested general approach to the management of adjuvant chemotherapy in older women with early stage breast cancer.
†Endocrine therapy is only appropriate for patient with hormone receptor positive tumors.
GA: Geriatric assessment.
After reviewing the goals of chemotherapy and eliciting the patient's treatment preferences, a brief GA can be performed to identify important deficits which may affect treatment tolerance, guide multidisciplinary intervention and aid treatment selection. GA can also help gauge a patient's functional status, burden of comorbidity, general health and overall fitness for therapy. Next, we suggest estimating life expectancy using the web-based, combined Lee–Schonberg index risk calculator [42]. In general, older women who are in good health with an average life expectancy greater than 10 years and no GA-identified deficits are considered fit and should be offered adjuvant chemotherapy perhaps with a second generation regimen such as docetaxel/cyclophosphamide. For these women, various prognostic indices (e.g., Adjuvant! Online and Predict) or molecular based assays (e.g., Oncotype DX) are available to help estimate the potential benefits of chemotherapy, while tools such as those developed by Hurria et al. and the CRASH score are available to aid in estimating the risk of chemotherapy toxicities. A discussion of the value of chemotherapy mitigated by the risk of toxicity and framed within the context of the patient's values and preferences is warranted. If the patient wishes to pursue chemotherapy, a third-generation anthracycline-based and taxane-based regimen either in a standard or dose dense fashion depending on the patient's goals and preferences is a reasonable option. However, given the modest additional survival benefit with these regimens and the risk of cardiac toxicities and secondary hematologic malignancy, a second generation regimen may be a more appropriate choice particularly in older women with pre-existing heart disease. The second-generation docetaxel/cyclophosphamide (TC) regimen was studied in older women (>65 years old) and was shown to be noninferior to the doxorubicin/cyclophosphamide regimen and therefore is an attractive option in older women when cardiac toxicity is a concern [93].
For older women with HER-2 positive tumors, the addition of adjuvant HER-2 directed therapy is recommended if baseline cardiac function is normal and there is periodic cardiac monitoring throughout therapy. The paclitaxel/trastuzumab (TH) regimen was evaluated in the adjuvant setting in patients with early (predominantly stage I) HER-2 overexpressing breast cancer and showed an approximately 2% risk of early recurrence [57] suggesting that TH is a reasonable option for older women with this breast cancer subtype. Higher risk HER-2 positive tumors should be treated with more established regimens. Prophylactic granulocyte stimulating growth factor is recommended with regimens where there is >20% estimated risk of febrile neutropenia and should be routinely prescribed for women over the age of 65 receiving such regimens [94].
For frail older women with limited life expectancy (<5 years) and multiple unmodifiable GA-identified deficits, such as a high burden of comorbidity or cognitive impairment, the benefits of chemotherapy are likely be modest and therefore chemotherapy is not recommended. For prefrail patients with high risk or unfavorable subtype breast cancer and average life expectancy of 5–10 years, we suggest multidisciplinary interventions prior to chemotherapy treatment for those with modifiable GA-identified deficits, or an adapted adjuvant treatment approach for those with unmodifiable GA identified deficits.
Adjuvant irradiation can be safely omitted in some older women without sacrificing a survival advantage if they are appropriately treated with adjuvant endocrine therapy. These women must be willing to accept a slightly higher risk of local recurrence (∼3% at 5 years and ∼8% at 10 years) [73,74]. Omitting radiation or pursuing shorter radiation schedules (hypofractionation) which require fewer treatment visits may be an appealing option for older women with limited mobility or concerns about radiation toxicity.
Because of the preponderance of hormone receptor positive tumors in older women and the fact that endocrine therapies are generally well tolerated and dramatically reduce the risk of recurrence, adjuvant endocrine treatments have become the cornerstone of therapy in older women with breast cancer. The majority of older women with this breast cancer subtype are generally considered for adjuvant endocrine therapy either with tamoxifen or an aromatase inhibitors after optimal surgery and radiation therapy (if appropriate). These endocrine agents lower risk of recurrence in both the breast and chest wall as well as in distant metastatic sites (bone, lung, liver or other organs) by about 50% [49].
Applying these principles to the patients in the clinical vignette, we note that despite their identical chronological age these patients have marked different estimated life expectancies (10 year: 92 vs 24%) based on the information provided (Table 5). This underscores the importance of GA in identifying variables not routinely assessed in clinical evaluation which affect life expectancy, a key consideration in adjuvant treatment decisions. Table 5 also shows that while Mrs A could likely have derived a reasonable estimated survival advantage with the addition of a 2nd generation chemotherapy regimen (10%) with acceptable estimated risk of toxicity (31%), Mrs B's estimated survival benefits are modest (5%) and risk of chemotherapy toxicity substantial (63%). Furthermore Mrs B has Alzheimer's disease which is likely to limit the overall survival benefits of chemotherapy [95] and make treatment compliance challenging. Therefore, if it is consistent with her goals and preferences, one could recommend chemotherapy for Mrs A but likely not offer it to Mrs B. Omitting radiation is a reasonable option for both women, although considering radiation perhaps with a hypofractionated schedule is a reasonable choice for Mrs A. Mrs A is also a good candidate for adjuvant AI therapy; however, worsening arthralgias due to underlying osteoarthritis is a concern and should be closely monitored. Because AIs are generally well tolerated, Mrs B could be considered for a closely monitored trial of AI therapy which can be truncated if significant toxicities develop. Tamoxifen is an option for either patient but carries the risk of venous thromboembolism and endometrial neoplasia particularly in the elderly [96].
Table 5.
Life expectancy and adjuvant chemotherapy/endocrine therapy benefit estimates of two hypothetical patients
| Variable | Mrs A | Mrs B |
|---|---|---|
| Estimated survival irrespective of cancer (%)† | ||
| 5 year | 98 | 65 |
| 10 year | 92 | 24 |
| 10-year mortality risk from cancer (%)‡ | ||
| Alive | 66 | 33 |
| Die from other causes | 11 | 50 |
| Die from cancer | 23 | 17 |
| Survival benefit (%)‡ | ||
| AI therapy | 6 | 3 |
| 2nd generation chemotherapy + AI | 10 | 5 |
| 3rd generation chemotherapy + AI | 12 | 6 |
| Risk of grade 3–5 chemotherapy toxicity (%)§ | ||
| Standard dose, polychemotherapy | 31 | 63 |
Estimated 5- and 10-year life expectancy using the combined Lee-Schonberg index.
Chemotherapy survival benefitestimates using Adjuvant! Online.
Chemotherapy toxicity estimates: model developed by Hurria et al.; assumes normal renal function and hemoglobin.
AI: Aromatase inhibitor.
Future perspective
The landscape of adjuvant breast cancer management in older adults is evolving rapidly with several major changes in the past two decades and several anticipated in the years ahead. The role of geriatric assessment in the care of older adults with cancer will likely broaden with emerging data showing improved outcomes, hence solidifying GA's role in oncology practice. Another anticipated development is the use of biological markers of aging such as p16INK4a, in clinical practice in addition GA to determine a patient's molecular age and predict tolerance for chemotherapy and other outcomes. Further, targeted therapies such as everolimus and palbociclib have both shown benefits in the metastatic hormone receptor positive setting and are being evaluated in the adjuvant setting. Likewise, chemotherapy conjugates of HER-2 directed therapies such as ado-trastuzumab are being investigated in the adjuvant setting as a way to better target cytotoxic chemotherapy. Overall the role of cytotoxic chemotherapy in the adjuvant setting will probably diminish but will likely not disappear completely. Caring for an increasing number of older cancer patients will remain a significant clinical challenge with the decreasing number of geriatrician and increasing, time, resource and personnel constraints. To overcome these challenge, health policy and workforce changes which bring together trained multidisciplinary teams prepared to care for an aging breast cancer population will be required.
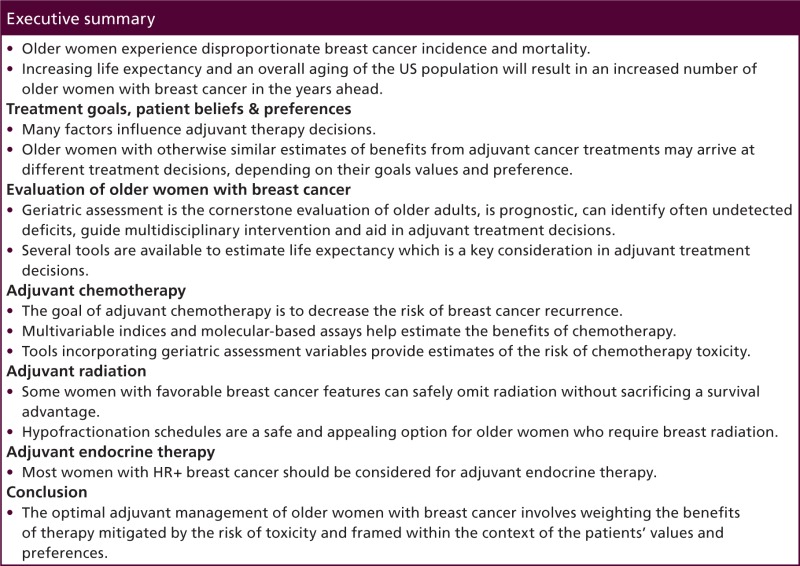
Executive summary
Older women experience disproportionate breast cancer incidence and mortality.
Increasing life expectancy and an overall aging of the US population will result in an increased number of older women with breast cancer in the years ahead.
Treatment goals, patient beliefs & preferences
Many factors influence adjuvant therapy decisions.
Older women with otherwise similar estimates of benefits from adjuvant cancer treatments may arrive at different treatment decisions, depending on their goals values and preference.
Evaluation of older women with breast cancer
Geriatric assessment is the cornerstone evaluation of older adults, is prognostic, can identify often undetected deficits, guide multidisciplinary intervention and aid in adjuvant treatment decisions.
Several tools are available to estimate life expectancy which is a key consideration in adjuvant treatment decisions.
Adjuvant chemotherapy
The goal of adjuvant chemotherapy is to decrease the risk of breast cancer recurrence.
Multivariable indices and molecular-based assays help estimate the benefits of chemotherapy.
Tools incorporating geriatric assessment variables provide estimates of the risk of chemotherapy toxicity.
Adjuvant radiation
Some women with favorable breast cancer features can safely omit radiation without sacrificing a survival advantage.
Hypofractionation schedules are a safe and appealing option for older women who require breast radiation.
Adjuvant endocrine therapy
Most women with HR+ breast cancer should be considered for adjuvant endocrine therapy.
Conclusion
The optimal adjuvant management of older women with breast cancer involves weighting the benefits of therapy mitigated by the risk of toxicity and framed within the context of the patients' values and preferences.
Adjuvant treatment for older women with invasive breast cancer
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/whe. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity evaluation: where 1 is strongly disagree and 5 is strongly agree.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| The activity supported the learning objectives. | |||||
| The material was organized clearly for learning to occur. | |||||
| The content learned from this activity will impact my practice. | |||||
| The activity was presented objectively and free of commercial bias. | |||||
-
Your patient is a 72-year-old woman with breast cancer. According to the review by Dr Jolly and colleagues, which one of the following statements about the effect of patient goals, beliefs, preferences, and quality-of-life considerations on adjuvant treatment options for older women with breast cancer is most likely correct?
-
□
A Estimated benefits from adjuvant cancer treatments are the sole factor affecting treatment decisions
-
□
B Older patients are less willing to undergo chemotherapy than younger patients
-
□
C Extended lifespan is the most important consideration for older patients when choosing adjuvant therapy
-
□
D Clinicians should ascertain and respect patient goals, beliefs, and preferences regarding adjuvant treatment
-
□
-
According to the review by Dr Jolly and colleagues, which one of the following statements about evaluation of the fitness and suitability of the older patient with breast cancer for adjuvant treatment, as defined by life expectancy, geriatric assessment, biologic, nodal, and tumor differences, is most likely correct?
-
□
A Geriatric assessment is prognostic, can identify otherwise undetected deficits affecting fitness for treatment, and can guide multidisciplinary intervention
-
□
B No good tools are available to estimate life expectancy
-
□
C Older women in good health with an average life expectancy between 5 and 10 years and no deficits identified on geriatric assessment are considered unfit for chemotherapy
-
□
D Women with hormone receptor-positive, HER-2-positive breast cancer have much worse long-term prognoses than those with hormone receptor negativity
-
□
-
According to the review by Dr Jolly and colleagues, which one of the following statements about the role of adjuvant treatment options, including chemotherapy, endocrine therapy, and radiation therapy would most likely be correct?
-
□
A The goal of adjuvant chemotherapy is to lessen the existing tumor burden
-
□
B Multivariable indices and molecular-based assays help estimate the benefits of chemotherapy, whereas geriatric assessment variables provide estimates of the risk for chemotherapy toxicity
-
□
C Radiation cannot be safely omitted without sacrificing a survival advantage
-
□
D Adjuvant endocrine therapy is usually unnecessary for most women with hormone receptor-positive breast cancer
-
□
Financial & competing interests disclosure
Editor: Laura Dormer, Future Science Group, London, UK.
Disclosure: Laura Dormer has disclosed no relevant financial relationships.
CME author: Laurie Barclay, MD, Freelance writer and reviewer, Medscape, LLC.
Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Author & credentials: Trevor A Jolly, MBBS, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Trevor A Jolly, MBBS, has disclosed no relevant financial relationships.
Grant R Williams, MD, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Grant R Williams, MD, has disclosed no relevant financial relationships.
Sita Bushan, MD, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Sita Bushan, MD, has disclosed no relevant financial relationships.
Mackenzi Pergolotti, PhD, OTR/L, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Mackenzi Pergolotti, PhD, OTR/L, has disclosed no relevant financial relationships.
No writing assistance was utilized in the production of this manuscript.
Kirsten A Nyrop, PhD, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Kirsten A Nyrop, PhD, has disclosed no relevant financial relationships.
Ellen L Jones, MD, PhD, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Ellen L Jones, MD, PhD, has disclosed no relevant financial relationships.
Hyman B Muss, University of North Carolina, Chapel Hill, NC, USA.
Disclosure: Hyman B Muss, has disclosed no relevant financial relationships.
No writing assistance was utilized in the production of this manuscript.
References
- 1.SEER Stat Fact Sheets. http://seer.cancer.gov/statfacts
- 2.Arias E. United States life tables, 2007. Natl Vital Stat. Rep. 59(9), 1–60 (2011). [PubMed] [Google Scholar]
- 3.Ortman JM, Velkoff VA, Howard H. An aging nation: the older population in the United States. www.census.gov
- 4.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 13(4), e148–e160 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 119(7), 1402–1411 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J. Natl Cancer Inst. 100(16), 1179–1183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BD, Jiang J, McLaughlin SS, et al. Improvement in breast cancer outcomes over time: are older women missing out? J. Clin. Oncol. 29(35), 4647–4653 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472), 1687–1717 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 11(6), 449–460 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part II. Cancer J. 11(6), 461–473 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Vlahiotis A, Barrett LB, Flood KL, Spitznagel EL, Steyerberg EW. The changing prevalence of comorbidity across the age spectrum. Crit. Rev. Oncol. Hematol. 67(2), 124–132 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blinman P, King M, Norman R, Viney R, Stockler MR. Preferences for cancer treatments: an overview of methods and applications in oncology. Ann. Oncol. 23(5), 1104–1110 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti J, Kenny FS, Syed BM, Robertson JF, Blamey RW, Cheung KL. A randomised trial of mastectomy only versus tamoxifen for treating elderly patients with operable primary breast cancer-final results at 20-year follow-up. Crit. Rev. Oncol. Hematol. 78(3), 260–264 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Johnston SJ, Kenny FS, Syed BM, et al. A randomised trial of primary tamoxifen versus mastectomy plus adjuvant tamoxifen in fit elderly women with invasive breast carcinoma of high oestrogen receptor content: long-term results at 20 years of follow-up. Ann. Oncol. 23(9), 2296–2300 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: Cochrane review. Br. J. Cancer 96(7), 1025–1029 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan JL, Reed MW, Wyld L. Primary endocrine therapy as a treatment for older women with operable breast cancer – a comparison of randomised controlled trial and cohort study findings. Eur. J. Surg. Oncol. 40(6), 676–684 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Mustacchi G, Scanni A, Capasso I, Farris A, Pluchinotta A, Isola G. Update of the Phase III trial ‘GRETA’ of surgery and tamoxifen versus tamoxifen alone for early breast cancer in elderly women. Future Oncol. 11(6), 933–941 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J. Natl Cancer Inst. 86(23), 1766–1770 (1994). [DOI] [PubMed] [Google Scholar]
- 19.Craig BM, Reeve BB, Cella D, Hays RD, Pickard AS, Revicki DA. Demographic differences in health preferences in the United States. Med. Care 52(4), 307–313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N. Engl. J. Med. 346(14), 1061–1066 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Evaluation Of Chemotherapeutic Agents. Macleod CM. (Ed.) Columbia University Press, New York, NY, USA, 191–205 (1948). [Google Scholar]
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J. Clin. Oncol. 5(6), 649–655 (1982). [PubMed] [Google Scholar]
- 23.Extermann M. Studies of comprehensive geriatric assessment in patients with cancer. Cancer Control 10(6), 463–468 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology Consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 32(24), 2595–2603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stotter A, Reed MW, Gray LJ, Moore N, Robinson TG. Comprehensive geriatric assessment and predicted 3-year survival in treatment planning for frail patients with early breast cancer. Br. J. Surg. 102(5), 525–533 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Aaldriks AA, Giltay EJ, Le Cessie S, et al. Prognostic value of geriatric assessment in older patients with advanced breast cancer receiving chemotherapy. Breast 22(5), 753–760 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Hamaker ME, Seynaeve C, Wymenga AN, et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: Results from the OMEGA study of the Dutch Breast Cancer Trialists' Group. Breast 23(1), 81–87 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Hamaker ME, Vos AG, Smorenburg CH, De Rooij SE, Van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist 17(11), 1439–1449 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J. Geriatr. Oncol. 4(3), 227–234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J. Clin. Oncol. 29(25), 3457–3465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the Task Force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 55(3), 241–252 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 342(8878), 1032–1036 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 20(4), 379–385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121(21), 4287–4294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J. Geriatr. Oncol. 6(3), 194–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hewitt ME, Bamundo A, Day R, Harvey C. Perspectives on post-treatment cancer care: qualitative research with survivors, nurses, and physicians. J. Clin. Oncol. 25(16), 2270–2273 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in Cooperative Group Clinical Cancer Trials: CALGB 360401. J. Clin. Oncol. 29(10), 1290–1296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolly TA, Chiu WK, Alston SM, Lacy AC, Muss HB. Geriatric assessment of older adults (> age 65) with diagnosed or suspected malignancy in a cancer center clinic. JCO ASCO Meeting Abstracts 29(Suppl. 15), e19546 (2011). [Google Scholar]
- 39.Williams G, Deal A, Jolly T, et al. Feasibility of geriatric assessment in community oncology clinics. J. Geriatr. Oncol. 5(3), 245–251 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Glare P, Virik K, Jones M, et al. A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ 327(7408), 195–198 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA 307(2), 182–192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ePrognosis. www.eprognosis.com
- 43.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295(7), 801–808 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J. Am. Geriatr. Soc. 59(8), 1444–1451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J. Gen. Intern. Med. 24(10), 1115–1122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. JAMA 309(9), 874–876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins EO, Deal AM, Anders CK, et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist 19(10), 1076–1083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J. Natl Cancer Inst. 92(7), 550–556 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Early Breast Cancer Trialists' Collaborative Group, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793), 771–784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J. Clin. Oncol. 28(3), 509–518 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J. Clin. Oncol. 33(1), 65–73 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N. Engl. J. Med. 332(14), 901–906 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J. Clin. Oncol. 24(34), 5381–5387 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J. Clin. Oncol. 8(9), 1483–1496 (1990). [DOI] [PubMed] [Google Scholar]
- 55.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 21(8), 1431–1439 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365(14), 1273–1283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 372(2), 134–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGhan LJ, McCullough AE, Protheroe CA, et al. Androgen receptor-positive triple negative breast cancer: a unique breast cancer subtype. Ann. Surg. Oncol. 21(2), 361–367 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J. Natl Cancer Inst. 100(4), 252–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adjuvant! Online. www.adjuvantonline.com
- 61.Predictwww.predict.nhs.uk
- 62.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 24(23), 3726–3734 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Tew WP, Muss HB, Kimmick GG, Von Gruenigen VE, Lichtman SM. Breast and ovarian cancer in the older woman. J. Clin. Oncol. 32(24), 2553–2561 (2014). [DOI] [PubMed] [Google Scholar]
- 64.O'Brien ME, Borthwick A, Rigg A, et al. Mortality within 30 days of chemotherapy: a clinical governance benchmarking issue for oncology patients. Br. J. Cancer 95(12), 1632–1636 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J. Support. Oncol. 1(4 Suppl. 2), 18–24 (2003). [PubMed] [Google Scholar]
- 66.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118(13), 3377–3386 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA 293(9), 1073–1081 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N. Engl. J. Med. 360(20), 2055–2065 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Howard RA, Gilbert ES, Chen BE, et al. Leukemia following breast cancer: an international population-based study of 376,825 women. Breast Cancer Res. Treat. 105(3), 359–368 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. 25(25), 3808–3815 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the united states, 1975–2008. Blood 121(15), 2996–3004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Network NCC. NCCN clinical practice guidelines in oncology (version 2.2015).
- 73.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N. Engl. J. Med. 351(10), 971–977 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J. Clin. Oncol. 31(19), 2382–2387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N. Engl. J. Med. 351(10), 963–970 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378(9804), 1707–1716 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, On Behalf of The PIII. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 16(3), 266–273 (2015). [DOI] [PubMed] [Google Scholar]
- 78.(ASTRO) TaSFRO. The American Society for Radiation Oncology (ASTRO).
- 79.Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother. Oncol. 75(1), 9–17 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 7(6), 467–471 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362(6), 513–520 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Whelan T, Mackenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J. Natl Cancer Inst. 94(15), 1143–1150 (2002). [DOI] [PubMed] [Google Scholar]
- 83.Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) trial a of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 9(4), 331–341 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 14(11), 1086–1094 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Bentzen SM, Agrawal RK, Aird EG, et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 371(9618), 1098–1107 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 348(24), 2431–2442 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Williams GR, Jones E, Muss HB. Challenges in the treatment of older breast cancer patients. Hematol. Oncol. Clin. North Am. 27(4), 785–804, ix (2013). [DOI] [PubMed] [Google Scholar]
- 88.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J. Natl Cancer Inst. 103(17), 1299–1309 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J. Clin. Oncol. 32(21), 2255–2269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381(9869), 805–816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 349(19), 1793–1802 (2003). [DOI] [PubMed] [Google Scholar]
- 92.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA. 17. J. Natl Cancer Inst. 97(17), 1262–1271 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Jones S, Holmes FA, O'shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J. Clin. Oncol. 27(8), 1177–1183 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J. Clin. Oncol. 24(19), 3187–3205 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Robb C, Boulware D, Overcash J, Extermann M. Patterns of care and survival in cancer patients with cognitive impairment. Crit. Rev. Oncol. Hematol. 74(3), 218–224 (2010). [DOI] [PubMed] [Google Scholar]
- 96.Hernandez RK, Sorensen HT, Pedersen L, Jacobsen J, Lash TL. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer 115(19), 4442–4449 (2009). [DOI] [PubMed] [Google Scholar]