Abstract
Objectives
To investigate the long term association of subthalamic beta activity with parkinsonian motor signs.
Methods
We recruited 15 patients with Parkinson’s disease undergoing subthalamic DBS for local field potential recordings after electrode implantation, and at 3 and 8 months post-operatively using the implantable sensing enabled Activa PC + S (Medtronic). Three patients dropped out leaving 12 patients. Recordings were conducted ON and OFF levodopa at rest. Beta (13–35 Hz) peak amplitudes were extracted, compared across time points and correlated with UPDRS-III hemibody scores.
Results
Peaks in the beta frequency band (13–35 Hz) in the OFF medication state were found in all hemispheres. Mean beta activity was significantly suppressed by levodopa at all recorded time points (P < 0.007) and individual beta power amplitude correlated with parkinsonian motor impairment across time points and dopaminergic states (pooled data; ρ = 0.25, P < 0.001).
Conclusions
Our results indicate that beta-activity is correlated with parkinsonian motor signs over a time period of 8 months.
Significance
Beta-activity may be a chronically detectable biomarker of symptom severity in PD that should be further evaluated under ongoing DBS.
Keywords: Deep brain stimulation, Basal ganglia, Local field potentials, Beta oscillations, Subthalamic nucleus
1. Introduction
Oscillatory activity in the human motor network is synchronized in the beta frequency band (13–35 Hz) at rest (Pfurtscheller and Lopes da Silva, 1999). Features like movement related desynchronization and post movement rebound synchronisation have led to the suggestion that beta activity serves to promote maintenance of the status quo and is, in these terms, antikinetic (Engel and Fries, 2010). Patients with Parkinson’s disease (PD) exhibit exaggerated beta oscillations in the basal ganglia, which have been related to bradykinesia and rigidity in line with the above hypothesis (Brittain and Brown, 2014). Beta activity recorded from implanted deep brain stimulation (DBS) electrodes in the subthalamic nucleus (STN) of PD patients correlates with signs of parkinsonian symptom severity (as assessed by UPDRS-III) in the hypodopaminergic state (Neumann et al., 2016a). Both, dopaminergic medication and DBS significantly decrease beta activity in parallel with the clinically apparent symptom alleviation (Brown et al., 2001; Neumann et al., 2016b), and the degree of beta suppression correlates with the change in UPDRS-III scores for either therapeutic procedure (Kühn et al., 2006; Kuhn et al., 2009; Oswal et al., 2016). Thus, beta amplitude may serve as a biomarker for instantaneous monitoring of concurrent therapeutic demand (Little and Brown, 2012). This has led to the trial of adaptive deep brain stimulation algorithms, utilizing a closed loop system that can trigger stimulation according to the level of beta activity in the STN. (Little et al., 2013, 2016a,b; Rosa et al., 2015) Although initial results have been promising, most of the relevant studies have been performed a few days after electrode implantation with DBS leads externalized. Therefore, little is known about the evolution of beta activity after chronic DBS and more importantly its relation to motor impairment over the long term. In the present study we aim to investigate the relation of subthalamic beta activity with parkinsonian motor signs directly after DBS surgery, after three months and eight months of chronic continuous DBS with an implantable sensing enabled pulse generator.
2. Materials and methods
Fifteen patients with Parkinson’s disease who underwent bilateral implantation of DBS electrodes in the STN were included in this study. All patients participated with informed consent, which was approved by the local ethics committee. The DBS macroelectrode used was model 3389 (Medtronic). Contacts 0 and 3 were the lowermost and uppermost contacts, respectively. Intraoperative microelectrode recordings were used for target mapping in all patients. Correct placement of the DBS electrodes was confirmed by three-dimensional electrode localization (Horn and Kuhn, 2015) using the in-house LEAD toolbox (LEAD-DBS; www.lead-dbs.org). In brief, preoperative and postoperative MR images are normalized to MNI space and electrode tips are identified and plotted on a three dimensional MNI version of the Morel Atlas (Jakab et al., 2012). All contact pairs included in the analysis had at least one contact inside the subthalamic nucleus. Three patients were excluded from further analysis (two subjects due to refusal of withdrawal from medication at 3 and 8 months, one subject did not complete the 8 months’ follow-up). Clinical details for the remaining 12 patients (age 63.16 ± 1.4 MEAN ± S.E.M.) are shown in Table 1. Subthalamic local field potential (LFP) recordings were performed at rest from adjacent contact pairs (01, 12, 23) ON and OFF dopaminergic medication at three time points: first at baseline after implantation of the Activa PC + S (Medtronic) pulse generator (MEAN 2.1 days ± 0.2 S.E.M; on average 7.6 ± 0.4 days after electrode implantation), after three months post-implantation and after 8 months post-implantation. Patients were left on their regular medication. If patients’ felt relatively OFF before the ON medication recording, a single dose of a fast-acting levodopa agent was administered (Case 7, 9 and 12 at 3 months and case 1 to 10 at 8 months). Nevertheless, some patients at some timepoints did not show a significant improvement of UPDRS-III scores through medication in the experiment. Notwithstanding this, the decrease of UPDRS-III through medication was highly significant across patients, when assessed with Wilcoxon’s signed rank tests (P < 0.007 at all timepoints). For OFF medication recordings, patients underwent a 12-h withdrawal from all dopaminergic medication. In three cases pramipexole treatment may have mildly affected the OFF state, as it has a half time of 8–12 h (case 2: 1.05 mg; case 7: 0.35 mg; case 9 1.4 mg). All other cases did not take dopamine agonists. LFPs were amplified (×2000), filtered at 1–100 Hz, and recorded at a sampling rate of either 422 or 800 Hz onto the pulse generator. During recordings, patients were seated comfortably in an armchair. LFP recordings of approximately one-minute length were obtained for each bilateral contact pair in subsequent sessions and cut to exact 60 s segments for analyses. Deep brain stimulation was turned off at least 30 min before the first recording. All data were temporarily stored to the IPG for the time of recording and thereafter downloaded to a personal computer for offline analysis using low frequency proximal (~4 cm) telemetry. Short recording lengths were chosen to keep battery discharge related to telemetric data transfer minimal. All sampled data traces were downsampled to 422 Hz where necessary and visually inspected for artefacts. Cardioelectric pulse artefacts were present in the majority of recordings from contact pairs 01, which led to the exclusion of all 01 contact pairs from the analysis. Segments with artefacts were rejected from the remaining contact pairs, leaving 54.3 ± 1 s (MEAN ± S.E.M.) per recording and data were analyzed using custom MATLAB (The Mathworks, Natick, MA, USA) code based on SPM12 for magnetoencephalography/ electroencephalography (Wellcome Trust Centre for Neuroimaging, UCL, London, UK) and FieldTrip (Donders Center for Cognitive Neuroimaging, University Nijmegen, Nijmegen, the Netherlands). The continuous rest recordings were divided into arbitrary epochs of 1 s (422 samples) and transferred into the frequency domain using Fourier transform-based methods. This resulted in a frequency resolution of 1 Hz over 100 frequency bins. Power-spectra were normalized to the percentage of total power of 5–45 Hz and 55–95 Hz and are further expressed as percent of total power (%). Therefore, the power in each power spectrum was summed across the frequency ranges 5–45 Hz and 55–95 Hz. Power values in each frequency bin were consecutively divided by that sum and multiplied with 100 resulting in % values representing a weight of each frequency bin on the spectrum. The 0–5 Hz and 45–55 Hz ranges were omitted to avoid contamination by movement artefact and mains noise, respectively. Relative rather than absolute power was analyzed to allow comparison across subjects, as absolute power is more likely to be dependent on the proximity to the oscillating neuronal population (LFP source) than relative power and to vary with local tissue properties. The higher frequency ranges (55–95 Hz) were not further evaluated in this study, as no relevant high frequency oscillatory activity was found in the data and the focus of this study was the long-term association of beta activity with PD symptoms. For a representative raw data trace from case 2 from contact pair 12 of the right hemisphere and the resulting power spectrum and time frequency plot see Fig. 1. Power spectra averaged across contact pairs per electrode (contact pairs 12 and 23) were visually inspected for peaks in the 13–35 Hz range. The OFF state peak frequency was used for further analysis, because all recording sites revealed beta peaks in the medication OFF state, but 7 of 24 recording sites did not show any peaks ON medication at each of the timepoints. In the remaining spectra average peak frequencies deviated from –0.7 Hz to 0.6 Hz ON medication, when compared to OFF medication across timepoints. Therefore, we analyzed a 3 Hz frequency window surrounding the peak. In many recordings a high noise floor was observable, leading to low amplitudes in peaks. To avoid any selection bias, the highest peak, defined as frequency bin higher than the surrounding bins was chosen for each recording time point. For visualization purposes power was averaged across all patients and smoothed (Fig. 2A–C). To highlight peak power differences, we aligned all spectra to the peak frequency per electrode and stacked them (Neumann et al., 2016b) (see Fig. 2D–F for averaged stacked data). All statistical analyses were conducted on averaged peak amplitude values from peak frequency and the two adjacent bins surrounding the peak. This method was chosen as peak beta power has been shown to be a more consistent marker for parkinsonian symptom severity, than averaged beta power. (Beudel et al., 2017; Neumann and Kuhn, 2017) and the high noise floor of the system would have dominated spectral averages in broader frequency ranges. Kolmogorov Smirnov tests revealed that the peak amplitudes were not normally distributed across patients. Therefore, non-parametric Wilcoxon’s signed rank tests were used to investigate the effect of medication on peak power at each timepoint. Non-parametric Kruskal-Wallis tests were conducted to test for a potential effect of the timepoint of the recording for each medication condition. All comparison P-Values were false discovery rate corrected for multiple comparisons (Benjamini et al., 2006).
Table 1.
Patient details.
| N | Age at OP | Sex | Disease duration (y) | UPDRS-III preop OFF/ON | UPDRS-III Baseline** mOFF/mON | UPDRS-III 3 months** FU mOFF/mON | UPDRS-III 8 months** FU mOFF/mON | Medication (LED) at 8 months FU | DBS effect at 8 months FU (% preop UPDRS) | Stimulation parameters at 8 months FU |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M | 10 | 46/26 | 43.5/16.5 | 34.5/25.5 | 35.5/24 | 1375 mg | 68 | L: 2-, 2 V; R: 2-,2.2 V; 60 μs; 130 Hz |
| 2 | 63 | M | 15 | 49/35 | 25/20.5 | 26/25 | 25.5/25 | 105 mga | 56 | L: 2-, 3 V; 2-,3-, 3.2 V; 60 μs; 130 Hz |
| 3 | 63 | F | 8 | 56/16 | 24/15.5 | 48/31.5 | 42/24 | 665 mg | 62 | L: 3-, 3.2 V; R: 1-, 3.5 V; 60 μs; 130 Hz |
| 4 | 66 | M | 8 | 26/17 | 28/29 | 20/18.5 | 19.5/12.5 | 1064 mg | 48 | L: 1-, 1.5 V; R: 1-, 3.3 V; 60 μs; 130 Hz |
| 5 | 73 | M | 9 | 35/20 | 23/17 | 33/22.5 | 27/18.5 | 1450 mg | 56 | L: 2-, 3.8 V; R: 2–3.9 V; 60 μs; 130 Hz |
| 6 | 63 | M | 16 | 42/29 | 36/32 | 26.5/29 | 39.5/29 | 950 mg | 57 | L: 1-,2-, 3.5 V; R: 1-,2–1.8 V; 60 μs; 130 Hz |
| 7 | 72 | F | 5 | 34/19 | 31/31 | 25.5/20 | 25/14 | 360 mga | 41 | L: 2-, 3.8 V; R: 1–3.9 V; 60 μs; 130 Hz |
| 8 | 61 | F | 10 | 20/4 | 19.5/13 | 18.5/20 | 21.5/13.5 | 500 mg | 43 | L: 2-, 3 V; R: 2–1 V; 60 μs; 130 Hz |
| 9 | 58 | F | 7 | 34/24 | 24/16 | 32.5/27.5 | 37/23.5 | 52 mga | 43 | L: 2-, 3 V; R: 2–1.8 V; 60 μs; 130 Hz |
| 10 | 56 | M | 18 | 41/20 | 20/16 | 51.5/33.5 | 41/17.5 | 665 mg | 45 | L: 1-, 2.2 V; R: 1–3.5 V; 60 μs; 130 Hz |
| 11 | 63 | M | 9 | 31/14 | 19/15.5 | 18/12 | 17/13.5 | 1207 mg | 60 | L: 1–2.8 V; R: 1–2.7 V; 60 μs; 130 Hz |
| 12 | 60 | M | 7 | 59/32 | 28.5/10 | 35/26.5 | 31.5/20 | 1325 mg | 80 | L: 1-, 3 V; R: 1-, 3.2 V; 60 μs; 130 Hz |
| Mean | 63.2 | 4 F | 10.2 | 27/19 | 31/24 | 30/20 | 817.2 mg | 54.9% |
All UPDRS-III scores were obtained at the time of recording after at least 30 min OFF DBS.
Cases 2/7/9 were on pramipexole treatment (1.05 mg/0.35 mg/1.4 mg); LED = Levodopa Equivalent Dose.
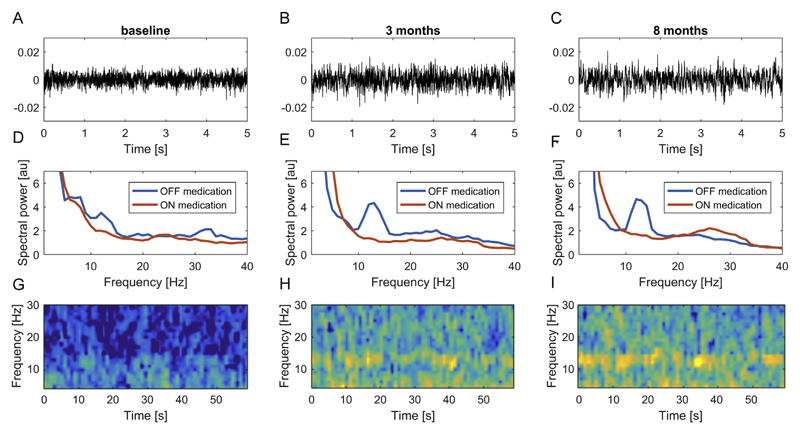
Fig. 1.
Representative example of subthalamic resting LFP data. Raw LFP data in the dopaminergic OFF condition from the right contact pair 12 in case 2 are shown for baseline (A), 3 months (B) and 8 months (C) conditions. The middle row (D-F) shows the resulting power spectra including the ON medication condition. Note that oscillatory activity can be visually identified in the time frequency representations (G-I) for OFF recordings, most notably at 3 (H) and 8 months (I).
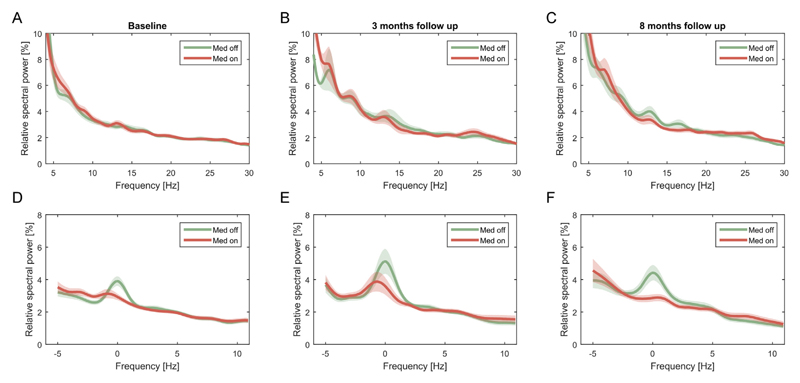
Fig. 2.
Average and peak aligned spectra for all three time points ON and OFF medication. Averaged power spectra failed to reveal distinct peaks at all time points (A–C), but clear peaks could be visualized in the medication OFF condition, when spectra were aligned to the peak centres (labelled 0 Hz) before averaging (D–F). Shaded areas depict S. E.M. across subjects.
Spearman correlation was calculated between the same beta peak amplitude averages of contact pairs 12 and 23 OFF and ON medication and UPDRS – III hemibody scores for all 24 hemispheres at each time point separately and all time points pooled. Furthermore, alleviation of motor signs through dopaminergic medication (UPDRS-III OFF – UPDRS-III ON) was correlated with beta peak power suppression (Peak power OFF – peak power ON). Statistical significance of all correlations was determined by Monte Carlo permutation (Good, 2005). Therefore, a test statistic was generated by calculating 10,000 replications of Spearman correlations from averaged spectral power and UPDRS scores with positions of UPDRS values randomly exchanged. P values are reported as the position of the original correlation coefficient in the distribution of the test statistic (Neumann et al., 2016a). All correlation P-Values were FDR corrected for multiple comparisons.
3. Results
Subthalamic deep brain stimulation lead to a 54.9% ± 3.4% (MEAN ± S.E.M) reduction. of UPDRS-III scores after 8 months of chronic stimulation (P < 0.001; preop UPDRS-III OFF medication: 39.4 ± 3.4; ON subthalamic stimulation, OFF dopaminergic medication at 8 months follow-up: 16.9 ± 1.2). When stimulation was turned off, medication still decreased UPDRS-III scores significantly at each timepoint (P < 0.007). Peaks in the beta frequency band were present in all patients (see Fig. 1 for an example of raw data traces at three recorded time points) in the medication OFF condition with a mean peak frequency of 17.6 Hz ± 1.0. In the ON medication condition, 7/24 recording sites did not show a peak at each timepoint. Furthermore, a peak frequency shift of 1 Hz was found in the ON medication condition in 4/2/2 of the remaining 17 recording sites at baseline, 3 months and 8 months, respectively. Averaged power spectra are shown in Fig. 2. Because of the low amplitude of the peaks and the variability in their precise peak frequency, no clear beta activity difference was visible in the group spectra, therefore we realigned all averaged spectra for each hemisphere to the peak frequency in the beta band OFF medication and stacked them for visualization. Individual peak frequencies were assessed across time points with an individual average absolute peak frequency change of 3.2 Hz ± 0.9 Hz between the three recorded time points (MEAN peak frequency at surgery, 16.6 ± 1.2 Hz; 3 months 17.8 ± 1.3 Hz; 8 months 18.3 ± 1.5). However, no statistically significant changes in peak frequency were observed for any time point. The Kruskal-Wallis tests revealed no significance for the effect of timepoint on beta peak power, neither ON, nor OFF medication (P > 0.1). Medication robustly suppressed beta peak amplitude (Fig. 3; MEAN power OFF 3.78 ± 0.2% vs ON 3.02 ± 0.2%.) with significant FDR corrected Wilcoxon signed-rank tests for each time point (P < 0.007 at all time points). Correlation analysis revealed a significant correlation of individual peak beta amplitude with pooled UPDRS-III hemibody scores across subjects, medication condition and time points (Fig. 4; ρ = 0.25, P = 0.0002). The correlation remained significant if only 3 months’ (ρ = 0.32, P = 0.01) and 8 months’ data (ρ = 0.36, P = 0.004) were considered. The correlation of change in UPDRS scores through medication with change in beta power did not reach significance.
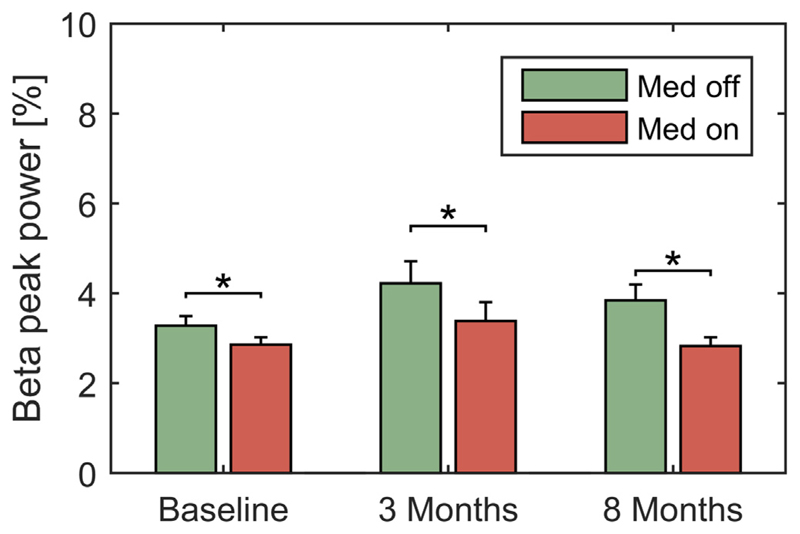
Fig. 3.
Beta peak amplitude is significantly reduced by medication at all time points. Averaged beta peak amplitudes were significantly modulated by medication across timepoints as revealed by Wilcoxon’s signed rank tests for baseline (P < 0.001), 3 months (P < 0.001) and 8 months (P = 0.0068) conditions after FDR correction for multiple comparisons. Error bars depict S.E.M. across subjects.
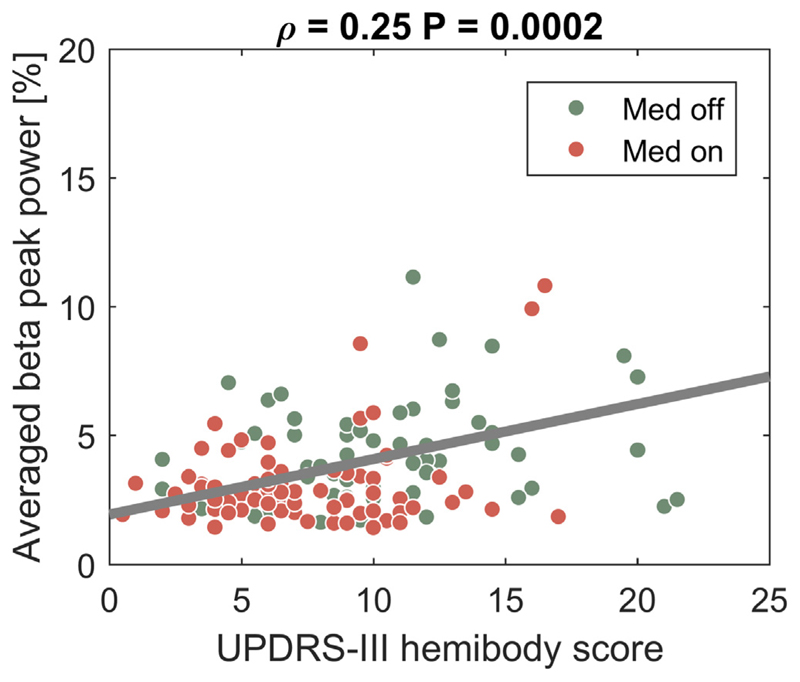
Fig. 4.
Beta peak amplitude is significantly correlated with parkinsonian motor signs across recording time points and medication conditions. A significant relation between UPDRS-III hemibody scores in the medication OFF (red) and ON (green) state with beta peak power averaged across the contralateral hemisphere was found across the three recording time points (Rho = 0.25, P = 0.0002). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We have demonstrated that beta oscillations can be consistently recorded in the subthalamic nucleus over 8 months in patients with Parkinson’s disease. Moreover, we have shown that beta peak frequency is stable across this time frame and that dopaminergic medication repeatedly suppressed beta activity at each time point. Finally, we have replicated a recent report that has shown that beta activity is directly correlated with parkinsonian motor signs as assessed by UPDRS-III. (Neumann et al., 2016a) Importantly, we extend the previous study to correlations including using clinical rating scores obtained directly at the time point of the recording, while our previous study in a large cohort of PD patients used preoperative archival UPDRS scores. Furthermore, both medication ON and OFF data were pooled for the correlation.
Before considering the clinical implications of our findings, we would like to highlight some of the most important limitations of our study. First of all, three subjects had to be excluded from the study because they declined to undergo recordings OFF medication at baseline or follow up. Furthermore, UPDRS-III ratings were not blinded to the stimulation condition in our report, which would have increased the objectiveness of the scores. Beta peak amplitudes were relatively small, which can be attributed to the lower amplification (2.000x), compared to externalized recordings (9.000x–50.000x), the higher noise floor of the system, and the short recording durations. This may have significantly more impact on the ON medication recordings, because suppressed beta values may have fallen under the noise floor and may thus not reflect the optimal quantitative estimation. This could explain that no significance for the correlation of symptom alleviation through dopaminergic medication with beta suppression. To avoid a selection bias, we chose the highest peak in the spectrum regardless of peak definition at other time points. Furthermore, as peaks were lacking in some of the recordings in the ON medication condition, peaks were defined in the medication OFF state. Future generations of implantable sensing enabled pulse generators should therefore increase the signal to noise ratio, by improving the noise floor and increasing amplification gain to robustly sense fluctuations in beta power (Neumann et al., 2016b). Nevertheless, the current system has enabled a number of studies on beta oscillatory activity in Parkinson’s disease. Thus it was recently shown that beta activity remains stable across different postures but is reduced by walking (Quinn et al., 2015). More importantly, the same study reported a voltage dependent decrease of beta activity through DBS, corroborating previous reports of DBS related beta suppression (Quinn et al., 2015; Neumann et al., 2016b). Here we show that correlations between beta amplitude and motor impairment remain consistent over time, even after successful long-term DBS. Converging evidence points to the potential usefulness of beta activity as a long term biomarker for concurrent symptom severity in PD. This could potentially be utilized for closed loop adaptive stimulation, which, in preliminary acute studies, has been shown to be even more efficient in symptom alleviation, when compared to continuous stimulation (Little et al., 2013), and to have potentially fewer stimulation-induced side effects such as dyskinesia (Rosa et al., 2015) and speech disturbance (Little et al., 2016a,b). Notwithstanding this, closed loop stimulation has not yet been tested in a clinical study with an implantable device and its’ efficacy may depend on more complex factors than resting beta activity, such as movement induced oscillatory modulations and additional spectral features. Divergent to previous studies (Rosa et al., 2011; Abosch et al., 2012; Trager et al., 2016), we did not find a significant reduction in beta activity over time. However, unlike our cohort symptom severity was improved over time even OFF stimulation in the previous report (Trager et al., 2016), which may have been obscured by the stun effect that may mimic subthalamic DBS in the perioperative phase. Importantly, this discrepancy is line with the notion that beta activity is correlated with the present symptom severity in patients with Parkinson’s disease.
5. Conclusions
We could confirm that beta oscillatory amplitudes in long term follow up recordings are correlated with symptom severity in PD patients across pooled time points and medication conditions. Future studies will have to investigate the robustness of beta activity during DBS as a measure of concurrent parkinsonian symptom severity to corroborate its role as a biomarker for long term adaptive stimulation.
Highlights.
Subthalamic beta activity was recorded with an implantable DBS pulse generator over 8 months in 12 patients with Parkinson’s disease.
Dopaminergic medication suppresses subthalamic beta activity at operation, 3 and 8 months after DBS.
Beta activity correlates with parkinsonian symptom severity over time.
Acknowledgements
WJN, FS, JS, AH and AAK were funded by the German Research Foundation (DFG, grant KFO 247). PB was funded by the Medical Research Council and National Institute for Health Research Oxford Biomedical Research Centre. Medtronic provided all pulse generators for free.
Funding
This work was supported by the German Research Foundation (DFG, grant KFO 247).
Footnotes
Conflict of interest
Medtronic provided all pulse generators for free.
References
- Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery. 2012;71:804–14. doi: 10.1227/NEU.0b013e3182676b91. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- Beudel M, Oswal A, Jha A, Foltynie T, Zrinzo L, Hariz M, et al. Oscillatory beta power correlates with akinesia-rigidity in the Parkinsonian subthalamic nucleus. Mov Disord. 2017;32:174–5. doi: 10.1002/mds.26860. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Brown P. Oscillations and the basal ganglia: motor control and beyond. Neuroimage. 2014;85:637–47. doi: 10.1016/j.neuroimage.2013.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21:1033–8. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol. 2010;20:156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Good P. Permutation, parametric and bootstrap tests of hypotheses. Springer Science+Business Media, Inc.; 2005. [Google Scholar]
- Horn A, Kuhn AA. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127–35. doi: 10.1016/j.neuroimage.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Jakab A, Blanc R, Berenyi EL, Szekely G. Generation of individualized thalamus target maps by using statistical shape models and thalamocortical tractography. AJNR Am J Neuroradiol. 2012;33:2110–6. doi: 10.3174/ajnr.A3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23:1956–60. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–7. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Little S, Beudel M, Zrinzo L, Foltynie T, Limousin P, Hariz M, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016a;87:717–21. doi: 10.1136/jnnp-2015-310972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann N Y Acad Sci. 2012;1265:9–24. doi: 10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74:449–57. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Tripoliti E, Beudel M, Pogosyan A, Cagnan H, Herz D, et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry. 2016b;87:1388–9. doi: 10.1136/jnnp-2016-313518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann WJ, Degen K, Schneider GH, Brucke C, Huebl J, Brown P, et al. Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease. Mov Disord. 2016a;31:1748–51. doi: 10.1002/mds.26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann WJ, Kuhn AA. Subthalamic beta power-Unified Parkinson’s disease rating scale III correlations require akinetic symptoms. Mov Disord. 2017;32:175–6. doi: 10.1002/mds.26858. [DOI] [PubMed] [Google Scholar]
- Neumann WJ, Staub F, Horn A, Schanda J, Mueller J, Schneider GH, et al. Deep brain recordings using an implanted pulse generator in Parkinson’s disease. Neuromodulation. 2016b;19:20–4. doi: 10.1111/ner.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Beudel M, Zrinzo L, Limousin P, Hariz M, Foltynie T, et al. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson’s disease. Brain. 2016;139:1482–96. doi: 10.1093/brain/aww048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, et al. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov Disord. 2015;30:1750–8. doi: 10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- Rosa M, Arlotti M, Ardolino G, Cogiamanian F, Marceglia S, Di Fonzo A, et al. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov Disord. 2015;30:1003–5. doi: 10.1002/mds.26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, et al. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson’s disease in hyperacute and chronic phases. Neurosignals. 2011;19:151–62. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- Trager MH, Koop MM, Velisar A, Blumenfeld Z, Nikolau JS, Quinn EJ, et al. Subthalamic beta oscillations are attenuated after withdrawal of chronic high frequency neurostimulation in Parkinson’s disease. Neurobiol Dis. 2016;96:22–30. doi: 10.1016/j.nbd.2016.08.003. [DOI] [PubMed] [Google Scholar]