Summary
Several prokaryotic Argonaute proteins (pAgos) utilize small DNA guides to mediate host defense by targeting invading DNA complementary to the DNA guide. It is unknown how these DNA guides are being generated and loaded onto pAgo. Here we demonstrate that guide-free Argonaute from Thermus thermophilus (TtAgo) can degrade dsDNA, thereby generating small dsDNA fragments that subsequently are loaded onto TtAgo. Combining single-molecule fluorescence, molecular dynamic simulations and structural studies, we show that TtAgo loads dsDNA molecules with a preference towards a deoxyguanosine on the passenger strand at the position opposite to the 5’-end of the guide strand. This explains why in vivo TtAgo is preferentially loaded with guides with a 5’-end deoxycytidine. Our data demonstrate that TtAgo can independently generate and selectively load functional DNA guides.
Introduction
Argonaute proteins are key components of eukaryotic RNA interference pathways (RNAi; reviewed in (Dueck and Meister, 2014; Swarts et al., 2014b)). These eukaryotic Argonaute proteins (eAgos) utilize small RNA guides to target complementary RNA molecules. Maturation and loading of RNA guides proceed via a series of conversions catalyzed by different enzymes (Dueck and Meister, 2014; Swarts et al., 2014b)). Argonaute proteins are also present in prokaryotes (pAgos) (Makarova et al., 2009; Swarts et al., 2014b), in which they are involved in host defense by interfering with invading DNA (Blesa et al., 2015; Olovnikov et al., 2013; Swarts et al., 2015a; Swarts et al., 2014a; Swarts et al., 2015b). In contrast to RNA-guided RNA-interfering eAgos, a number of recently characterized pAgos uses DNA guides to interfere with DNA targets (Ma et al., 2005; Sheng et al., 2014; Swarts et al., 2015a; Swarts et al., 2014a; Swarts et al., 2015b; Wang et al., 2008a; Wang et al., 2009; Wang et al., 2008b; Yuan et al., 2005; Zander et al., 2014). Unlike the well characterized biogenesis of small RNAs in eukaryotes, it remains elusive how the small interfering DNA guides (siDNAs) are generated and loaded onto pAgo proteins.
Argonaute of the bacterium Thermus thermophilus (TtAgo) uses siDNAs to interfere with plasmid transformation and propagation (Blesa et al., 2015; Swarts et al., 2014a; Swarts et al., 2015b). These siDNA guides are 5’ phosphorylated and are 13 to 25 nucleotides (nt) in length. A large fraction (89%) of TtAgo-bound siDNAs has a deoxycytidine at the 5’-end, suggesting a specific mechanism for guide generation and/or guide loading. TtAgo acquires these siDNAs upon heterologous expression in Escherichia coli, indicating either that siDNA acquisition is dependent on common host factors, shared between T. thermophilus and E. coli, or that TtAgo is solely responsible for processing of its siDNA guides. The latter explanation is supported by the observation that a catalytic double mutant TtAgoD478A,D546A (TtAgoDM) does not acquire siDNAs in vivo (Swarts et al., 2014a).
Here we elucidate the mechanism for guide generation and loading by TtAgo. Apo-TtAgo is able to degrade unstable dsDNA targets, generating short DNA products. These products are selectively loaded onto TtAgo and guide subsequent DNA target cleavage.
Results
Apo-TtAgo degrades partially unwound DNA targets
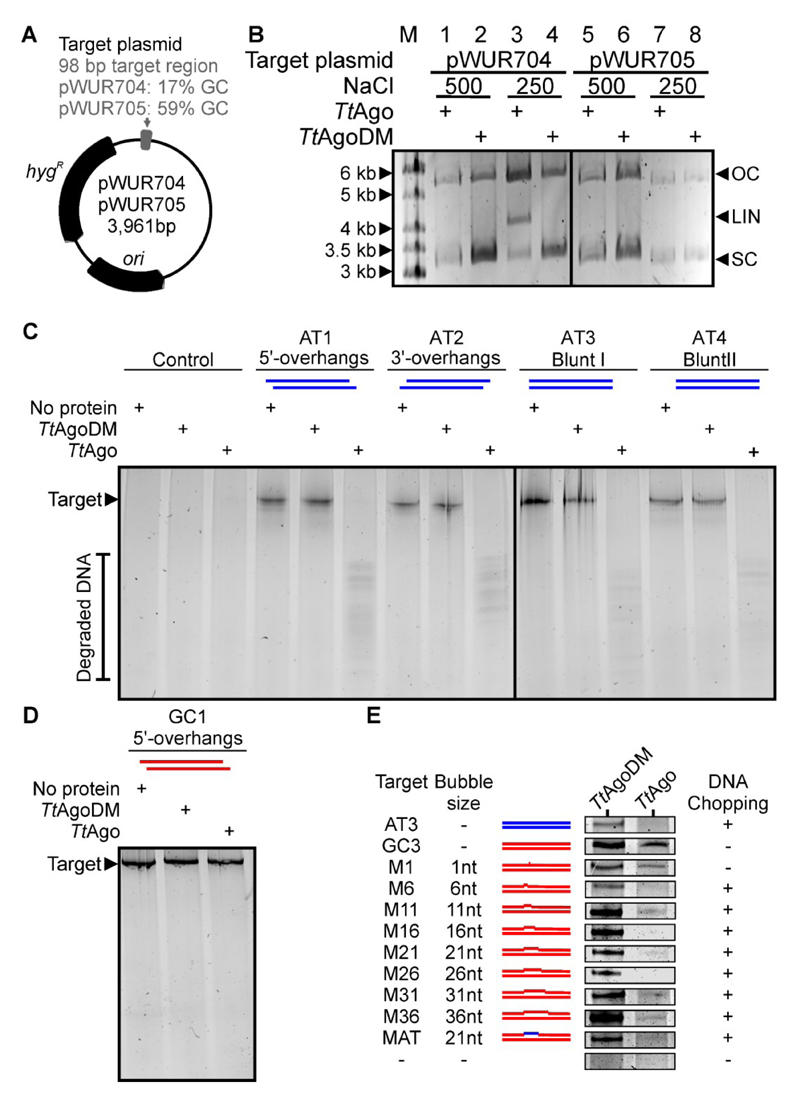
We previously observed that TtAgo co-purifies with siDNAs after heterologous expression in E. coli, whereas no siDNAs co-purify with the catalytically inactive TtAgoDM (Swarts et al., 2014a). Based on that result, we investigated whether apo-TtAgo has the potential to cleave plasmid DNA. TtAgo (with an N-terminal strep tag II) was purified using a modified protocol that precluded co-purification of cellular nucleic acids. We incubated the guide-free TtAgo with plasmids pWUR704 and pWUR705 (Figure 1A) at various NaCl concentrations (Figure 1B). In a low salt buffer, it was observed that apo-TtAgo is able to linearize pWUR704 (AT-rich insert), but not pWUR705 (Figure 1B). This cleaving activity was not observed for TtAgoDM, indicating its dependence on the canonical active site of TtAgo. To explore the cleavage mechanism, we incubated apo-TtAgo with each of the ssDNA strands that form the pWUR704 insert. We did not detect any degradation of these ssDNA strands (Figure S1A), indicating that apo-TtAgo-mediated degradation of pWUR704 does not rely on the presence of undetectable co-purified siDNAs. Furthermore, it implies that the double-stranded nature of plasmid DNA is required for apo-TtAgo mediated DNA degradation. To distinguish dsDNA degradation by apo-pAgo from canonical target cleavage by pAgo-guide complexes, we coin the term ‘chopping’.
Figure 1. Guide-free TtAgo can cleave unstable DNA.
(A) Schematic representation of target plasmids pWUR704 and pWUR705. ori: Origin of replication. hygR: Hygromycin resistance marker.
(B) Plasmids pWUR704 and pWUR705 incubated with apo-TtAgo in buffer containing 500 mM or 250 mM NaCl resolved on 0.8% agarose gels. M: GeneRuler 1 kb DNA Ladder (Thermo Scientific). OC: Open circular. LIN: Linear. SC: Supercoiled.
(C-E) 98 bp AT-rich dsDNAs (C), a 98 bp GC-rich dsDNA (D) or 98 bp GC-rich dsDNAs with internal mismatches (E), incubated with TtAgo or TtAgoDM and resolved on 20% denaturing polyacrylamide gel. AT-rich and GC-rich DNA is colored blue and red, respectively. ‘Control’ samples include no target DNA, ‘No protein’ samples contain no protein. For a detailed overview of the dsDNA targets, see Table S1. For the uncropped gels of (E) and additional chopping experiments, see Figure S1E.
In line with earlier observations (Swarts et al., 2014a), apo-TtAgo is unable to linearize pWUR704 in a high salt buffer (500 mM NaCl) (Figure 1B). Because siDNA-guided TtAgo can cleave both ssDNA and plasmid DNA targets in a buffer with 500 mM NaCl (Swarts et al., 2014a), these results imply that chopping relies on a mechanism other than canonical guide-mediated target cleavage. The target plasmid pWUR704 that is linearized by apo-TtAgo has a 98 bp insert with a GC content of 17%. In contrast, the target plasmid pWUR705 that is not linearized by apo-TtAgo, has a 98 bp insert with a GC content of 59%. This suggests that pWUR704 is cut in the AT-rich region, which is possibly unwound at the incubation temperature of 65 °C. At high NaCl concentrations, positively charged sodium ions mask the negative charge of the DNA, reducing the repulsion (‘breathing’) between the two DNA backbones (Owczarzy et al., 2004). The resulting increase of dsDNA stability might hamper guide-free plasmid degradation by TtAgo. These findings suggest that apo-TtAgo can only cleave dsDNAs that have a certain degree of unwinding.
To confirm that apo-TtAgo chops unstable dsDNA, apo-TtAgo was incubated with various 98 bp long dsDNA fragments (Table S1 and Table S2). GC-poor dsDNA fragments identical to the pWUR704 insert (17% GC) are completely chopped into multiple small DNA fragments (Figure 1C). Chopping is dependent on the canonical catalytic site of TtAgo, as the catalytic mutant TtAgoDM is unable to chop dsDNA (Figure 1C). Like guide-free TtAgo-mediated plasmid linearization, the GC-poor dsDNA target is chopped by TtAgo in a low salt buffer (250 mM NaCl), but not in high salt buffers (500 mM or 750 mM NaCl) (Figure S1B). DNA overhangs do not appear to play a role during chopping since targets with blunt ends, 5’ overhangs, and 3’ overhangs are all chopped by apo-TtAgo (Figure 1C).
TtAgo does not efficiently chop GC-rich dsDNA fragments identical to the pWUR705 insert (59% GC) (Figure 1D and Figure S1C). To determine whether DNA instability indeed promotes chopping of the dsDNAs, apo-TtAgo was incubated with GC-rich dsDNAs with internal mismatched regions (1 to 36 bp mismatches, Figure 1E and Table S1). In contrast to perfectly base-paired or single-mismatch GC-rich DNA, GC-rich DNAs with an internal mismatch of 6 bp or larger are completely chopped (Figure 1E, Figure S1D and S1E). These observations confirm that a certain degree of dsDNA unwinding is required for dsDNA chopping by TtAgo.
Canonical target cleavage by TtAgo requires binding of the 5’ phosphate of the siDNA in the MID domain. Replacing the siDNA 5’-end phosphate with a fluorophore prevents canonical target cleavage, probably at the level of guide loading (Figure S1F). In contrast, guide-free TtAgo chops dsDNA targets with 5’-end fluorophores on both strands (Figure S1F), which suggests that loading of the dsDNA 5’-ends onto the MID domain does not play a role during chopping. We also compared the rate of TtAgo-mediated chopping of dsDNA to the rate of canonical TtAgo-siDNA complex-mediated ssDNA cleavage (Figure S1G). This shows that TtAgo-mediated DNA chopping is less efficient than canonical target cleavage.
DNA chopping generates functional DNA guides
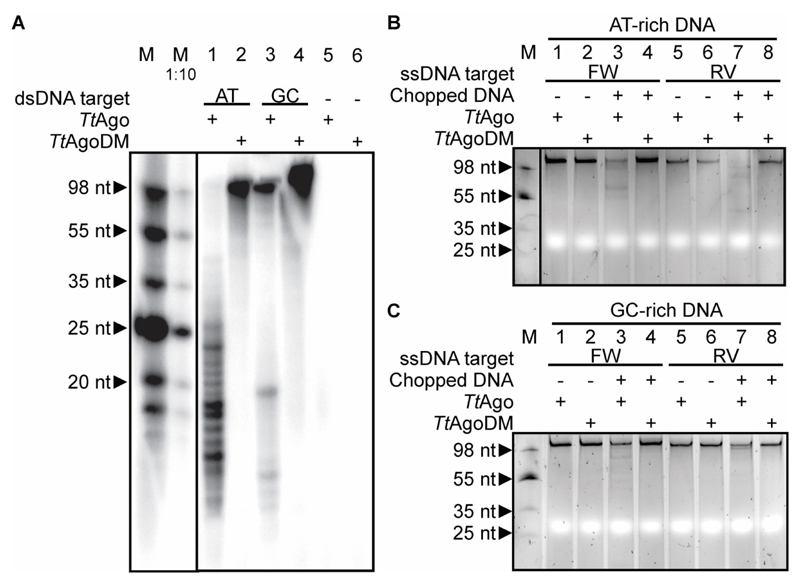
To determine the size of chopping products, they were purified and 5’-end 32P-labeled in a polynucleotide kinase exchange reaction. Remarkably, the DNA chopping products have a length of ~13-25 nucleotides (Figure 2A), which matches very well the size range of siDNAs that have been found to associate with TtAgo in vivo (Swarts et al., 2014a). In addition, this more sensitive detection method reveals that TtAgo also chops the GC-rich target, although at much lower levels compared to the GC-poor target (Figure 2A, lane 3).
Figure 2. DNA chopping generates functional siDNA guides.
(A) AT-rich dsDNA target ‘AT3’ or GC-rich dsDNA target ‘GC3’ (Table S1) were incubated with TtAgo or TtAgoDM, after which the purified and [γ-32P] ATP labeled nucleic acids were resolved on a 20% denaturing polyacrylamide gel. M: ssDNA marker. M 1:10: 10 times diluted ssDNA marker.
(B) TtAgo incubated with chopped AT-rich dsDNA (as in panel a, lane 1) and ssDNA targets. The ssDNA targets have the same sequence as the forward (FW, BG4262) or reverse (RV, BG4724) strand of the chopped dsDNA.
(C) TtAgo incubated with chopped GC-rich dsDNA (as in panel a, lane 3) and ssDNA targets. The ssDNA targets have the same sequence as forward (FW, BG4264) or reverse (RV, BG4726) strand of the chopped dsDNA. M: ssDNA marker.
For sequence analysis of the generated fragments, see Figure S2.
We verified whether DNA chopping generates products that guide TtAgo activity. TtAgo was pre-incubated with purified DNA chopping products, after which ssDNA targets complementary to the chopped DNA were added. Chopped AT-rich DNA (Figure 2B, lanes 3 and 7) and, to a lesser extent, chopped GC-rich DNA (Figure 2C, lanes 3 and 7) is utilized by TtAgo to guide ssDNA target cleavage. As cleavage is observed in ssDNA targets identical to both the forward (FW) and the reverse (RV) strands of the chopped dsDNA, it is concluded that siDNAs are acquired from both strands of chopped dsDNA. These results indicate that chopping of dsDNA results in the generation of functional siDNAs.
DNA chopping is governed by DNA instability rather than by specific DNA sequences
When expressed in E. coli, TtAgo is preferentially loaded with guides with a 5’-end deoxycytidine (hereafter termed g1C for guide with nucleotide 1 deoxycytidine) (Swarts et al., 2014a). To investigate if DNA chopping preferentially generates fragments with a 5’-end deoxycytidine, TtAgo-chopped DNA was successfully cloned into vectors, indicating that the DNA is present as dsDNA after chopping by TtAgo. A total of 120 plasmid inserts (harboring one or more chopped DNA fragments) were sequenced. The shortest inserts were only 8 bp long, while the longest were intact 98 bp targets. GC-poor DNA chopping generated considerably shorter inserts (average length 17 bp (±11.6)) compared to GC-rich DNA chopping (average length 59 bp (±33.6)). These findings are in agreement with the difference in efficiency at which these targets were chopped by TtAgo (Figure 2B and 2C). The 5’-ends of the inserts allowed for the identification of a total of 202 and 48 chopping sites for GC-poor and GC-rich target DNAs, respectively (Figure S2). Although there seems to be a preference for generating 5’-end deoxycytidines in the AT-rich dsDNA target (26% observed versus 10% randomly expected; Figure S2C), the chopping position is not strictly determined by recognition of specific sequences.
Chopping positions appear to be enriched at the 5’ border of GC-rich stretches within the GC-poor DNA target (Figure S2A and S2B). The 10 bp upstream the observed chopping sites have a GC-content comparable to what would be expected if the same dsDNA target would be chopped at random locations (17% observed vs 18% random; p = 0.45; Figure S2H). In contrast, the 10 bp downstream the cleavage site have a GC content significantly higher than what would be expected if the dsDNA target would be randomly chopped (35% observed vs 18% random; p < 0.001; Figure S2D). The necessity of GC-poor dsDNA upstream the chopping site most likely reflects the aforementioned requirement for DNA unwinding for DNA chopping.
TtAgo has no preference for a specific 5'-end deoxynucleoside of a guide DNA
As chopping does not strictly generate fragments with a 5’-deoxycytidine, we hypothesized that TtAgo preferentially binds g1C siDNAs with a nucleotide specificity loop, as has been described for some eAgos (Frank et al., 2012; Frank et al., 2010).
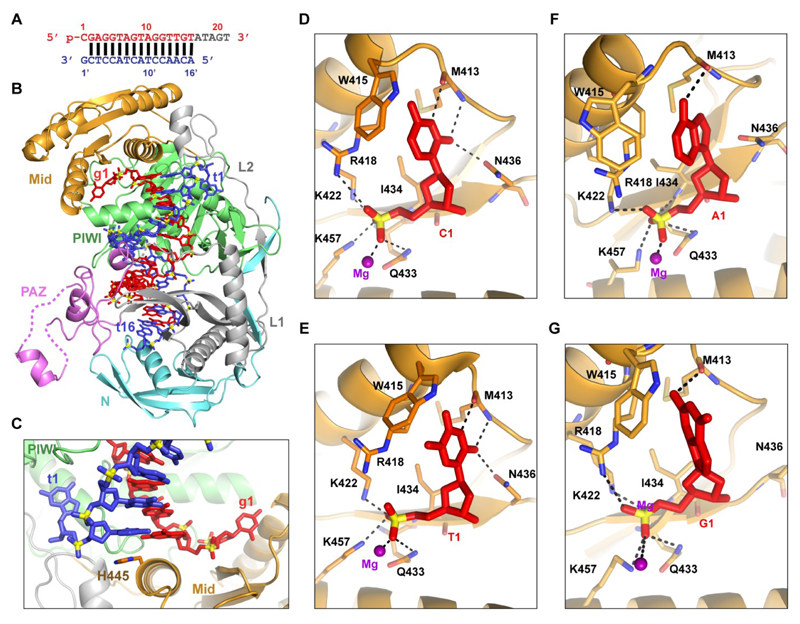
The available crystal structures of TtAgo bound to a siDNA contain a g1T siDNA. To address the specificity of the guide 5’-end nucleotide, we set out to solve the crystal structure of TtAgo bound to a 21 nt g1C siDNA and a complementary 16 nt ssDNA target. In the obtained 2.7 Å crystal structure (schematic in Figure 3A; structure in Figure 3B; x-ray statistics in Table 1), the guide DNA strand (in red) can be traced from nucleotide positions 1 to 16. The target DNA strand (in blue) can be traced from nucleotide positions 1’ to 16’, with guide-target pairing spanning base pair positions 2-2’ to 16-16’. The terminal base pair 2-2’ of the guide-target duplex is stacked over the side chain of H445, while the base of g1C and the opposing base of target strand nucleotide 1 (t1G for target with nucleotide 1 deoxyguanosine) are splayed out relative to the g2G-t2C base pair. g1C and t1G are positioned in separate binding pockets in the MID and PIWI domains, respectively (Figure 3C). The 5’-end phosphate of the guide is anchored in the MID binding pocket via hydrogen bonds and salt bridges formed with the residues K457, K422, Q433, I434 and R418, and a Mg2+ atom bound in this pocket (Figure 3D). The base of g1C forms hydrogen bonds with the main chain of M413 and the side-chain of N436.
Figure 3. Ternary complex of TtAgo bound to a 21 nt siDNA with g1C and a 16 nt ssDNA target complementary to the guide strand.
(A) Sequence of 5’-phosphorylated guide DNA (red, with disordered segment in gray) and complementary target DNA (blue).
(B) 2.7 Å ternary complex of TtAgo bound to guide DNA and 16-mer target DNA (PDB 5GQ6). The guide DNA and target DNA are in a stick representation, with same colors as in (A). See also Table 1.
(C) The 5’-end phosphorylated guide DNA deoxycytidine (g1C) and the opposing nucleotide on the target DNA strand (t1G) are splayed out relative to the g2G-t2C base pair and are positioned in separate binding pockets in the MID and PIWI domains, respectively.
(D and E) TtAgo MID domain residues interacting with the guide 5’-end phosphate and deoxycytosine (g1C, (D), PDB 5GQ6) and thymidine (g1T, (E), PDB 4NCB) residues of the guide strand (red).
(F and G) Modeled structures of TtAgo MID domain residues interacting with the guide 5’-end phosphate and deoxyadenine (g1A, (F)) and deoxyguanine (g1G, (G)). The model is based on molecular dynamics simulations of the crystal structure of TtAgo with 21 nt g1C guide strand and 16 nt target strand (PDB 5GQ6). See also Figure S3.
Table 1. Crystallographic data collection and refinement statistics.
| Ago-7C | |
|---|---|
| Data collection | |
| Space group | P212121 |
| Cell dimensions | |
| a,b, c (Å) | 111.8, 116.5, 161.0 |
| α, β, γ (º) | 90, 90, 90 |
| Resolution (Å) | 100-2.7 (2.75-2.7) |
| Rsym or Rmerge | 0.057 (0.418) |
| I/σI | 22.5 (2.2) |
| Completeness (%) | 98.7 (97.6) |
| Redundancy | 4.5 (3.3) |
| Refinement | |
| Resolution (Å) | 40-2.7 |
| No. reflections | 56,058 |
| Rwork/Rfree | 19.2/24.3 |
| No. atoms | |
| Protein | 10,289 |
| Ligand/ion | 1320 |
| Water | |
| B-factors | |
| Protein | 51.4 |
| Ligand/ion | 55.6 |
| Water | |
| R.m.s deviations | |
| Bond lengths (Å) | 0.005 |
| Bond angles (º) | 0.995 |
Highest resolution shell is shown in parentheses.
We compared the current ternary complex (TtAgo with 16 nt target strand and 21 nt g1C guide strand, Figure 3D) with previously determined analogous ternary complex (TtAgo with 16 nt target strand and 21 nt g1T guide strand, Figure 3E) (Sheng et al., 2014). The overall structures of these two ternary complexes are very similar; g1T, like g1C, forms hydrogen bonds with the main chain of M413 and side-chain of N436. These findings imply that TtAgo is unable to structurally distinguish a 5’-end deoxycytidine from a 5’-end thymidine.
To further investigate the role of the g1N base, we performed a series of molecular dynamics simulations with models in which the g1N or the t1N were varied (Tables S3 and S4). Two sets of simulations with or without t1N/g1N variances from different initial crystal structures (PDB 4NCB and PDB 5GQ6) were performed. The two sets of simulations give similar results, confirming the validity of these simulations (Table S4 and Figure S3). Mutation of g1N and t1N did not significantly affect the overall conformation of complexes as shown in our ~100 ns simulations. However, the hydrogen bonding networks among the two bases and the TtAgo protein are reorganized when the bases are mutated. As a result, three to five stable hydrogen bonds can be formed between TtAgo and a g1C base, one to two between TtAgo and a g1T base or g1A base, and none or one between TtAgo and a g1G base (Figure S3A and S3B). Differences between the g1N bases and the interacting amino acids may affect the strength of the corresponding hydrogen bonds. The base of g1A forms one hydrogen bond with the oxygen atom and none with the nitrogen atom of M413. The interaction between g1G and the backbone of M413 is similar to g1A. However, the side-chain of M413 is additionally rotated away from the binding pocket (Figure 3F and 3G). It should be mentioned that the strength of an H-bond depends on its local environment, and that the number of H-bonds does not necessarily reflect binding energy. Still, the combined data on the number of interactions in the simulations and the structures (three stable hydrogen bonds between TtAgo and both g1C and g1T bases) suggest that the affinity between the g1N bases and TtAgo follows C≅T>A>G (Figure 3 and S3), which can be tested with further experimental studies. It should, however, be noted that there are more interactions between TtAgo and the 5’-end phosphate than between TtAgo and the g1N bases. This might explain why the g1N base does not significantly affect TtAgo activity (Swarts et al., 2014a).
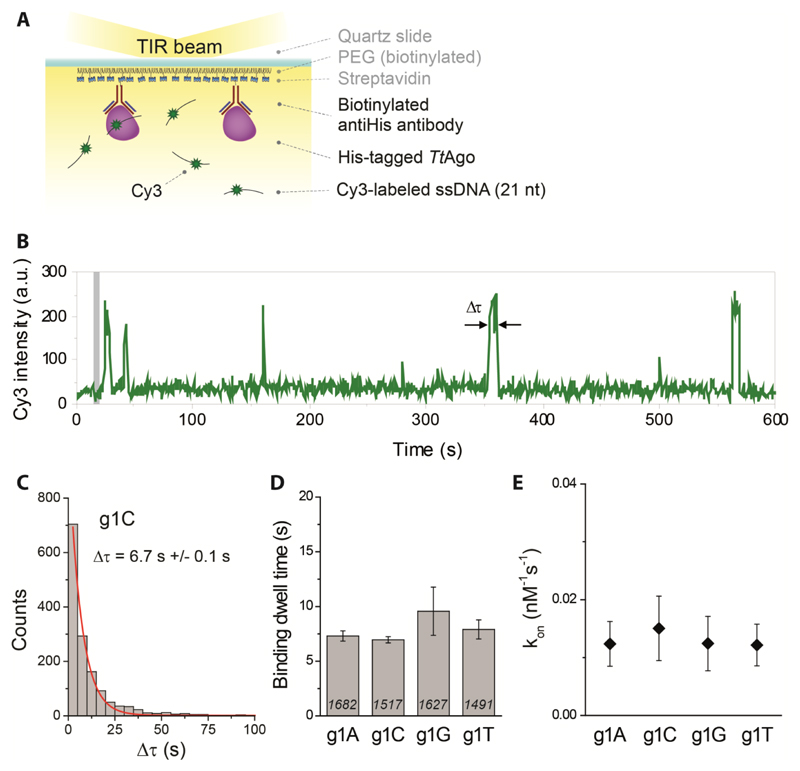
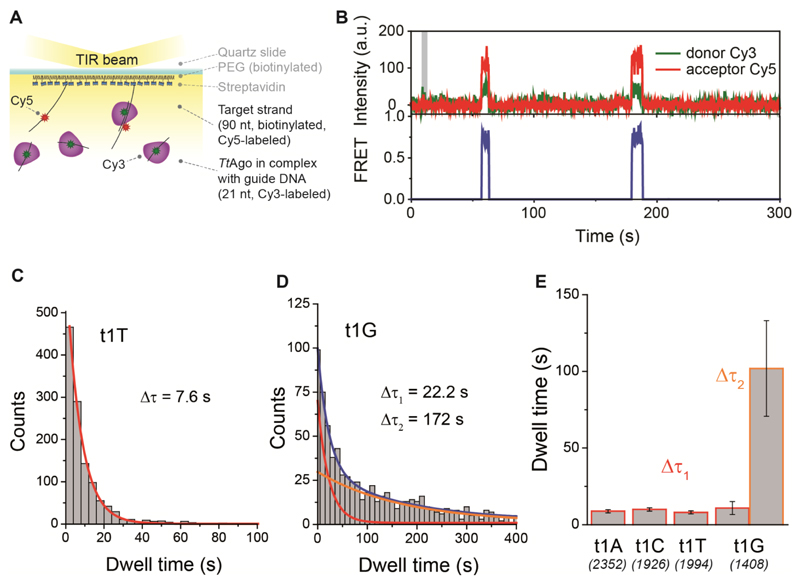
To further corroborate our structural interpretation, we performed single-molecule fluorescence measurements to determine if guides with specific 5’-end deoxynucleosides are preferred by TtAgo. We immobilized His-tagged TtAgo on a polymer-coated quartz surface of four separate microfluidic chambers using a biotinylated anti-His antibody (Figure 4A). In each chamber we introduced one variant of a 5’-end phosphorylated, 21-nt long Cy3-labeled ssDNA, with the first nucleotide (g1N) on the 5’-end being one of the four nucleotides (A, C, G, or T). Individual interactions of ssDNA with TtAgo were recorded in real time using a total internal reflection microscope. The resulting Cy3 fluorescence time traces show short interactions between protein and ssDNA (Figure 4B). Binding dwell times for each ssDNA construct demonstrate a single-exponential distribution (Figure 4C), with fitted mean interaction times ranging from 7.3 to 9.6 s (Figure 4D), showing little variation between guide DNAs with different g1N. A similar conclusion can be drawn from comparison of binding rates (kon, Figure 4E). Combined with the earlier finding that TtAgo can utilize guide DNA with different g1Ns to cleave ssDNA targets equally well (Swarts et al., 2014a), and the aforementioned lack of specificity with respect to the DNA guide g1N in the available crystal structures, these results indicate that TtAgo does not predominantly bind a guide DNA with a g1C. As in vivo TtAgo is mostly loaded with siDNAs with a g1C after heterologous expression, it is concluded that an alternative mechanism for preferential guide loading is in place.
Figure 4. TtAgo does not specifically interact with a g1C.
(A) (left panel) TtAgo immobilization scheme. (right panel) Sequence of the guide DNA. The Cy3 fluorophore is positioned on the 9th nt of the guide DNA, counting from the guide DNA 5'-end.
(B) Representative Cy3 fluorescence trace showing multiple short binding events. Binding dwell time, Δτ, is indicated with the arrows and time point at which ssDNA is introduced into the chamber is indicated with a grey bar.
(C) Dwell time distribution of g1C binding events was fitted with a single-exponential function (red line); Δτ - mean dwell time.
(D) Comparison of binding dwell times for all four ssDNA constructs differing only with the first nucleotide on the 5’-end. Numbers in each column represent the number of analyzed TtAgo molecules, while error bars represent SEM, n=3.
(E) Corresponding binding rate (kon) of all four ssDNAs. Error bars represent SEM, n=3.
TtAgo is preferentially loaded with dsDNAs
Although TtAgo utilizes ssDNA guides, it has not been investigated whether it acquires guides from a single-stranded or a double-stranded form of DNA. In living organisms, DNA rarely exists as a single-stranded polymer (Travers and Muskhelishvili, 2005; Watson and Crick, 1953; Zimmerman, 1982). We observed that DNA chopping generates small dsDNA fragments and that apo-TtAgo has only short interactions with ssDNA. We therefore consider it most likely that apo-TtAgo binds dsDNAs for guide acquisition. This would resemble guide acquisition by eAgos, which in many cases are loaded with dsRNA substrates (such as siRNA or miRNA/miRNA* duplexes), after which one strand (the passenger strand) is removed while the other strand (the guide strand) remains bound (Dueck and Meister, 2014).
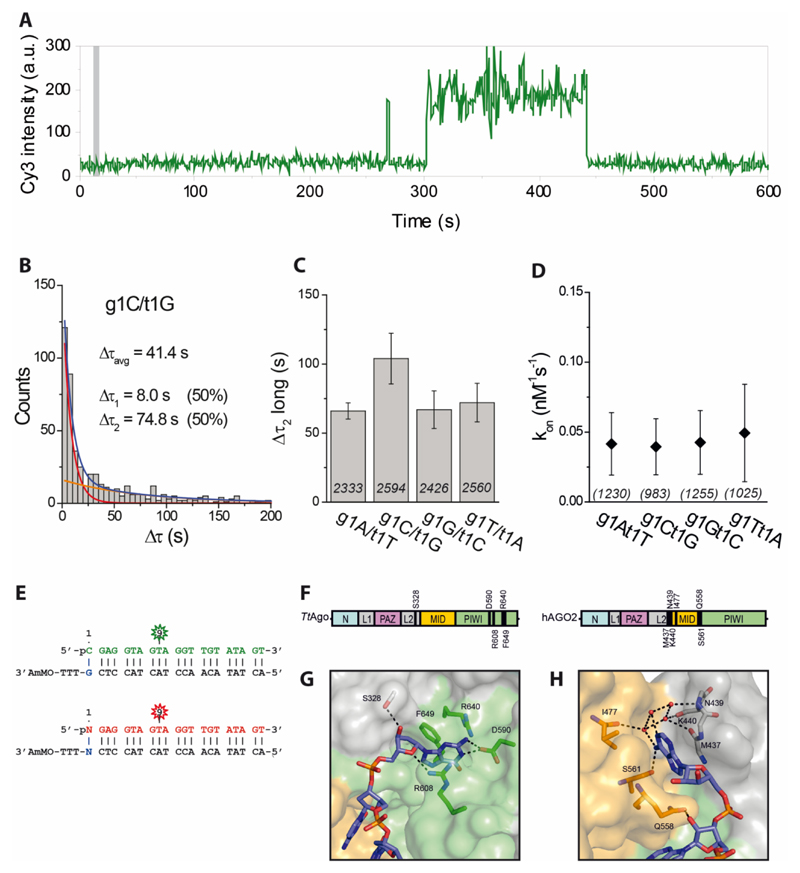
To test our hypothesis, we performed additional single-molecule fluorescence measurements in which apo-TtAgo interacts with dsDNAs. We immobilized His-tagged TtAgo in four separate microfluidic chambers and in each chamber introduced a different dsDNA construct (g1N/t1N, where g1N stands for the 5’-end phosphorylated nucleotide on the guide strand and t1N represents a complementary nucleotide on the target/passenger strand directly opposite of g1N; N stands for A, C, G or T) labeled with Cy3 (see sequence information in Figure 5E). A representative fluorescence time trace shows not only short binding events, as in the case of ssDNA (Figure 4A), but also longer-lasting interactions (Figure 5A). Distributions of binding dwell times of four complementary g1N/t1N constructs show that TtAgo-dsDNA interactions are described with two characteristic times, as demonstrated for the g1C/t1G construct in Figure 5B. In all cases the shorter time (Δτ1) falls in the range 8-11 s (Figure S4A), similar to the binding time of ssDNA, and thus might represent non-specific and non-productive interactions. The longer binding time (Δτ2), ranging from 60 s to 120 s (Figure 5C), represents stronger interactions that might be crucial for the proper loading of the guide strand. In addition, all four dsDNAs exhibit comparable binding rates (Figure 5D).
Figure 5. TtAgo interactions with dsDNA show a preference for a deoxyguanosine on the target strand.
(A) Representative fluorescence trace showing short and long binding events. Time point at which dsDNA is introduced into the chamber is indicated with a grey bar.
(B) Dwell time distribution of g1C/t1G binding events with a double-exponential fit (blue line) with short- and long-bound populations indicated as red and orange lines, respectively. The average dwell time (Δτavg) was obtained from short (Δτ1) and long (Δτ2) binding dwell times and their corresponding amplitudes. See also Figure S4A.
(C) Comparison of longer binding dwell times (Δτ2) for four fully complementary dsDNA constructs. Numbers in each column represent the number of analyzed TtAgo molecules, while error bars represent SEM, n=6.
(D) Binding rate (kon) of all four fully complementary dsDNAs. Numbers in parentheses represent the number of analyzed TtAgo molecules, while error bars represent SEM, n=3.
(E) Sequences of guide DNA and target DNA. The fluorophore (Cy3 or Cy5) is positioned on the 9th nt of guide DNA, counting from guide DNA 5'-end. Vertical lines denote contiguous base pairs between the guide and target strands. Cy3-labeled g1C/t1G was used in the competition assay as a reference for all four fully complementary dsDNA constructs labeled with Cy5 (see also Figure S4D).
Control experiments for the single-molecule experiments displayed in this figure are given in Figures S5 and S6.
(F) Schematic representation of TtAgo and hAGO2 proteins. Residues involved in direct or water-mediated (*) t1N interactions are indicated in the figure.
(G) Crystal structure of TtAgo with bound 21 nt DNA guide and 16 nt DNA target (PDB 5GQ6). TtAgo residues specifically interact with t1G. Domains colored as in (E); DNA target is colored blue. See also Figure S6.
(H) Crystal structure of hAGO2 with bound 21 nt RNA guide and 9 nt RNA target (PDB 4W5O). hAGO2 residues specifically interact with t1A. Domains are colored as in (E); RNA target is colored blue.
TtAgo selects dsDNA with t1G passenger strand
In ternary structures of TtAgo, the target strand t1G nucleoside, which is located opposite the g1N nucleoside, is specifically recognized and bound by a binding pocket formed by a residue on loop L2 (S328) and residues in the PIWI domain (D590, R608, F649) (Figure 5F and 5G) (Sheng et al., 2014). Both previous and present ternary structures of TtAgo bound to guide and target DNA strands demonstrate that nucleotide t1G is stabilized via hydrogen bonds between its base and the side-chain of D590, and between its deoxyribose sugar and the side-chain of S328. Residues R608 and F649 sandwich the base, further stabilizing the interaction with t1G.
The observations that TtAgo interacts longer with dsDNA than with ssDNA, and that it has a binding pocket that specifically binds t1G led to a hypothesis that the preference for g1C observed in vivo (Swarts et al., 2014a) could be explained if TtAgo acquires siDNAs by specifically binding dsDNAs with a t1G, after which the target strand is removed and a guide with g1C remains bound to TtAgo. Molecular dynamics simulation in which the t1N is varied (Table S3 and Table S4) demonstrate that about four to six stable hydrogen bonds are formed between the t1N base and TtAgo in the case of t1G, and the number of hydrogen bonds decreases to one to three for t1T, one to two for t1A, and none for t1C (Figure S3C and S3D). In the molecular simulation models, t1A and t1T are bound in the same pocket as t1G, although compared to t1G, there are reduced interactions between these bases and the PIWI domain. In addition, t1A does not interact with the side-chain of D590. In contrast, t1C is located outside of the binding pocket as no hydrogen bonds are formed between t1C and the PIWI domain (Figure S6). These simulations suggest that the affinity between TtAgo and the t1 base at the 3’-end of the target strand follows the order G>T>A>C.
Our single-molecule fluorescence data corroborates the observation of the molecular dynamic simulation that TtAgo has a higher affinity for a t1G. The g1C/t1G construct exhibits approximately 2-fold longer binding dwell time (Δτ2) than any of the other g1N/t1N combinations (Figure 5C). This effect, however, is statistically significant only when g1C/t1G is compared with g1A/t1T (Table S7). To further test our hypothesis, we designed two additional sets of g1N/t1N constructs by reducing the extent of base-pairing between guide and target strands from 21 bp to 16 bp and 12 bp. We compared binding kinetics of partially destabilized dsDNA constructs (g1C-16/t1G vs. g1A-16/t1T and g1C-12/t1G vs. g1A-12/t1T, see Figure S4B and S4C, respectively). Irrespective of the extent of base-pairing, dsDNAs with t1G show approximately 2 times longer binding than dsDNAs with t1T. This preference towards g1C/t1G is further supported by a single-molecule competition assay (Figure S4D), in which we immobilized TtAgo on the surface and introduced an equimolar mixture of g1C/t1G labeled with Cy3 and g1N/t1N labeled with Cy5 (see sequence and labeling scheme in Figure 5E). We counted the number of dsDNA molecules interacting with immobilized protein 5 min and 60 min after incubation, using Cy3-labeled g1C/t1G as a reference. The results show that, even though there is no observable difference between all four dsDNAs after 5 min incubation, two times more g1C/t1G molecules become associated with TtAgo than any other dsDNA after 60 min (Figure S4D). This suggests that longer-lasting interactions are determined by t1G. Although the observed 2-fold effect is small, together with the bias towards GC-rich sequence downstream the chopping site (and possibly other, so far undetermined factors), it contributes to the observed preference for the acquisition of siDNAs with a g1C in vivo.
Based on the combined results, we hypothesize that the t1N binding pocket might also play a role in stabilization of the interaction between TtAgo-siDNA complex and ssDNA targets. We thus performed single-molecule FRET experiments to visualize the possible influence of t1N on binding dwell time and binding rate. We employed an assay in which one of four Cy5-labeled target ssDNA (each varying at t1N, N = A, C, G, or T) was immobilized on a quartz surface of one of four separate microfluidic channels via streptavidin-biotin conjugation (Figure 6A), as described previously (Chandradoss et al., 2015). We then introduced TtAgo, pre-loaded with a Cy3-labeled single-stranded guide DNA (in all channels a g1C guide was used). In this assay, a high FRET signal is only observed when the guide strand base-pairs with the complementary part of the target DNA strand (see Figure 6A and 6B). Remarkably, binding dwell time analysis revealed much stronger interactions between the TtAgo-guide complex and a target DNA strand with t1G than with any other t1N target (Figure 6C-E). This result supports our hypothesis that TtAgo residues form a binding pocket that preferentially interacts with deoxyguanosine. However, even though the presented structure of TtAgo in a ternary complex with guide and target DNA strands does not show base-pairing between the g1C and t1G nucleotides (Figure 3C), at this point we cannot exclude the possibility that such extended base-pairing between g1C guide DNA and t1G target DNA contributed to the observed increase in the binding dwell time.
Figure 6. TtAgo-guide complex preferentially binds to a target strand with a t1G.
(A) (top panel) Immobilization scheme. (bottom panel) Sequences of guide and target DNA strands. Donor fluorophore (Cy3) is positioned on the 9th nt of the g1C guide DNA, counting from the guide DNA 5'-end. The acceptor (Cy5) is positioned on the t1N target DNA opposite nt 17 of the guide DNA. Vertical lines denote contiguous base pairs between the guide and target strands, while dots represent nonconsecutive pairs. For immobilization, the 3'-end of the target strand is annealed to a biotinylated DNA anchor.
(B) Representative time trajectory showing binding events: (top panel) Cy3 and Cy5 fluorescence intensities (time point at which TtAgo-siDNA complex is introduced into the chamber is indicated with a grey bar); (bottom panel) FRET calculated from Cy3 and Cy5 intensities. FRET was set to zero value outside of the binding events.
(C) Dwell time distribution of TtAgo-guide binding to the target strand with t1T was fitted with a single-exponential function (red line).
(D) Dwell time distribution of TtAgo-guide binding to the target strand with t1G was fitted with a double-exponential function (blue line) with short- and long-binding populations indicated as red and orange lines, respectively. Characteristic short (Δτ1) and long (Δτ2) binding times are indicated.
(E) Comparison of binding dwell times for four target DNA constructs. Numbers in parentheses represent the number of analyzed immobilized target DNA molecules, while error bars represent SEM, n=4.
Control experiments for the single-molecule experiments displayed in this figure are given in Figures S5and S6.
(F) Efficiency of t1G and t1T cleavage in competition assays, relative to cleavage efficiency of the same targets in single-target assays. Cleavage efficiency was determined after incubation of the targets with TtAgo-siDNA (g1T) complexes at 65 ºC for 16 h. Gel images from which efficiencies were calculated are displayed in Figure S7.
To further verify this data we performed cleavage assays with two ssDNA targets, which have identical sequences except for a variation at t1N (t1G vs t1T). To elucidate the putative role of base-pairing between g1 and t1, a g1T guide was used. The targets are labeled with different 5’-end fluorophores, which allows for tracing of the individual targets. Although t1T targets are cleaved slightly more efficient than t1G targets by TtAgo-siDNA complexes in single-target cleavage assays (Figure S7), TtAgo-siDNA complexes have a strong preference for t1G targets in target competition assays (Figure 6F). This effect is only observed when the targets compete for TtAgo-siDNA, and is lost when reactions are performed with an excess of TtAgo-siDNA. These findings show that, even in the absence of hypothetical g1-t1 base-pairing, TtAgo-siDNA complexes in direct competition experiments prefer a target with a t1G over a target with a t1T.
Discussion
Model for DNA guide acquisition by TtAgo
Prokaryotic Argonaute proteins have been demonstrated to play a role in host defense by interfering with invading nucleic acids (Blesa et al., 2015; Olovnikov et al., 2013; Swarts et al., 2015a; Swarts et al., 2014a; Swarts et al., 2015b). In this study, we demonstrated that as a first step in the guide loading process TtAgo actively generates its own siDNAs from dsDNA molecules. We hypothesize that this ‘chopping’ activity of pAgo is reminiscent of the RNase H activity (Cerritelli and Crouch, 2009; Tadokoro and Kanaya, 2009). RNase H is unable to cleave single-stranded nucleic acids but catalyzes the cleavage of the RNA strand from a DNA/RNA duplex. Similarly, apo-TtAgo is unable to cleave single-stranded nucleic acids, but catalyzes hydrolysis of a DNA strand from a DNA/DNA duplex. This activity is dependent on the catalytic site that resides in the TtAgo PIWI domain, and that has evolved from an RNase H-like nuclease (Makarova et al., 2009; Swarts et al., 2014b).
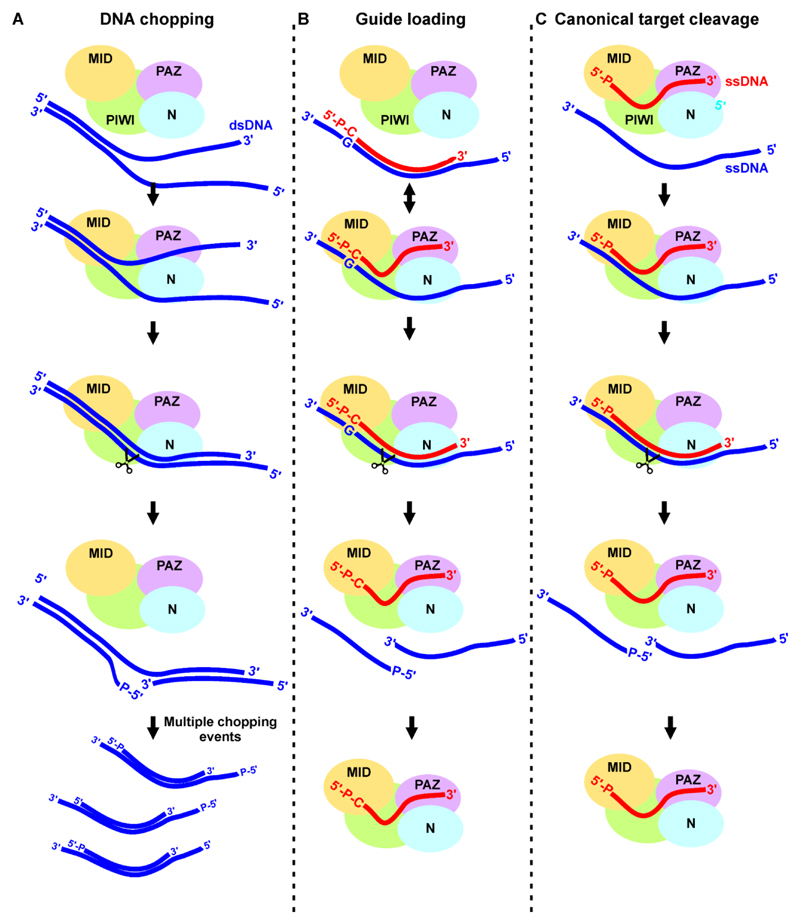
Based on the experimental data presented here, the following working hypothesis is proposed for chopping (Figure 7A) and guide loading (Figure 7B). Apo-TtAgo-mediated DNA chopping requires partially unwound DNA duplexes, possibly because steric hindrance by the N domain prevents binding of a fully base-paired DNA duplex binding (Sheng et al., 2014). Upon unwinding of the DNA duplex, the PIWI domain of TtAgo binds the more stable part of the duplex DNA. The 5’ and 3’ ends of the guide strand are not specifically bound to the pAgo protein, but rather protrude from the protein, resembling substrate binding by RNase H. We speculate that this unusual configuration does not prevent TtAgo from further processing of the bound dsDNA since it is a requirement for the observed apo-TtAgo mediated plasmid chopping. Next, the guide strand is released from the PAZ domain, leading to (partial) re-hybridization of the DNA duplex. In the canonical Argonaute mechanism, these target binding events are associated with the conformational changes required for target cleavage (Sheng et al., 2014). We predict that during DNA chopping such induced conformational changes result in a ‘cleavage-competent state’ (Sheng et al., 2014), and in nicking of the passenger strand of the DNA duplex. Multiple chopping events eventually will generate small duplex DNAs.
Figure 7. Models for DNA chopping, guide loading, and target cleavage by TtAgo.
(A) Proposed model for DNA chopping. TtAgo binds the stable part of partially unwound dsDNA with at least its PIWI domain. Duplex re-hybridization of the DNA associated with conformational changes results in chopping of the target strand. Multiple chopping events will generate small duplex DNAs.
(B) Proposed model for DNA guide loading. Duplex DNA substrates are bound by TtAgo: The 5’-end phosphorylated g1N is bound by the MID domain, while the t1N is bound in the t1N binding pocket. Duplex DNAs with a t1G have longer dwell times than other duplex DNAs. The passenger strand is released either by N domain-mediated duplex destabilization or after target cleavage, while the guide strand (siDNA) remains bound.
(C) Canonical target cleavage by TtAgo. The 5’-phosphorylated end and the 3’ end of the guide strand are bound by TtAgo MID and PAZ domains respectively. Target binding initiates at the seed segment of the guide, after which zippering in the direction of the 3’ end of the guide takes place. The 3’ end of the guide is released from the PAZ domain and accompanying conformational changes lead to correct positioning of the catalytic residues and eventually target cleavage.
Partial unwinding of dsDNA, a prerequisite for DNA chopping, is facilitated in vitro by lowering salt concentrations, by lowering GC-content, and by using partially mismatched DNA. In vivo, other proteins such as RNA and DNA polymerases as well as DNA helicases may facilitate the chopping action of TtAgo by transiently unwinding dsDNA. Since invading DNA such as plasmid and viral DNA generally is more actively replicated than genomic DNA, DNA replication might play a role in the preferential generation of siDNAs from invader DNA. It has previously been demonstrated that TtAgo mainly acquires guides from plasmid DNA. Moreover, the extent of siDNA source preference appears to reflect the copy number of the source per cell (Swarts et al., 2014a).
The second step in the process, guide loading (Figure 7B), might take place during or after DNA chopping. We hypothesize that small duplex DNAs are bound to TtAgo such that a phosphorylated 5’-end of g1N is anchored in the MID domain, while the opposing t1N deoxynucleoside on the passenger strand is bound in the t1N binding pocket of the PIWI domain. As the nucleic acid binding groove of TtAgo does not allow duplex DNA binding past nucleotide 16 of the guide strand, the loaded dsDNA has to be partially unwound during binding. In this process, the N domain possibly plays a role in duplex destabilization as described for eAgos (Wang et al., 2009). DNA duplex unwinding during guide acquisition might be connected to the conformational changes in TtAgo postulated by the induced fit mechanism of guide binding (Zhu et al., 2016). Similar to specific eukaryotic Argonautes (Matranga et al., 2005), TtAgo-mediated passenger strand cleavage might result in the release of this strand, leading to formation of a TtAgo-siDNA complex. Such a binary complex can then perform canonical guide-mediated target cleavage (Figure 7C).
Sequence-specific loading of guides on Argonaute proteins
TtAgo is preferentially loaded with siDNAs with a g1C in vivo (Swarts et al., 2014a). Other pAgos and eAgos also preferentially associate with guides with a specific g1N (Batista et al., 2008; Czech et al., 2008; Czech et al., 2009; Ghildiyal et al., 2010; Olovnikov et al., 2013). It has been suggested that a structural feature in the MID domain termed the ‘nucleotide specificity loop’ is essential in selective binding of a specific g1N in some eAgos (Frank et al., 2012; Frank et al., 2010). Although our molecular dynamic modeling suggests that TtAgo also might have a preference for specific g1Ns, we were unable to detect preferential binding of any g1N in single-molecule experiments or in previous in vitro activity assays (Swarts et al., 2014a). This indicates that the proposed g1C-specific interactions do not result in preferential g1C guide loading. Instead, we have demonstrated that specific interactions between TtAgo and t1G play an important role during dsDNA loading. As t1G target strands are naturally bound to g1C guide strands, preferential loading of t1G dsDNA subsequently results in the preferential loading of guide strands with a g1C.
In contrast with earlier reports (Felice et al., 2009), it has been demonstrated that hAGO2 can also be loaded with guides with any g1N (Kawamata et al., 2011). In addition, hAGO2 cleaves target RNAs equally well when loaded with a g1U or g1G guide RNA, and molecular dynamics simulations demonstrate that hAGO2 can accommodate any g1N (Kalia et al., 2015). Combined, this downplays the importance of a nucleotide specificity loop in hAGO2, and it suggests that other determinants for preferential guide loading are in place. hAGO2 specifically interacts with target strands with a t1A, thereby enhancing the binding dwell time of targets with a t1A (Schirle et al., 2015). Given the observations that, like TtAgo, hAGO2 can accommodate any g1N guide (Kawamata et al., 2011; Schirle et al., 2015), it is tempting to speculate that the t1N binding pocket of hAGO2 also plays a role in guide acquisition.
The residues that make up the t1A binding pocket of hAGO2 are located in the MID and L2 domains (Figure 5F and 5H), and are conserved in a subset of Ago-like eAgos (Schirle et al., 2015). In contrast, the residues that form the t1G binding pocket of TtAgo are located in the L2 loop and PIWI domain (Figure 5F and 5G) and do not appear to be conserved, even within its phylogenetic clade (data not shown). This demonstrates a high flexibility for the t1N binding pocket that, despite its poor sequence and structural conservation, plays an important role in target/passenger strand binding in both pAgos and eAgos.
View on the function and evolution of Argonaute proteins
The discovery that apo-TtAgo is able to degrade dsDNA in the absence of a guide raises the question why siDNAs are required to degrade invading DNA in the first place. In theory, dsDNA chopping might be enough to provide innate immunity against invading nucleic acids. However, DNA chopping is much less efficient than canonical siDNA-guided target cleavage. As DNA chopping could also target host DNA, we speculate that low efficiency of the chopping process may be required to protect the host organism against toxic or potentially even lethal effects of DNA chopping. In addition, siDNA-guided pAgo proteins might allow for more efficient removal of multiple copies of the same invader DNA, or they might provide immunity against previously encountered invaders for multiple generations.
It should be noted that many other pAgos are catalytically inactive and thus rely on other mechanisms for guide generation. For example, many genes encoding catalytically inactive pAgos co-occur with genes encoding nucleases (Makarova et al., 2009; Miyoshi et al., 2016; Olovnikov et al., 2013; Swarts et al., 2014b). It is expected that these nucleases are responsible for guide generation and/or for target degradation. We cannot rule out that in vivo chopping is aided by other processes such as transcription and replication, during which DNA gets temporarily unwound. Nevertheless, the finding that TtAgo can generate its own siDNAs implies that at least some pAgos can function as stand-alone proteins, and that the requirement for interactions with other essential RNAi proteins required for guide generation and loading has most likely evolved eukaryotic organisms. We hypothesize that the development of a separate mechanism for guide generation, possibly required to reduce toxicity and to improve efficiency, has been essential for the transformation of Argonaute proteins from a stand-alone host defense system to the key enzyme in RNA interference.
Supplementary Material
Acknowledgements
This work was financially supported by grants from the Netherlands Organization of Scientific Research (NWO) to JvdO (NWO-TOP, 854.10.003), the European Molecular Biology Organization (EMBO) to DCS (EMBO ALTF 179-2015), and CJ was funded by European Research Council under the European Union’s Seventh Framework Programme [FP7/2007-2013] / ERC grant agreement n° [309509] and by the Netherlands Organization for Scientific Research (Vidi 864.14.002), YW was funded by the Natural Science Foundation of China (91440201) and the Strategic Priority Research program of the Chinese Academy of Sciences (XDB08010203). The authors thank Martin Jinek for the permission to use laboratory space and reagents. The authors thank the staff from BL17B beamline of National Center for Protein Sciences Shanghai (NCPSS) at Shanghai Synchrotron Radiation Facility, for assistance during data collection.
Footnotes
Author Contributions
D.C.S. and J.v.d.O. designed in vitro chopping experiments. Proteins were purified and chopping experiments were performed by D.C.S., E.M.T., and Yi.Z.. Single-molecule experiments were designed by M.S., S.C., and C.J. and performed by M.S. and S.C.. Structural experiments were designed by G.S. and Y.W., and performed by G.S. and H.Z.. Molecular Dynamics simulation was designed by J.L. and Y.W., and performed by Yo.Z. and J.L.. D.C.S., M.S., Y.W., C.J., and J.v.d.O. wrote the manuscript. All authors read and approved the manuscript.
Data deposition
Atomic coordinates and structure factors have been deposited in the Protein Data Bank under accession number 5GQ6.
References in the Supplementary information and STAR methods
(Kibbe, 2007), (Chandradoss et al., 2014), (Jung et al., 2013), (Adams et al., 2002; Best et al., 2012; Humphrey et al., 1996; MacKerell et al., 1998; Mackerell et al., 2004; Mccoy et al., 2007; Otwinowski and Minor, 1997; Phillips et al., 2005)
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best RB, Zhu X, Shim J, Lopes PE, Mittal J, Feig M, Mackerell AD., Jr Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. Journal of chemical theory and computation. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa A, Cesar CE, Averhoff B, Berenguer J. Noncanonical cell-to-cell DNA transfer in Thermus spp. is insensitive to argonaute-mediated interference. J Bacteriol. 2015;197:138–146. doi: 10.1128/JB.02113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerritelli SM, Crouch RJ. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 2009;276:1494–1505. doi: 10.1111/j.1742-4658.2009.06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandradoss SD, Haagsma AC, Lee YK, Hwang JH, Nam JM, Joo C. Surface passivation for single-molecule protein studies. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/50549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandradoss SD, Schirle NT, Szczepaniak M, MacRae IJ, Joo C. A Dynamic Search Process Underlies MicroRNA Targeting. Cell. 2015;162:96–107. doi: 10.1016/j.cell.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, Perrimon N, Kellis M, Wohlschlegel JA, Sachidanandam R, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, Zhou R, Erlich Y, Brennecke J, Binari R, Villalta C, Gordon A, Perrimon N, Hannon GJ. Hierarchical rules for Argonaute loading in Drosophila. Mol Cell. 2009;36:445–456. doi: 10.1016/j.molcel.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck A, Meister G. Assembly and function of small RNA - argonaute protein complexes. Biol Chem. 2014;395:611–629. doi: 10.1515/hsz-2014-0116. [DOI] [PubMed] [Google Scholar]
- Felice KM, Salzman DW, Shubert-Coleman J, Jensen KP, Furneaux HM. The 5' terminal uracil of let-7a is critical for the recruitment of mRNA to Argonaute2. Biochem J. 2009;422:329–341. doi: 10.1042/BJ20090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank F, Hauver J, Sonenberg N, Nagar B. Arabidopsis Argonaute MID domains use their nucleotide specificity loop to sort small RNAs. EMBO J. 2012;31:3588–3595. doi: 10.1038/emboj.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank F, Sonenberg N, Nagar B. Structural basis for 5'-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010;465:818–822. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, Xu J, Seitz H, Weng ZP, Zamore PD. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. Rna-a Publication of the Rna Society. 2010;16:43–56. doi: 10.1261/rna.1972910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. Journal of molecular graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. 27-38. [DOI] [PubMed] [Google Scholar]
- Jung SR, Kim E, Hwang W, Shin S, Song JJ, Hohng S. Dynamic anchoring of the 3'-end of the guide strand controls the target dissociation of Argonaute-guide complex. J Am Chem Soc. 2013;135:16865–16871. doi: 10.1021/ja403138d. [DOI] [PubMed] [Google Scholar]
- Kalia M, Willkomm S, Claussen JC, Restle T, Bonvin AM. Novel Insights into Guide RNA 5'-Nucleoside/Tide Binding by Human Argonaute 2. Int J Mol Sci. 2015;17 doi: 10.3390/ijms17010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Yoda M, Tomari Y. Multilayer checkpoints for microRNA authenticity during RISC assembly. EMBO Rep. 2011;12:944–949. doi: 10.1038/embor.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbe WA. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35:W43–46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ. Structural basis for 5 '-end-specific recognition of guide RNA by the A-fulgidus Piwi protein. Nature. 2005;434:666–670. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKerell AD, Bashford D, Bellott M, Dunbrack RL, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha S, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- Mackerell AD, Jr, Feig M, Brooks CL., 3rd Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. Journal of computational chemistry. 2004;25:1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, van der Oost J, Koonin EV. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol Direct. 2009;4:29. doi: 10.1186/1745-6150-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Mccoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Ito K, Murakami R, Uchiumi T. Structural basis for the recognition of guide RNA and target DNA heteroduplex by Argonaute. Nat Commun. 2016;7 doi: 10.1038/ncomms11846. 11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olovnikov I, Chan K, Sachidanandam R, Newman DK, Aravin AA. Bacterial argonaute samples the transcriptome to identify foreign DNA. Mol Cell. 2013;51:594–605. doi: 10.1016/j.molcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Owczarzy R, You Y, Moreira BG, Manthey JA, Huang L, Behlke MA, Walder JA. Effects of sodium ions on DNA duplex oligomers: improved predictions of melting temperatures. Biochemistry. 2004;43:3537–3554. doi: 10.1021/bi034621r. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. Journal of computational chemistry. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, Sheu-Gruttadauria J, Chandradoss SD, Joo C, MacRae IJ. Water-mediated recognition of t1-adenosine anchors Argonaute2 to microRNA targets. eLife. 2015;4 doi: 10.7554/eLife.07646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, Zhao H, Wang J, Rao Y, Tian W, Swarts DC, van der Oost J, Patel DJ, Wang Y. Structure-based cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proc Natl Acad Sci U S A. 2014;111:652–657. doi: 10.1073/pnas.1321032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts DC, Hegge JW, Hinojo I, Shiimori M, Ellis MA, Dumrongkulraksa J, Terns RM, Terns MP, van der Oost J. Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA. Nucleic Acids Res. 2015a;43:5120–5129. doi: 10.1093/nar/gkv415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts DC, Jore MM, Westra ER, Zhu Y, Janssen JH, Snijders AP, Wang Y, Patel DJ, Berenguer J, Brouns SJ, et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature. 2014a;507:258–261. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts DC, Koehorst JJ, Westra ER, Schaap PJ, van der Oost J. Effects of Argonaute on Gene Expression in Thermus thermophilus. PLoS One. 2015b;10 doi: 10.1371/journal.pone.0124880. e0124880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts DC, Makarova K, Wang Y, Nakanishi K, Ketting RF, Koonin EV, Patel DJ, van der Oost J. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol. 2014b;21:743–753. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro T, Kanaya S. Ribonuclease H: molecular diversities, substrate binding domains, and catalytic mechanism of the prokaryotic enzymes. FEBS J. 2009;276:1482–1493. doi: 10.1111/j.1742-4658.2009.06907.x. [DOI] [PubMed] [Google Scholar]
- Travers A, Muskhelishvili G. DNA supercoiling - A global transcriptional regulator for enterobacterial growth? Nature Reviews Microbiology. 2005;3:157–169. doi: 10.1038/nrmicro1088. [DOI] [PubMed] [Google Scholar]
- Wang YL, Juranek S, Li HT, Sheng G, Tuschl T, Patel DJ. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. 2008a;456:921–U972. doi: 10.1038/nature07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Juranek S, Li HT, Sheng G, Wardle GS, Tuschl T, Patel DJ. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. 2009;461:754–U753. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Sheng G, Juranek S, Tuschl T, Patel DJ. Structure of the guide-strand-containing argonaute silencing complex. Nature. 2008b;456:209–U234. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- Yuan YR, Pei Y, Ma JB, Kuryavyi V, Zhadina M, Meister G, Chen HY, Dauter Z, Tuschl T, Patel DJ. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol Cell. 2005;19:405–419. doi: 10.1016/j.molcel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander A, Holzmeister P, Klose D, Tinnefeld P, Grohmann D. Single-molecule FRET supports the two-state model of Argonaute action. RNA Biol. 2014;11:45–56. doi: 10.4161/rna.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Jiang H, Sheong FK, Cui X, Gao X, Wang Y, Huang X. A Flexible Domain-Domain Hinge Promotes an Induced-fit Dominant Mechanism for the Loading of Guide-DNA into Argonaute Protein in Thermus thermophilus. J Phys Chem B. 2016;120:2709–2720. doi: 10.1021/acs.jpcb.5b12426. [DOI] [PubMed] [Google Scholar]
- Zimmerman SB. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.