Abstract
This unit provides a step-by-step protocol for constructing cell-type and mitochondria-targeted GCaMP genetically-encoded Ca2+ indicators (GECIs) for mitochondrial Ca2+ imaging in astrocytes. Mitochondrial Ca2+ plays a critical role in controlling cytosolic Ca2+ buffering, energy metabolism, and cellular signal transduction. Mitochondrial Ca2+ overload contributes to various pathological conditions including neurodegeneration and apoptotic cell death in neurological diseases. Live cell mitochondrial Ca2+ imaging is an important approach to understand mitochondrial Ca2+ dynamics and thus cell physiology and pathology. We implement astrocyte-specific mitochondrial targeting of GCaMP5G/6s (mito-GCaMP5G/6s). By loading X-Rhod-1 in astrocytes, we can simultaneously image mitochondrial and cytosolic Ca2+ signals. The current protocol provides a novel approach to image mitochondrial Ca2+ dynamics as well as Ca2+ interplay between the endoplasmic reticulum and mitochondria.
Keywords: Mitochondrial Ca2+ uptake, mitochondrial matrix, astrocyte, endoplasmic reticulum (ER), GCaMP5G/6s, X-Rhod-1, ATP
INTRODUCTION
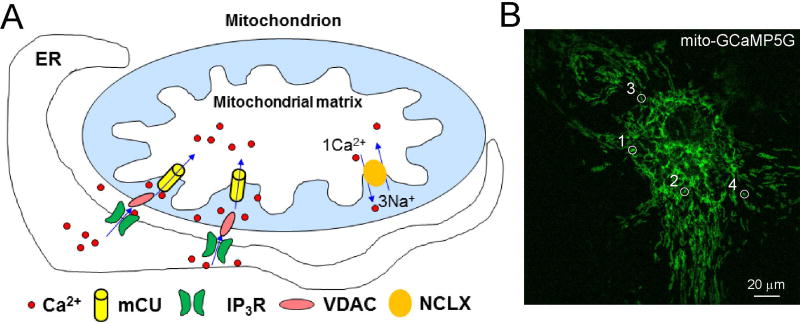
It has been established that astrocytes participate in synaptic transmission as a part of tripartite synapse through Ca2+-mediated gliostransmitter release. Mitochondria can be used as Ca2+ sinks to buffer local or cytosolic Ca2+ rises in astrocytes. Mitochondrial Ca2+ uptake into its matrix [see Figure 1] affects metabolic processes by regulating oxidative phosphorylation and stimulates ATP production under physiological conditions (Burkeen et al., 2011;Griffiths and Rutter, 2009;Llorente-Folch et al., 2013;Pizzo et al., 2012). Mitochondrial Ca2+ uptake is also a determinant for inducing necrotic and apoptotic cell death in brain disorders (Duchen, 2012;Gouriou et al., 2011;Qiu et al., 2013). Mitochondrial Ca2+ overloading opens mitochondrial permeability transition pores (mPTPs) which can initiate apoptotic cell death. Therefore, studying mitochondrial Ca2+ dynamics in living cells is important for understanding cellular (patho)physiological function. Under physiological conditions, mitochondria maintain matrix Ca2+ homeostasis through a balanced Ca2+ uptake and efflux. Mitochondrial Ca2+ uptake is mainly mediated by mitochondrial Ca2+ uniporters (MCUs) while mitochondrial Ca2+ efflux is mediated by Na+-Ca2+-Li+ exchangers (NCLXs). The balance can be perturbed through the stimulation of GPCRs (Finkel et al., 2015) (Figure 1A).
Figure 1. Brief illustration of mitochondrial Ca2+ signalling.
A) Mitochondrial Ca2+ uptake basically is mediated by MCUs while mitochondrial efflux is primarily attributed to the function of the Na+-Ca2+-Li+ exchangers NCLXs IR3R functions to release Ca2+ from intracellular store of endoplasmic reticulum (ER). B) A cultured astrocyte expressed with GCaMP5G in the mitochondria. Figure 1B is adapted from Li et al (Li et al., 2014).
Many synthetic organic Ca2+ indicators have been used for cellular Ca2+ imaging (for reviews see Contreras et al., 2010;Paredes et al., 2008); however, these indicators are partitioned between mitochondria, other organelles and the cytosol, making absolute measurements difficult. In contrast, genetically encoded Ca2+ indicators (GECIs) can be expressed in different cell types and subcellular compartments including mitochondria for cell- and compartment-specific Ca2+ imaging in vitro or in vivo. Fluorescence intensity-based GCaMP Ca2+ indicators have recently emerged as major GECIs (Akerboom et al., 2012;Chen et al., 2013;Tian et al., 2009;Yamada et al., 2011). In this unit, we provide protocols for astrocyte- and mitochondria-specific targeting of GCaMP5G and GCaMP6s (GCaMP5G/6s) to image mitochondrial Ca2+ dynamics in primary cultured astrocytes. Combined with organic Ca2+ indicator X-Rhod-1, we also provide a protocol for simultaneous imaging of mitochondrial and cytosolic Ca2+ signals. Using this novel approach, mitochondrial Ca2+ uptake in individual mitochondria in cultured astrocytes can be revealed after ATP stimulation. We demonstrate that mitochondrial Ca2+ signal is tightly coupled to IP3R-mediated Ca2+ release from the ER in astrocytes, indicating the dependence of mitochondrial Ca2+ dynamics on cytosolic Ca2+ changes.
BASIC PROTOCOL 1
IMAGING MITOCHONDRIAL AND CYTOSOLIC Ca2+ IN ASTROCYTES USING GCAMP5/6
To image mitochondrial matrix and cytosolic Ca2+ simultaneously in astrocytes, we constructed DNA plasmids with astrocyte-specific promoter combined with mitochondrial targeting sequence to encode GCaMP5G and GCaMP6s (GCaMP5G/6s) (Li et al., 2014). The DNA plasmids are transfected in cultured astrocytes to express GCaMP5G/6s. The transfected astrocytes are ready for imaging one day later. To simultaneously image mitochondrial matrix and cytosolic Ca2+ signaling, the astrocytes are then loaded with organic red fluorescence Ca2+ indicator X-Rhod-1. Time-lapse imaging is conducted using two-photon (2-P) or confocal microscopy. ATP will be applied to astrocytes through a solution perfusion system to stimulate Ca2+ signal in astrocytes. Both mitochondrial fluorescent GCaMP5G/6s signal and cytosolic X-Rhod-1 fluorescent signal will be acquired through an imaging/acquisition system.
Materials
Papain (20 IU/ml; Sigma)
EBSS - Earle's Balanced Salt Solution (Invitrogen)
Glass coverslips (12 mm in diameter; Fisher Scientific)
Dulbecco’s Modified Eagle Medium (DMEM) medium (Invitrogen)
Fetal bovine serum (FBS) (Hyclone)
Cultured astrocytes grown on glass coverslips in 24-well plate
Endofree plasmid maxi kit (Qiagen)
DNA plasmids with astrocyte-specific promoter and mitochondrial targeting sequence to express GCaMP5G/6s in the mitochondria in astrocytes. DNA construction is outlined below but if additional details are needed see Li et al., 2014.
Transfection reagent: Lipofectamine® 2000 Transfection Reagent (Thermo Fisher)
Artificial cerebrospinal fluid (ACSF) at room temperature, see recipe in reagents and solutions
ATP stock solution (10 mM) prepared with distilled water or ACSF kept at −20°C
Perfusion chamber (PH1, Warner Instruments, MA)
Perfusion system (ALA-VM8, ALA Scientific, NJ) which can control solution change and drug application using pinch valves
Two-photon (2-P) or confocal fluorescence microscope with imaging and acquisition system
Weigh out sodium bicarbonate and place it into a 100mL (200 mL) bottle. Add the α–MEM, L-glutamine, D-glucose, sodium pyruvate, and penn/strep. Mix until sodium bicarbonate goes into solution. Sterile filter the solution through a 0.2µm cellulose acetate bottle top filter into a sterile media bottle. Add the serum. Store the modified media at 4°C. Do not leave media in an incubator for more than a day.
Construction of DNA plasmid for mitochondrial expression of GCaMP5G/6s
-
1
Insert mitochondrial matrix (MM)-targeting sequence (mito-) ATGT CCGTCCTGAC GCCGCTGCTG CTGCGGGGCT TGACAGGCTC GGCCCGGCGG CTCCCAGTGC CGCGCGCCAA GATCCATTCG TTG (Rizzuto et al., 1995) in the backbone of AAV plasmid pZac2.1 containing astrocytic gfaABC1D promoter (Lee et al., 2008;Li et al., 2014;Xie et al., 2010).
-
2
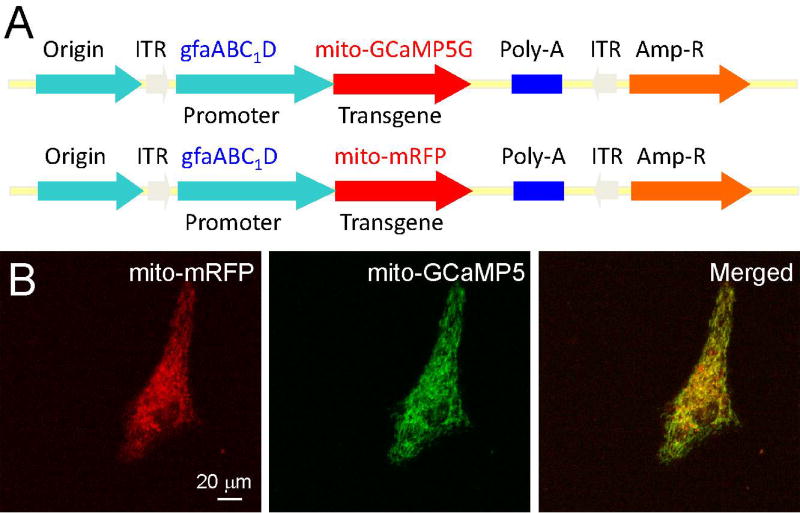
Subclone GCaMP5G/6s or monomeric red fluorescence protein (mRFP) as a control into the cloning sites BamH I and Not 1 using PCR to obtain new expressing plasmids that can target transgene expression in mitochondria of astrocytes (Li et al., 2014) (Figure 2).
-
3
Plasmids are purified for transfection using Endofree plasmid maxi kit.
Figure 2. DNA constructs for astrocyte-specific and mitochondria-targeted transgene expression.
A) Construction maps of genetically encoded Ca2+ indicator GCaMP5G (or GCaMP6s) and mRFP in pZac2.1 plasmid under gfaABC1D promoter for delivering transgenes to astrocytic and mitochondrial matrix. Organelle-specific targeting is achieved using a mitochondrial matrix specific sequence (mito) appended to the N-terminus of the fluorescent proteins. B) Two-photon images showing the coexpression of mito-mRFP (left) and mito-GCaMP5G (right) in primary cultured astrocytes after DNA transfection. Notice exquisite organelle-specific colocalization of mito-mRFP (left) and mito-GCaMP5G. Data is adapted from Li et al (Li et al., 2014). Note: While this protocol focuses on astrocytes, using neuron-specific promotor CaMKII, GCaMP5G or GCaMP6s can also be expressed specifically in the mitochondrial in neurons.
Primary cultured astrocytes
Astrocytic cell cultures were prepared as previously described (Gottipati et al., 2012). All procedures were performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Missouri Animal Care Quality Assurance Committee.
-
4
Remove brain from 0–2 day old C57BL/6J mouse pups and place into a small culture dish containing 1 ml cold EBSS (4 °C). [Note: one pup is enough for culturing one 25 cm2 flask of astrocytes. The gender of pups is not considered].
-
5
Isolate cortices (bilateral) and place into a 15 ml centrifuge tube containing 10 ml of cold EBSS.
Mince cortices with a scissor.
-
6
Decant EBSS and replace with 3 ml papain (20 IU/ml; Sigma) in the presence of L-cysteine (0.2 mg/ml).
-
7
Incubate at 37°C for 1 h.
Gently mix with finger tapping for about 1 min/15 min or with continuous shaking with an orbital shaker at low speed for 1 h during the papain treatment.
-
8
Neutralize with 5 ml trypsin inhibitor (type II-O, 10 mg/ml; Sigma) for 5 min at room temperature (22–25°C).
Triturating the tissue gently to avoid disrupting the cells or injury.
-
9
Decant trypsin inhibitor and replace with 2 ml DMEM medium.
-
10
Gently triturate the tissue using a glass seriological pipet.
-
11
Plate 1 ml of the resulting cell suspension into a 25 cm2 tissue culture flasks containing 15 ml α-MMEM (see Table 1) and place the flask in an incubator maintained at 37°C in a 95% air/ 5% CO2 atmosphere.
-
12
One hour post plating, replace with fresh α-MMEM.
Be careful when replacing medium since the cells do not attach to the bottom of plate very strongly at this time point.
-
13
Put back in the tissue flask in the incubator and add 1ml of fresh MMEM every 5 days to maintain astrocyte cultures.
It should take about 10–14 days to obtain cell growth and proliferation to ~60% confluency.
-
14
Shake the flasks twice on an orbital shaker, first for 1.5 h followed by two exchanges with culture media and again for 18 hours, at 37°C and 260 rpm to remove microglia.
This is a necessary step to obtain purified astrocytes (McCarthy and de Vellis, 1980). The purity of astrocyte can be quantified by immunostaining with anti-glial fibrillary acidic protein (GFAP) and nuclear counterstaining with Dapi. A purity of 95% should be obtained with this procedure.
-
15
Plate these astrocytes on the glass coverslips (12 mm in diameter; Fisher Scientific) (Xie et al., 2010) in 24-well plates and culture the plates in the incubator.
-
16
Feed the astrocytes every 48 h with fresh Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS as described previously.
Table 1.
α-Modified Minimal Essential Media (α-MMEM)
| Ingredient | Supplier | Caltalog # | Concentration | Amount/100 ml |
|---|---|---|---|---|
| α-MEM | Gibco | 41061-019 | 86% | 86 ml |
| Serum | Hyclone | SV30014.03 | 10% | 10 ml |
| Glutamine (200 mM) | Gibco | 25030-081 | 2 mM | 1 ml |
| D-glucose (2M) | Sigma | G7021 | 20 mM | 1 ml |
| Na-pyruvate (100 mM) | Gibco | 11360-070 | 1 mM | 1 ml |
| Penn/Strep | Gibco | 15140-122 | 1% | 1 ml |
| Na-bicarbonate | Sigma | S5761 | 14 mM | 117.6 mg |
Expression of mito-GCaMP5G/6s in astrocytes by DNA transfection
-
17
Change the medium of cultured astrocytes (up to 60% confluence) right before transfection.
-
18
For transfecting 4 wells in 24 well-plate, prepare two tubes: 1st tube: 100 µl DMEM (serum free)+2 µg DNA; 2nd tube: 100 µl DMEM (serum free)+ 4–6 µl lipofectamine 2000, gentle mixing for 5 min.
-
19
Add 2nd tube mixture to 1st tube dropwise while gently tapping the tube, and incubate the mixture at room temperature for 20 min (it remains stable for up to 6 h).
In the mixture, the ratio of DNA (µg):Lipofectamine 2000 (µl)=1:2–3.
-
20
Gently add 50 µl mixtures dropwise to astrocytes in each well for transfection.
-
21
Change culture medium 6 h later.
-
22
The cells are ready to use for imaging 24 h later. Transfected astrocytes can be visualized using a fluorescent microscope (Figure 1B, 2B and 3A).
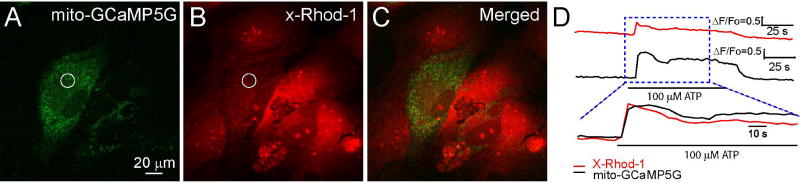
Figure 3. Simultaneous cytosolic and mitochondrial Ca2+ imaging in cultured astrocytes.
A-C) Two-photon images of astrocytes expressing mito-GCaMP5G (A) and loaded with x-Rhod-1 (B) and the merged images (C) for simultaneous mitochondrial and cytosolic Ca2+ imaging. D) The time courses (ΔF/Fo, see Figure 4) of Ca2+ changes in cytosol (upper) and mitochondria (middle) in a region marked by a circle in (A-B). The bottom panel is normalized peak ΔF/Fo of mitochondrial and cytosolic Ca2+ signals showing the matching kinetics of mitochondrial and cytosolic Ca2+ increase after ATP stimulation. Data is adapted from Li et al (Li et al., 2014).
Loading astrocytes with organic Ca2+ indicator X-Rhode-1 dye for simultaneous mitochondrial and cytoplasmic Ca2+ imaging
-
23
Make a X-Rhode-1 working solution of 1 µg/ml by diluting 1 µl stock solution with ACSF.
-
24
Transfer a glass coverslip grown with astrocytes to a well on a 24-well plate, and gently wash away the residue medium with 0.5 ml ACSF.
-
25
Add 0.5 ml X-Rhode-1 working solution to the astrocytes grown on the glass coverslip in the 24-well plate and incubate at room temperature for 45 min.
-
26
Wash away excess X-Rhode-1 with ACSF and incubate in ACSF for 30 min for the de-esterification of the dye.
Under a fluorescent microscope, both astrocytes expressing and not expressing mito-GCaMP5G/6s will be labeled with X-Rhod-1 (Figure 3A-C).
Imaging mitochondrial and cytosolic Ca2+ signaling (setup)
-
27
Make 100 µM ATP in 200 ml ACSF as working solution for stimulation of astrocytes.
-
28
Set the perfusion system and put 100 ml ACSF solution in one barrel and 100 ml 100 µM ATP-containing ACSF in the other barrel.
-
29
Test the perfusion system to make sure the solution flow into chamber freely with gravity and can be removed from chamber with vacuum pump (see Figure 4 for setup).
Two important issues: 1) Make sure there are no bubbles in the tube; and 2) make sure that the solution can be removed so that there is no overflow in the chamber.
-
30
Transfer the glass coverslip grown with astrocytes to a perfusion chamber for imaging.
-
31
Perfuse the cells with ACSF.
Note: the speed should be ~ 1–2 ml/min and make sure that ACSF does not overflow and the glass coverslip does not move.
-
32
Normally after a few minutes of ACSF perfusion, time-lapse Ca2+ imaging can be performed. After 1–2 minutes of data acquisition with ACSF perfusion, switch to ACSF with 100 µM ATP for 1–2 min to stimulate astrocytes.
Usually the imaging acquisition speed is 1 frame/second
-
33
Switch back to ACSF solution and image for 2 minutes.
The mito-GCaMP5G and X-Rhode-1 fluorescence should go back to the baseline when imaging is finished.
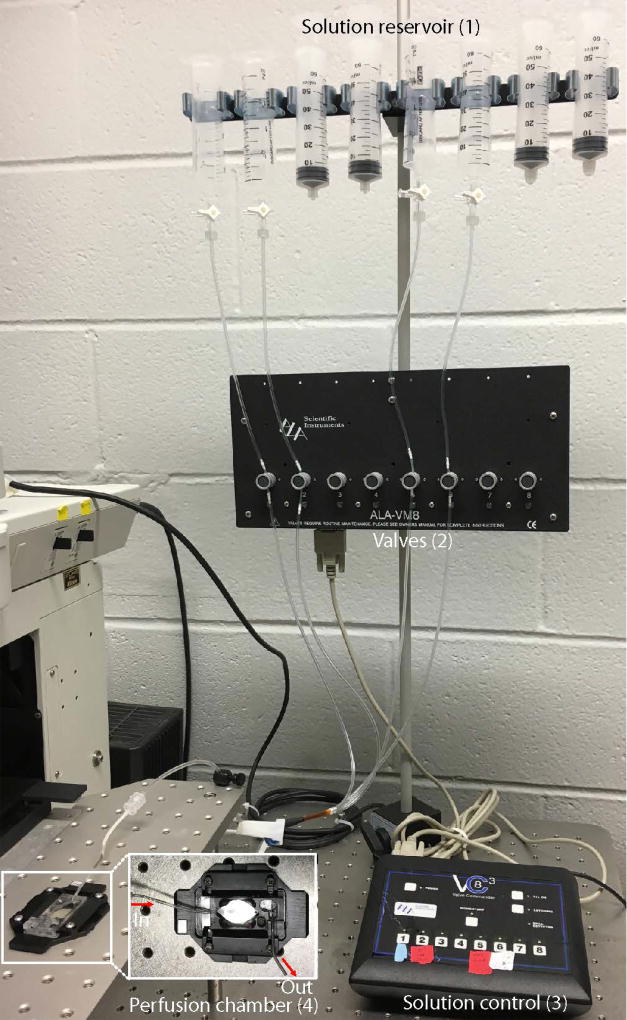
Figure 4. The setup of solution perfusion system.
The system consists of 4 parts, i.e., reservoirs (1), pinch valve (2), solution control unit (3) and perfusion chamber (4). The vacuum pump that removes solution is not indicated.
The 2-P microscope (Prairie Technologies, WI) should be turned on 30 min before step 18. We use a 40×/NA0.8 or 60×/NA0.95 Olympus water immersion objective. Excitation (850 nm) for x-Rhod-1 and mito-GCaMP5G/6s was generated by a pulse laser beam from a Chameleon Ti:Sapphire Ultra I laser (Coherent, CA). Emission was detected using two photo-multiplier tubes (PMTs). Images were acquired with a speed of one frame per second. All the raw fluorescence images should have pixel intensities without saturation and within the PMT’s dynamic range (0–4095). Depending on the availability, a confocal microscope is also ideal for imaging.
Analysis of mitochondrial and cytosolic Ca2+ signal
-
34
Define the regions of interest (ROI) around the mitochondria or cytoplasm, or a blank region without any cultured astrocytes as a background on the acquired time-lapse images using of free software ImageJ or Metamorph software (Molecular Device, CA).
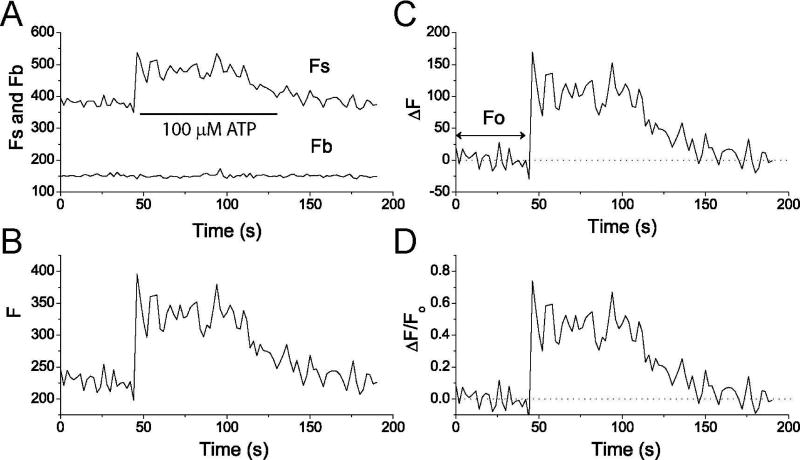
Figure 4 is the procedure for analysis of a single mitochondrion in region 2 in Figure 1B. The same procedure is used for the analysis cytosolic Ca2+ signals.
-
35
Using software, measure the mean pixel fluorescent intensities of each ROI as overall fluorescence signal (Fs (t)) meanwhile measure background intensity (Fb) of a blank region where no cell is grown (Figure 5A).
-
36
Export Fb(t) from Fs(t) to other software such as Origin software (OriginLab Corporation, MA) and subtract Fb(t) from Fs(t), i.e., Fs(t)-Fb(t). We define this as background subtracted fluorescence F(t) (Figure 5B).
-
37
Calculate the baseline fluorescence Fo, and then subtract Fo from F(t), i.e., F(t)-Fo=ΔF (t), which is defined as the baseline subtracted fluorescence (Figure 5C).
Fo is the average fluorescence before ATP stimulation.
-
38
Ca2+ changes were calculated and expressed as ΔF(t)/Fo values vs. time (Figure 5D).
Figure 5. Analysis of mitochondrial Ca2+ signal based on mito-GCaMP5G fluorescence.
A) Raw data of F(t)s and F(t)b. B) background subtracted fluorescence F(t)=F(t)s-F(t)b. C) Baseline subtracted fluorescence change ΔF (t)=F(t)-Fo. D) Ca2+ changes expressed as ΔF(t)/Fo.
REAGENTS AND SOLUTIONS
Artificial cerebrospinal fluid (ACSF)
120 mM NaCl, 3.1 mM KCl, 2 mM CaCl2, 1.3 mM MgCl2, and 10 mM glucose, 10 mM Hepes, pH 7.4). ACSF should be made fresh for imaging experiments and remain at room temperature.
ATP stock solution (10 mM) and working solution (100 µM)
Stock solution (10 mM) is made with ACSF or water and stored at −20 °C up to one year. Working solution (100 µM) is made by diluting stock solution with ACSF and used fresh.
X-Rhode-1 stock solution (1 µg/µl)
Dissolve 50 µg X-Rhode-1 (X14210, Thermo Fisher) in 50 µl DMSO and mix well.
Aliquot into 25 tubes with 2 µl in each tube and store them at −20 °C up to one year.
Use distilled and deionized water in all protocol steps during imaging.
COMMENTARY
Background Information
Many synthetic organic Ca2+ probes have been used for cellular Ca2+ imaging (for reviews see Contreras et al., 2010;Paredes et al., 2008); the major disadvantage of these probes is the partitioning between mitochondria, other organelles and the cytosol, making measurements in specific regions difficult. Rhod-2 is the most widely used organic dye to image mitochondrial Ca2+ uptake, which preferentially locates to the mitochondrial matrix (for review see Contreras et al., 2010;Davidson and Duchen, 2012), but it also shows partial localization in the cytosol which causes errors in measurements. There are other problems generally associated with synthetic organic Ca2+ probes; these include cytotoxicity resulting from the de-esterification of reaction products and photodamage due to a prolonged exposure to high energy excitation light (Thomas et al., 2000).
Genetically encoded Ca2+ indicators (GECIs) provide substantial advantages over synthetic organic Ca2+ probes due to their high sensitivity, brightness and dynamic range of fluorescence. For these reasons, fluorescence intensity-based GCaMP Ca2+ indicators have recently emerged as major GECIs (Akerboom et al., 2012;Chen et al., 2013;Tian et al., 2009;Yamada et al., 2011). For instance, GCaMP5G and GCaMP6s can readily detect cytosolic Ca2+ increases in a neuron after triggering a single action potential (Akerboom et al., 2012;Chen et al., 2013). On the other hand, using molecular biology, these GECIs can be targeted to subcellular compartments such as the cytoplasm, mitochondria and nuclei. For in vivo studies, they can be expressed in different cell type using cell-type specific promotors (Xie et al., 2010). In this protocol, we employed astrocyte- and mitochondria-specific targeting of GCaMP5G/6s to assess mitochondrial Ca2+ dynamics in primary cultured astrocytes. ATP stimulates astrocytic Ca2+ increase through Ca2+ release from ER via activation of IP3Rs (Figure 1)(Ding et al., 2007;Ding, 2013;Li et al., 2014). Large increases in Ca2+ signals can be observed in individual mitochondria in astrocytes with stimulation of ATP. Combined with cytoplasmic loading of red fluorescence Ca2+ probe X-Rhod-1, we can study the coupling between mitochondrial and cytosolic Ca2+ signals in astrocytes. Our results demonstrate that GCaMP5G/6s are suitable probes for detecting Ca2+ uptake in individual mitochondria in astrocytes. This protocol can be used to study mitochondrial Ca2+ uptake and efflux for other cell types such as neurons and in vivo with slight modifications.
Critical Parameters
Microscope
Fluorescence imaging with multiple channel acquisition usually uses confocal and 2-P laser scanning microscope equipped with two or more PMTs. Either inverted or upright microscopes are suitable for imaging cultured astrocyte. A 40x or 60x high numerical aperture (NA) objective with high light transmission is desirable so that the maximal amount of fluorescence can be collected.
Image acquisition speed
Compared with neuronal Ca2+ signaling, Ca2+ signaling in astrocytes is relative slow. It is usually fast enough to acquire one image per second.
Perfusion system
For live cell imaging, it is important to have a good perfusion system for exchanging solutions with different drugs to stimulate or inhibit Ca2+ signals in astrocytes. Solution changes can be controlled with pinch values either manually or by computer. The speed of solution change will affect rising and decay time of fluorescence signals. If experiments focus on studying steady-state Ca2+ signals, the speed of solution exchange is less important.
Temperature
For Ca2+ imaging, the experiments are usually performed at room temperature.
Photobleaching
Theoretically all fluorescent indicators are subject to photobleaching. GCaMP5G/6s and X-Rhod-1 are quite stable but they will bleach under continual exposure to excitation light. For time-lapse imaging, it usually takes several minutes with acquisition rates of one image per second. Thus, profound photobleaching could take place. A general practice to reduce photobleaching is to avoid over-exposure of cells to laser light while enough fluorescence is collected. Exposure time can be reduced if high sensitivity PMTs and high light transmission objective are used. Photobleaching can also be reduced by closing shutter between images.
Troubleshooting
Astrocytes sometimes have low fluorescent intensity of X-Rhode-1. This can happen for a couple reasons. First, it may result from the decay of X-Rhode-1 in the working or stock solutions. One should remember, the working solution can be used for only a few hours after it is made when kept at room temperature. To eliminate the possibility of decayed stock solution, make new stock solution and then new working solution. Normally, stock solution can be used for one year when it is kept in a −20 °C freezer. Second, the astrocyte culture is not healthy or too old. Ideally cultured astrocytes should be used in 7–10 days after plating. To make sure astrocytes are healthy, one should examine their morphology under phase-contrast microscopy before the experiment. Unhealthy astrocytes may have vacuoles and affected membrane integrity. Unhealthy astrocytes will also have poor expression of mito-GCaMP5G and consequently low mito-GCaMP5G fluorescence.
If fluorescence does not increase for a long time (such as 2 minutes) after switching to ATP solution during the live experiments, check the perfusion system to make sure solution is flowing normally and no bubble in the tubing.
If the fluorescence of mito-GCaMP5G/6s is poor due to low expression, the signal/noise will be low and this will in turn cause a low sensitivity to ATP stimulations.
Understanding Results
Typical results for the Basic Protocol are shown in Figure 1B and Figure 3A-C. The GCaMP5G/6s fluorescence should show mitochondrial structure since they are expressed in the mitochondria. When stimulated with ATP, both GCaMP5G/6s and X-Rhod-1 fluorescence will increase at similar time (Figure 3D). If accurate time courses of mitochondrial and cytosolic Ca2+ increases are required, one would use line-scan imaging with fast solution perfusion system.
Time Considerations
If the DNA plasmid and cultured astrocytes are ready, the astrocytes can be used one day after transfection. For imaging, the basic protocol should take about 1 h per experiment. It will take ~3 weeks from the generation of astrocyte cultures to conduct imaging experiments.
Significance Statement.
Astrocytes are the predominant glial cell type in the central nervous system. Although astrocytes are electrically inexcitable, their excitability is manifested by Ca2+ signaling. Mitochondria can uptake cytosolic Ca2+, thus regulating the function of astrocytes. On the other hand, mitochondrial Ca2+ overloading could induce cellular death. Here we developed novel imaging technologies that express genetically encoded Ca2+ indicator (GECI) GCaMP5G/6s in the mitochondria of cultured astrocytes. By loading red fluorescence organic Ca2+ dye X-Rhod-1 in the cytoplasm, we can simultaneously image mitochondrial and cytosolic Ca2+ signals. This protocol demonstrates that GCaMP5G/6s are suitable probes for detecting Ca2+ uptake in individual mitochondria in astrocytes and could help to understand astrocytic function.
Acknowledgments
This work was supported by the National Institutes of Health [R01NS069726] and [R01NS094539], and the America Heart Association awards [16IRG27780023] and [16GRNT31280014] to SD.
TERATURE CITED
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderön NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SSH, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. The Journal of Neuroscience. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial Calcium Signaling Mediates Rhythmic Extracellular ATP Accumulation in Suprachiasmatic Nucleus Astrocytes. The Journal of Neuroscience. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: The calcium connection. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Davidson S, Duchen M. Imaging Mitochondrial Calcium Signalling with Fluorescent Probes and Single or Two Photon Confocal Microscopy. In: Palmeira CM, Moreno AnJ, editors. Mitochondrial Bioenergetics. Humana Press; 2012. pp. 219–234. [DOI] [PubMed] [Google Scholar]
- Ding S. In vivo astrocytic Ca2+ signaling in health and brain disorders. Future Neurology. 2013;8:529–554. doi: 10.2217/fnl.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced Astrocytic Ca2+ Signals Contribute to Neuronal Excitotoxicity after Status Epilepticus. J. Neurosci. 2007;27:10674–10684. doi: 10.1523/JNEUROSCI.2001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers Arch - Eur J Physiol. 2012;464:111–121. doi: 10.1007/s00424-012-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Menazza S, Holmström KM, Parks RJ, Liu J, Sun J, Liu J, Pan X, Murphy E. The Ins and Outs of Mitochondrial Calcium. Circ Res. 2015;116:1810. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottipati MK, Kalinina I, Bekyarova E, Haddon RC, Parpura V. Chemically Functionalized Water-Soluble Single-Walled Carbon Nanotubes Modulate Morpho-Functional Characteristics of Astrocytes. Nano Lett. 2012;12:4742–4747. doi: 10.1021/nl302178s. [DOI] [PubMed] [Google Scholar]
- Gouriou Y, Demaurex N, Bijlenga P, De Marchi U. Mitochondrial calcium handling during ischemia-induced cell death in neurons. Biochimie. 2011;93:2060–2067. doi: 10.1016/j.biochi.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Rutter GA. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2009;1787:1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M, Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. GLIA. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Li H, Wang X, Zhang N, Gottipati MK, Parpura V, Ding S. Imaging of mitochondrial Ca2+ dynamics in astrocytes using cell-specific mitochondria-targeted GCaMP5G/6s: Mitochondrial Ca2+ uptake and cytosolic Ca2+ availability via the endoplasmic reticulum store. Cell Calcium. 2014;56:457–466. doi: 10.1016/j.ceca.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, Satröstegui J. Calcium-Regulation of Mitochondrial Respiration Maintains ATP Homeostasis and Requires ARALAR/AGC1-Malate Aspartate Shuttle in Intact Cortical Neurons. The Journal of Neuroscience. 2013;33:13957–13971. doi: 10.1523/JNEUROSCI.0929-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. Chemical calcium indicators. Methods. 2008;46:143–151. doi: 10.1016/j.ymeth.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca2+ homeostasis: mechanism, role, and tissue specificities. Pflugers Arch - Eur J Physiol. 2012;464:3–17. doi: 10.1007/s00424-012-1122-y. [DOI] [PubMed] [Google Scholar]
- Qiu J, Tan YW, Hagenston AM, Martel MA, Kneisel N, Skehel PA, Wyllie DJA, Bading H, Hardingham GE. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Commun. 2013;4 doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Current biology : CB. 1995;5(6):635–642. doi: 10.1016/s0960-9822(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Meth. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S. Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience. 2010;170:992–1003. doi: 10.1016/j.neuroscience.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Michikawa T, Hashimoto M, Horikawa K, Nagai T, Miyawaki A, Hausser M, Mikoshiba K. Quantitative comparison of genetically encoded Ca2+ indicators in cortical pyramidal cells and cerebellar Purkinje cells. Frontiers in Cellular Neuroscience. 2011;5 doi: 10.3389/fncel.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]