Abstract
A large number of medications and medical devices removed from the market by the US Food and Drug Administration over the past 4 decades specifically posed greater health risks to women. This article reviews the historical background of sex and gender in clinical research policy and describes several approved drugs and devices targeted for use in women that have caused major morbidity and mortality. The intended population for the medications and devices, population affected, approval process, and the basic and legal actions taken against the medication/drug company are also discussed. It is recognized that women are still at risk for harm from unsafe medications and devices, and continued improvements in legislation that promotes inclusion of sex and gender into the design and analysis of research will improve safety for both men and women.
Keywords: sex and gender, medication and device safety
INTRODUCTION
Inequitable sex and gender distribution in pharmacologic research and subsequent clinical trials can result in downstream toxic effects in women. Unanticipated adverse events or fetal injury, associated with significant morbidity and mortality, continue to be reported in mainstream media, peer-reviewed literature, and government-sponsored reporting systems. The present commentary describes drugs and devices targeted for use in women that have caused major morbidity and mortality. The controversy surrounding many of these drugs and devices has altered the current landscape of research policy and drug and device approval. We review the historical background of the effects of sex and gender in clinical research policy and present a series of notable medications and devices removed from the market due to serious adverse drug reactions in women, as well as several undergoing public scrutiny due to current safety concerns in women and their offspring.
HISTORICAL BACKGROUND
The metabolism of pharmaceutical drugs, which includes the duration and intensity of a drug’s pharmacologic action, varies between men and women. These variations may be due to sex differences in reproductive physiology, hormone variances, and genetic polymorphisms, and they have led to numerous safety issues in women because they experience more frequent and more severe adverse drug events than male subjects.1,2 Historically, women have been under represented in clinical trials, with resulting research predominantly being performed on male animals and men. In many cases, this inherent bias in research design has led to catastrophic consequences for women. In the 1960s and 1970s, women of childbearing age and their offspring suffered debilitating health outcomes due to the use of several unsafe medications.
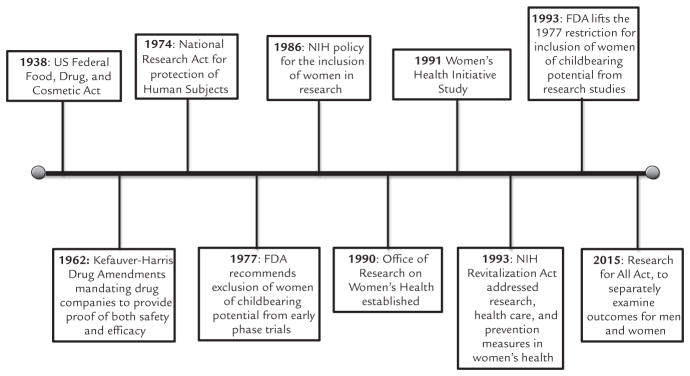
The bias against including women in clinical trials was initially intended to be protective to prevent potentially negative consequences for women if they became pregnant during the study.3 Owing to political, legal, and social forces, these protectionist policies led to a disparity between sexes. Although policies have advanced to be more inclusive of female subjects in research, sex-specific adverse events still occur. This phenomenon may be due to undetected or unrecognized adverse events that occur during initial approval and subsequent off-label use. In particular, this scenario may occur when novel therapeutic agents are not tested in the intended population using the pharmaceutical. In light of these adverse drug reaction reports, advancements over the past decades have promoted more inclusive safety studies, although there is still a lag with resultant safety issues in women. The Figure outlines historically important policy changes.
Figure.
Historical timeline of important sex- and gender-related clinical research policy. FDA = US Food and Drug Administration; NIH = National Institutes of Health.
Morbidity to offspring caused by 2 medications in particular, thalidomide and diethylstilbestrol (DES), fueled the desire to protect fetuses and women of childbearing potential.3 The adverse events associated with thalidomide and DES became examples for the importance and creation of drug safety policy leading to numerous changes in legislation in the United States. At the time the thalidomide application was filed with the US Food and Drug Administration (FDA) in 1960, the agency did not have the authority to require proof of efficacy of drugs.4 After thalidomide was recognized to cause significant birth defects, legislation was introduced that provided the FDA authority to require efficacy and safety data before drug approval.4,5 The Kefauver-Harris Drug Amendments to the US Federal Food, Drug, and Cosmetic Act of 1938 were signed into law on October 10, 1962, mandating that drug companies provide proof of both safety and efficacy of their products before approval.6 In addition, it required consent of human subjects during clinical trials and stated that investigational drugs must be tested in animals before human subjects.7
The creation of new drug safety policy in the United States continued with the National Research Act of 1974, which established a National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Their obligations included identifying ethical principles and developing guidelines for research involving human subjects. One year later, regulations created by the national commission regarding research on fetuses and pregnant women were incorporated into the Department of Health and Human Services regulations. Although the original drafts included restrictions on the inclusion of women of childbearing potential, these were removed from the final regulations.8 Despite removal of restrictions, the FDA issued guidelines in 1977 for drug development which recommended that women of childbearing potential be excluded from the early phases of drug trials, arguably setting back advances in women’s health.9
With continued recognition of adverse drug events in women, advocates of women’s health began to speak out against the disparity. In 1985, the US Public Health Service Task Force on Women’s Health Issues reported that the “historical lack of research focus on women’s health concerns has compromised the quality of health information available to women as well as the health care they receive.”10 This statement provoked the National Institutes of Health (NIH) to create a policy in 1986 urging researchers applying for grants to include women in clinical research and to evaluate their studies according to sex and gender differences. However, in 1990, the General Accounting Office released a report stating that the NIH policy was ineffective.11 After publication of this report, the NIH created an Office of Research on Women’s Health, which was mandated to support and enhance research on diseases affecting women’s health, to ensure that women were adequately represented in research studies and to help increase the enrollment of women in biomedical research. Similar changes were implemented in the following years, including the establishment of the Women’s Health Initiative in 1991 and the NIH Revitalization Act of 1993, which contained 6 bills that addressed research, health care, and prevention measures in women’s health. However, despite these changes, the General Accounting Office issued another report in 1992 stating that although women were often included in drug trials, far fewer were represented than the number of women in the population with the disease of interest for >60% of the drugs. The report also noted that pharmaceutical manufacturers often did not analyze data for sex and gender differences.
With all of these criticisms circulating, in 1993, the FDA finally lifted the 1977 restriction on the inclusion of women of childbearing potential in early clinical trials. They also reinforced their expectations regarding analysis of trials according to sex and gender differences in pharmacokinetic and pharmacodynamic principals, and the need for an increase of studies involving women.10 Efforts to equalize men and women with respect to clinical research have continued through to the present day. The Research for All Act of 2015 amends the Public Health Service Act to require the NIH to ensure that basic research projects include both male and female cells, tissues, or animals.12
Between 1997 and 2000, eight of the ten drugs withdrawn from the market posed a greater health risk for women either due to unanticipated gender-prescribing trends or sex-specific adverse drug reactions.13 Despite the immense progress made with respect to equality in human research, women are still at greater risk of harm then men by unsafe medications and devices approved for their use. The Table outlines the medications and devices discussed here.
Table.
Selected approved drugs and devices that demonstrated health risks to women.
| Drug/Device | Years on Market | Intended Use | Health Risk |
|---|---|---|---|
| Thalidomide | Did not gain FDA approval in United States | Treatment of morning sickness associated with pregnancy | Fetal phocomelia (absent or shortened limbs) |
| Diethylstilbestrol | 1940–1971 | Prevent miscarriage, premature labor, and other pregnancy complications | Increased risk of breast cancer in female subjects using medication and increased clear cell adenocarcinoma, birth defects, and other developmental abnormalities in offspring |
| Fenfluramine | 1973–1997 | Appetite suppressant | Valvular heart disease |
| Alosetron | February 2000–November 2000 | Treatment of irritable bowel syndrome | Ischemic colitis, severe constipation, mesenteric ischemia, death |
| Tegaserod | 2002–2007 | Irritable bowel syndrome with constipation | Increased risk for heart attack, stroke, and unstable angina |
| Ondansetron | Approval in 1991, presently available | Nausea and vomiting in pregnancy | Fetal birth defects |
| Essure® | Approval in 2002, presently available | Permanently implanted birth control device for women (female sterilization) | Persistent pain, perforation of the uterus and/or fallopian tubes, device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions |
| Transvaginal mesh | Approval in 1996, presently available | Pelvic organ prolapse and urinary incontinence | Persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions |
THALIDOMIDE
Thalidomide is a synthetic drug that was first marketed in Germany in 1957 by the company Chemie Grünenthal under the name “Contergan.”14 Thalidomide, synthesized in 1953, was initially explored for bactericidal properties. Although the initial animal models failed to demonstrate antimicrobial activity, Chemie Grünenthal noted that the drug was nonfatal in extremely high doses in animals and determined the drug to be nontoxic.15,16 Initial human trials explored thalidomide as an antiepileptic agent but failed.14 It was, however, observed to be an excellent sedative and treatment of morning sickness in female subjects, and it quickly became the third best-selling drug in Europe. The company distributed free samples of the drug to physicians in West Germany and Switzerland in 1955 without a plan for monitoring or follow-up.16 Starting on October 1, 1957, thalidomide was made available over the counter in Germany. It was reported to be extremely safe because it was never observed to cause a fatality in overdose.17 However, data were based on limited animal studies. Thalidomide was initially available for nearly 5 years, being sold in 46 countries and under at least 37 different names, before it was withdrawn from the market for its teratogenic associations.14,18
Although thalidomide was primarily sold as a sedative and for the treatment of morning sickness in pregnant female patients, it was also marketed as effective for the treatment of numerous other conditions, including alcoholism, anxiety, arthritis, asthma, depression, emotional instability, influenza, premature ejaculation, premenstrual symptoms, stress headaches, and tuberculosis.4,17 As a result, numerous pharmaceutical companies manufactured the drug and sold it under a variety of names for various conditions across the world over the next few years.17
The US FDA received an application from the William S. Merrell Company to market thalidomide in September 1960.14,19 Although the drug was widely used in Europe, Dr. Frances Oldham Kelsey was concerned about the lack of safety data presented in the application and requested more data. While the application was under review by the FDA, thalidomide stopped being sold over the counter and required a prescription in Germany due to reports of associated peripheral neuritis with chronic use.14 In November 1961, Dr. Widukind Lenz of Germany wrote to the manufacturer regarding his concern that thalidomide was responsible for the outbreak of phocomelia (absent or shortened limbs) in children born to mothers taking thalidomide in Europe at the time. Shortly thereafter, the Lancet published a letter written by Dr. William McBride from Australia reporting an association between thalidomide use during pregnancy and congenital abnormalities.20 Once the association was confirmed, the drug was withdrawn from the world market. The William S. Merrell Company withdrew their application to market the drug in March 1962 before the drug ever received approval in the United States.14 During its time on the market, thalidomide is believed to have caused congenital abnormalities in > 10,000 children worldwide.4
After withdrawal of the drug from the market, legal action was brought against Chemie Grünenthal and 9 of its officials for their role in the distribution and sales of thalidomide.21 This was brought to trial as a criminal action case based on alleged violation of drug laws in West Germany, homicide, and negligence. In December 1969, a financial settlement was reached in an amount close to $30,000,000.21 The settlement was distributed among parents of nearly 2000 surviving children (approximately $19,000 per child), 800 adults, and court costs. However, the court dropped the criminal charges on the basis that criminal intent had never been proven. In addition to legal actions taken against the drug makers, the thalidomide experience helped develop the postmarketing surveillance culture, as well as having a significant effect on the regulatory process of pharmaceuticals in the United States and abroad.5,7
Currently, thalidomide is approved by the FDA for the treatment of erythema nodosum and leprosy.16 In 1965, Dr. Jacob Sheskin treated some of his critically ill patients with thalidomide and observed resolution of their symptoms, including skin lesions.18 After further studies, the drug was approved by the FDA in July 1998 with a restricted prescribing and dispensing program called the System for Thalidomide Education and Prescribing Safety (S.T. E.P.S). This program required patients to view an educational video. Female patients are instructed to use 2 methods of contraception and undergo regular pregnancy testing, while male patients are required to use condoms during sexual intercourse with female subjects of childbearing age. Since its approval in 1998, ongoing investigations are exploring the use of thalidomide as an anti-angiogenic, anti-inflammatory, and immunomodulatory agent.
DIETHYLSTILBESTROL
DES, a nonsteroidal, high potency estrogen compound, was first synthesized in 1938.22 DES exerts it effects by interacting with intracellular estrogen receptors. The drug was initially FDA-approved for the treatment of vaginitis, postmenopausal symptoms, and postpartum lactation suppression.23,24 In the early 1940s, literature published by Smith et al25,26 suggested that estrogen deficiency was responsible for miscarriage and that DES administration during pregnancy decreased fetal mortality. This report prompted off-label prescribing of DES by physicians to pregnant women with high-risk features (eg, previous miscarriages, diabetes, a history of gynecologic surgical procedures) for the prevention of miscarriage. DES was eventually FDA-approved for this indication in 1947. The trials on which the approval was based have been widely criticized for poor design and inadequate controls. Between the early 1940s and late 1960s, an estimated 5 to 10 million individuals were exposed to the drug, including pregnant mothers and their offspring.27 The use of DES in pregnancy began to decline in the 1950s as clinical trial results disputed claims of its efficacy.28 However, it was still prescribed to pregnant female subjects until the publication of the hallmark case series by Herbst et al29 that described a cluster of unusual cases of clear cell vaginal adenocarcinoma (CCA) in young female subjects who were exposed to DES in utero. This series, as well as subsequent epidemiologic studies, demonstrated the DES–CCA correlation, which led to the FDA’s recommendation against its use in pregnancy in 1971.30 DES is currently only used in veterinary medicine and research trials.31
Extensive epidemiologic studies have followed up with DES-exposed mothers and their offspring (the later often referred to as DES daughters and DES sons) to determine the long-term effects. The female patients who took DES during pregnancy are at a moderately increased risk for breast cancer.32 DES daughters are the most extensively studied group among those exposed, particularly with regard to CCA risk, with a current estimated risk of CCA of 1 in 1000.33 In addition, DES daughters have also been shown to be at increased risk for breast cancer, genital tract abnormalities, complications of pregnancy (eg, ectopic pregnancy, preterm delivery, neonatal death), and melanoma.34–36 DES sons have higher rates of genital tract abnormalities, such as hypospadias, epididymal cysts, and cryptorchidism, than nonexposed male subjects.37,38 Animal studies and early epidemiologic studies also questioned an increased risk of testicular cancer in DES sons, although there is currently no evidence to support this assertion. Current studies have shown no increased risk of cancer or genital tract abnormalities associated with third-generation DES descendants (those born to DES sons and daughters); however, female subjects in this group may have increased risk of menstrual irregularities.39,40
With millions of individuals affected years after the exposure has ended, the current chapter of the DES story focuses on caring for and compensating those affected. A major barrier to early litigation and attempts at compensation for the affected individuals was the difficulty proving the exact source of exposure (and thus the responsible party). Dodds et al41 first synthesized DES but never patented the drug due to regulations from their federal funding source. Consequently, numerous pharmaceutical companies simultaneously manufactured and marketed DES between the 1940s and the 1960s. This made it difficult or impossible for offspring to determine the source of their mothers’ DES exposure decades later. However, in the landmark 1980 case of Sindell v Abbott Laboratories, the Supreme Court of California determined that plaintiffs can hold multiple manufacturers of an identical drug responsible for the adverse outcome if the exact source cannot be identified, which is referred to as the market share liability doctrine.42 More recently, DES ligation has focused on breast cancer cases. In 2013, a total of 51 women brought suit against multiple pharmaceutical companies, including Eli Lily, for breast cancer as a result of in utero DES exposure; many of these cases have been settled.
Federal agencies such as the Centers for Disease Control and Prevention and the NIH continue to follow up DES-exposed female subjects and their descendants and to monitor their health outcomes. More research is needed to determine if DES exposure will continue to affect further generations of DES descendants.
FENFLURAMINE-PHENTERMINE
Fen-phen, the common name for the combination drug fenfluramine-phentermine, was a weight loss agent that was sold in the United States in the mid-1990s. Despite its popularity, the use of this medication was off-label because studies were never submitted to the FDA demonstrating the effectiveness or safety of the combination drug, and the FDA never approved the combination product or the long-term use of fenfluramine.43,44 Despite the lack of FDA approval, the drug became widely prescribed in the United States during the mid-1990s, with >18 million prescriptions for the combination medication by 1996.45 The prescribing of fen-phen was based on a single-center study of 121 patients in which patients received treatment with fen-phen or a placebo for up to 4 years.46 Despite less than one third of the patients completing the study, many of whom regained weight in later phases of the trial, the results were published in 1992, and phen-fen was viewed as an effective weight loss agent in the popular press. This study set the precedent for the use of phen-fen in the long-term treatment of obesity.
Phentermine was approved by the FDA in 1959, fenfluramine was approved in 1973, and dexfenfluramine (the d-isomer of fenfluramine) was approved in 1996.46 Fenfluramine was approved by the FDA after the Amphetamine Anorectic Drug Project. The project was a meta-analysis of >10,000 patients that studied weight loss data. It determined that amphetamines and amphetamine congeners, including fenfluramine, were efficacious, but recommendations were made to limit their use to the short term in an effort to limit the risk of addiction. The application to market dexfenfluramine was not reviewed by the FDA until September 1995. At the time, the FDA advisory committee recommended that at least 1500 obese patients be studied for 1 year in a placebo-controlled study and that 200 to 500 of these patients be followed up for a second year. Dexfenfluramine was granted approval in 1996 and was on the market for 16 months before being voluntarily withdrawn.
Fenfluramine is a fluorinated amphetamine derivative believed to lack the psychomotor stimulant effects and abuse potential traditionally recognized with amphetamines.44 Fenfluramine’s activity is mediated through serotonergic pathways by promoting release of serotonin and inhibiting its reuptake.44,45 Phentermine is a noradrenergic drug that directly stimulates the sympathomimetic pathway and also interferes with the pulmonary clearance of serotonin.45,47
While the risks of developing pulmonary hypertension associated with the use of fenfluramine and phentermine were already known, the first reports of cardiac valvular disease associated with the medications were published in July 1997.45 The Mayo Clinic described 24 cases of an unusual cardiac valvular disease in patients taking fen-phen.43,45 All 24 cases described in this initial case series were women.45 At the same time, an additional 28 cases in 18 states were also published in the New England Journal of Medicine.48 Of these patients, all had a cardiac valvular insufficiency involving a left-sided valve, and ≥2 valves were affected in 78% of patients. These findings led the FDA to issue a public health advisory requesting other cases to be reported, further epidemiologic studies to be initiated, and for prevalence studies of valvular disease in patients taking fen-phen, fenfluramine or dexfenfluramine to be performed.43,48 Although additional studies demonstrated minimal increased risk of developing valvular insufficiencies in patients who took fenfluramine or dexfenfluramine for <3 months, the risk increased greatly if the medications were taken for ≥4 months.49
In September 1997, the FDA asked manufacturers to voluntarily withdraw fenfluramine and dexfenfluramine from the market.44 By the summer of 1999, there were thousands of class action lawsuits against fen-phen manufacturers being reviewed in the courts. In October 1999, approval was granted to the plaintiff attorneys for a single nationwide class action lawsuit.50 After the announcement of the nationwide class action lawsuit, a settlement was reached. The settlement plan divided patients into those who had received the drug for ≤60 days and those who had received the drug for >60 days. It involved compensation for patients with a diagnosis of a valvular regurgitation by September 30, 1999, and a medical monitoring program for patients without diagnosed valvular insufficiency. It also allowed individuals to opt out of the class action suit and pursue their own claims for damages.
ALOSETRON AND TEGASEROD
Alosetron* and tegaserod†,‡ are prescription medications marketed for relief of irritable bowel syndrome (IBS). Early clinical research suggested that these medications were predominately effective in women, with little benefit to men. Therefore, further clinical trials focused on women, and both medications were only approved by the FDA for use in women.51,52 Sub sequently, both medications were removed from the market due to concerns for major health risks that were overlooked (in the case of alosetron) or unknown before FDA approval.
Alosetron was approved by the FDA in 2000 as a medication for the treatment in women with severe diarrhea-predominant type IBS. It is a 5-HT3 antagonist that slows the movement of fecal matter through the large intestine, increasing the extent to which water may be absorbed from the fecal content, thereby decreasing the moisture and volume of the remaining waste products.53 During clinical trials, constipation among the alosetron group was the most frequently reported reason for withdrawal from the study.51,54 Although initial trials also reported cases of ischemic colitis, drug makers claimed that there was no evidence to support an association of alosetron with ischemic colitis.55 Within 9 months of FDA approval, the drug was voluntarily withdrawn from the market by its manufacturers after multiple cases of morbidity and mortality were reported, including: 143 hospital admissions, 113 reports of severe constipation, 84 instances of ischemic colitis, and 7 deaths.56 The FDA approved a supplemental New Drug Application for alosetron in 2002 that allows restricted marketing of the drug, only to treat women with severe diarrhea-predominant type IBS. The FDA received intense criticism for its handlings of alosetron. Editorials published in the Lancet and British Medical Journal suggested that the FDA failed its mission, that the approval process was an example of regulatory capture, approval should have been revoked earlier, and the reinstatement of alosetron was negotiated in confidential with representatives from GlaxoSmithKline.55,57
Tegaserod is manufactured by Novartis Pharmaceuticals Corporation and was approved in 2002 by the FDA for the short-term treatment of women with IBS whose primary bowel symptom is constipation. Its mechanism of action is through activation of 5-HT4 receptors of the enteric nervous system in the gastrointestinal tract; it stimulates gastrointestinal motility, the peristaltic reflex, and is purported to reduce abdominal pain. Five years later, in 2007, tegaserod was removed from the market due to concerns regarding possible adverse cardiovascular effects. The manufacturer denies an association, although the Phase III clinical trials were not powered to detect the adverse events that were later recognized by using a pooled data analysis. Tegaserod exhibited a higher risk of serious cardiovascular adverse events compared with placebo. In tegaserod-treated patients, 13 experienced cardiovascular ischemic events (0.11%), including 6 cases of severe cardiac ischemia, 4 myocardial infarctions, 3 strokes, and 1 death (secondary to myocardial infarction).58 These outcomes are compared with 1 patient who received placebo who had symptoms of stroke (0.01%). Tegaserod was reintroduced on an emergency basis in 2007.
ONDANSETRON
The popular anti-nausea mediation ondansetron,§ manufactured by GlaxoSmithKline, was approved for use by the FDA in 1991 to treat nausea and vomiting after chemotherapy, radiation therapy, and surgery. Ondansetron is prescribed off-label to treat nausea and vomiting in pregnancy and is used by ~1 million female patients each year throughout the world.59 Ondansetron started being used in pregnant female subjects despite lack of FDA approval in pregnancy and before any studies examining its safety were conducted among this population. Moreover, there are allegations that early animal studies showed evidence of congenital heart defects in fetuses, with human studies showing the medication’s ability to cross the placenta.
An early study by investigators from Motherisk Program at The Hospital for Sick Children in Toronto 2004 showed no increased risk for fetal malformations; however, this study was an underpowered sample unable to detect small differences.60 In 2011, Anderka et al61 published data showing a 2-fold increased risk in cleft palate for offspring of mothers using ondansetron. Danielsson et al62 found a statistically significant increase in fetal risk for a cardiovascular defect (primarily a septal defect). Although multiple studies show a small, but significant, risk for ondansetron causing fetal teratogenesis after administration to pregnant female subjects, other studies refute this finding.62
The drug manufacturer GlaxoSmithKline agreed to plead guilty in 2012 to federal charges of fraud and illegal promotion, with financial inducements to physicians for off-label unapproved use of several drugs, including ondansetron. It paid $3 billion as part of this legal settlement.59 Further litigation by families affected by birth defects are currently proceeding due to concerns that drug makers marketed the mediation to pregnant female subjects without FDA approval in this population, and claimed its safety in the absence of safety data, and with the knowledge that the medication could be harmful and cross the human placenta.
ESSURE
Effective, permanent female sterilization has traditionally relied on laparoscopic interventions such as tubal ligation. Improving technology and the desire to perform sterilization in the most minimally invasive manner has led to multiple attempts of an endocervical approach to sterilization.63 Hysteroscopic electrocautery, mechanical obstruction of the fallopian tubes, and drug-based sclerosis of the uterotubal junction have all been attempted, with varying degrees of success.64–66 In response to advances in sterilization techniques, development of a non-invasive method of sterilization, the Essure® device (marketed by Conceptus Incorporated), was approved by the FDA in 2002.67 Essure is a stainless steel, nickel titanium elastic 4 cm × 2 cm flexible coil with polyethylene fibers that is hysteroscopically inserted into the fallopian tubes bilaterally. The nickel titanium and polyethylene fibers induce a local fibrosis with proliferation of benign epithelium over ~3 months that ultimately completely occludes the fallopian tube, resulting in sterilization. Advantages of the Essure device include the ability to place coils in the outpatient setting with oral analgesics and minimal local anesthesia. Female subjects who receive Essure are encouraged to use an alternative form of birth control for 3 months to allow time for tissue overgrowth, at which time hysterosalpingography or ultrasound is performed to confirm tubal occlusion and sterilization.68
Two industry-sponsored nonrandomized, non-blinded prospective trials enrolling a total of 926 female subjects piloted the Essure device before FDA approval.69,70 These early studies in 2002 suggested that Essure placement was simple and effective in 90% of female subjects.71 In those who did not experience subsequent sterilization, it was determined that the likely etiology was failure of one of the devices; a second procedure to achieve bilateral placement improved success rates to 97%.70 Although Essure is marketed as a lifelong, permanent sterilization technique, early studies focused on short-term (1-year) outcomes. Reliability was determined based solely on a subgroup of female subjects who successfully had bilateral Essure devices implanted and completed a 3-month follow-up to confirm fibrosis at the uterotubal junction.69 Perhaps more concerning, long-term follow-up in the initial Essure trials was poor: only 85% of individuals were evaluated at the 1-year follow-up, and just 25% of individuals completed the 2-year follow-up. Despite significant limitations in these trials, the FDA fast-tracked the Essure device for approval on the condition that the company extend monitoring to 5 years in the original cohort of female subjects who received the device.67 Data from the 5-year follow-up studies have been limited; neither study was registered under Clinicaltrials.gov, 1 postapproval study remains unpublished, and a second study was published nearly 7 years after study completion and 13 years after device approval.69,72
Adverse events related to Essure include unintended pregnancy, allergic reactions to the nickel titanium elastic, persistent pain, and migration of Essure devices.73,74 A true understanding of the magnitude of complications associated with Essure is difficult because most adverse events remain unreported. Published case reports detail bowel injury, device migration, nickel allergy, and unintended pregnancy after successful Essure placement.74–76 Unintended intra-uterine pregnancy remains a significant concern after Essure placement. One postmarketing subgroup analysis on female subjects who reported unintended pregnancy to Conceptus Incorporated after Essure placement revealed poor adherence with 3-month follow-up and misinterpretation of hysterosalpingogram by physicians as leading etiologies of unintended pregnancy.77 A query of the FDA’s Manufacturer and User Facility Device Experience (MAUDE) Database from 2002 to 2012 reported 61 unintended pregnancies after Essure placement, of which 47.5% were ectopic gestations.67 In addition, nearly 5000 adverse events related to Essure have been submitted through the MAUDE Database maintained by the FDA since the device’s approval.69,78 Given these concerns, the FDA convened a meeting of the Obstetrics and Gynecology Devices panel in 2015 to review safety data on the Essure device, which lead to revised guidelines for providers and patients seeking Essure placement.79 Given the increasing number of adverse events reported to MAUDE, the panel ordered the Essure parent company (Bayer, Whippany, New Jersey) to conduct extended postmarketing surveillance studies regarding the benefits and risks of Essure placement. In addition, the FDA provided a draft patient checklist to improve understanding of the risks and benefits of Essure placement.
TRANSVAGINAL MESH
Pelvic organ prolapse (POP) and urinary incontinence remain major causes of morbidity among females worldwide.80 In female subjects, one of the most common etiologies of POP or urinary incontinence remains injury to the pelvic floor muscles during childbirth. Up to 30% to 50% of female subjects will develop POP in their lifetime, with ~2% developing symptoms necessitating pharmacologic treatment, device inserts (pessary), pelvic muscle exercises, or surgical repair. When pharmacologic treatments and mechanical solutions to POP and urinary incontinence fail, surgical approaches to reinforce pelvic floor muscles and prevent recurrent prolapse or incontinence are available.81,82 Since the 1950s, surgeons have experimented with a variety of synthetic and natural grafts to reinforce the pelvic floor to prevent POP and incontinence. Adaptation of tantalum mesh used for hernia repair for POP was first described in 1955, although persistent defects in the vaginal wall after surgery were common.83 Existing mesh used for abdominal hernia repair were custom-cut by surgeons to approximate and help reconstruct pelvic floor muscles. High-density, non-absorbable polypropylene or polyethylene mesh gained popularity in the early 1990s.84 Long-term follow-up of individuals with POP repaired by using high-density, nonabsorable polypropylene or poly-ethylene showed that despite improved sexual function and resolution of prolapse, there remained poor satisfaction (only 42% of individuals reported satisfaction with mesh at 23 months’ postprocedure).82,85
The first preconfigured surgical mesh devices specifically designed to treat urinary incontinence in female subjects was cleared by the FDA through the 510(k) pathway, a mechanism for the FDA to determine safety of medical devices intended to enter the consumer market, in 1996. Since this initial approval in 1996, multiple urogynecologic mesh devices have cleared the 510(k) pathway, with up to 168 different devices currently available in the US market. Mesh devices come in 4 different categories: nonabsorbable synthetic, absorbable synthetic, biologic, and a composite device incorporating features from other categories.81,82 These devices are created to match common defects in the pelvic floor that cause POP or incontinence. With increased utilization of mesh products, an increasing number of reports of adverse events have been reported to the FDA, prompting a warning issued in 2008 detailing a rare but present risk of mesh erosion, recurrent prolapse, wound infection, and debilitating dyspareunia. Despite the FDA notification, studies show that mesh procedures continued to remain popular in the surgical treatment of POP and urinary incontinence despite a complication rate of nearly 30%.86
In the interim from 2008 to 2011, the FDA completed a second audit of the MAUDE database and discovered an additional 2874 cases of adverse events related to mesh slings. Of these adverse events, erosion of the mesh remained the most commonly reported event, followed by persistent pain and surgical site infection. The most frequent intervention required for reported adverse events was operative intervention with mesh revision or removal. After review, the FDA issued an update on transvaginal mesh devices upgrading their previous notice in 2008 from “rare adverse events experienced after mesh placement” to “complications after mesh placement are not rare.”
CONCLUSIONS
Gender differences in research policies have historically put women and their offspring at risk for severe associated morbidity and mortality. Significant changes to regulatory measures have been enacted to ensure that novel xenobiotics are carefully evaluated and that sex differences in drug effect and metabolism are considered. Despite research and legislative advances, significant adverse drug events continue to be reported in association with novel medications and devices. Recognizing the persistent gender bias in clinical trials and drug discovery trials, the NIH and FDA continue to develop methods to reach and encourage women to participate in studies. Notably, the NIH asks companies requesting new drug applications to present safety effectiveness data according to sex and gender. Continuing education that describes novel methods to ensure gender equality in clinical trials and the creation of a sex and gender analysis work group demonstrate the continued need for inclusion of women in clinical trials and analysis of sex differences in drug metabolism.
It is increasingly recognized that adverse drug events occur at varying rates among men and women. Novel drug development, clinical trials, and advanced device development should seek to enroll and study both men and women equitably and separately. Historical precedence has described a generation of morbidity and mortality associated with unrecognized sex- and gender-specific adverse events that continue to affect individuals today. Although significant advances have been made to recognize and prevent additional gender bias in future drug and device trials, continued scrutiny and vigilance should be practiced among manufacturers and researchers as novel sex-and gender-specific adverse events continue to occur.
Acknowledgments
Alyson McGregor, MD. Authors: Carey, Nader, Chai, Carreiro, Boyle were responsible for literature review, writing, and editing. Carey, Nader created the figure and table. Griswold was responsible for literature review, editing and technical aspects.
Footnotes
Trademark: Lotronex® (Abbott Laboratories Corporation, Abbott Park, Illinois).
Trademark: Zelnorm® (Novartis Pharmaceuticals Corporation, East Hanover, New Jersey).
Trademark: Zelmac® (Novartis Pharmaceuticals Corporation).
Trademark: Zofran® (GlaxoSmithKline, Research Triangle Park, North Carolina).
CONFLICTS OF INTEREST
None.
References
- 1.Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol. 2013;38:1003–1009. doi: 10.1177/009127009803801103. [DOI] [PubMed] [Google Scholar]
- 2.Martin RM, Biswas PN, Freemantle SN, et al. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 2002;46:505–511. doi: 10.1046/j.1365-2125.1998.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parekh A, Fadiran EO, Uhl K, Throckmorton DC. Adverse effects in women: implications for drug development and regulatory policies. Expert Rev Clin Pharmacol. 2011;4:453–466. doi: 10.1586/ecp.11.29. [DOI] [PubMed] [Google Scholar]
- 4.Avorn J. Learning about the safety of drugs—a half-century of evolution. N Engl J Med. 2011;365:2151–2153. doi: 10.1056/NEJMp1110327. [DOI] [PubMed] [Google Scholar]
- 5.Emanuel M, Rawlins M, Duff G, Breckenridge A. Thalidomide and its sequelae. Lancet. 2012;380:781–783. doi: 10.1016/S0140-6736(12)60468-1. [DOI] [PubMed] [Google Scholar]
- 6.Turner JR. The 50th anniversary of the Kefauver-Harris amendments: efficacy assessment and the randomized clinical trial. J Clin Hypertens (Greenwich) 2012;14:810–815. doi: 10.1111/jch.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelsey FO. Thalidomide update: regulatory aspects. Teratology. 1988;38:221–226. doi: 10.1002/tera.1420380305. [DOI] [PubMed] [Google Scholar]
- 8.Mastroianni A. Women and health research: ethical and legal issues of including women in clinical studies (2 vols): children as research subjects: science, ethics and law. BMJ. 1994;309:1095. [Google Scholar]
- 9.Merkatz RB. Inclusion of women in clinical trials: a historical overview of scientific, ethical, and legal issues. J Obstet Gynecol Neonatal Nurs. 1998;27:78–84. doi: 10.1111/j.1552-6909.1998.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 10.Mastroianni AC, Faden R, Federman D. Women and health research: a report from the Institute of Medicine. Kennedy Inst Ethics J. 1994;4:55–62. doi: 10.1353/ken.0.0121. [DOI] [PubMed] [Google Scholar]
- 11.Hathaway C. A patent extension proposal to end the underrepresentation of women in clinical trials and secure meaningful drug guidance for women. Food Drug Law J. 2012;67:143–176. [PubMed] [Google Scholar]
- 12.Cooper JHR. 2101—Research Act for All 2015. https://www.congress.gov/bill/114th-congress/house-bill/2101.
- 13.Heinrich J, Gahart MT, Rowe EJ, Bradley L. A letter to The Honorable Tom Harkin. Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women. 2001 Available at: http://www.gao.gov/products/GAO-01-286R.
- 14.Taussig HB. Thalidomide and phocomelia. Pediatrics. 1962;30:654–659. [PubMed] [Google Scholar]
- 15.Dally A. Thalidomide: was the tragedy preventable? Lancet. 1998;351:1197–1199. doi: 10.1016/S0140-6736(97)09038-7. [DOI] [PubMed] [Google Scholar]
- 16.Silverman WA. The schizophrenic career of a “monster drug. Pediatrics. 2002;110:404–406. doi: 10.1542/peds.110.2.404. [DOI] [PubMed] [Google Scholar]
- 17.Taussig HB. The evils of camouflage as illustrated by thalidomide. N Engl J Med. 1963;269:92–94. doi: 10.1056/NEJM196307112690207. [DOI] [PubMed] [Google Scholar]
- 18.Matthews SJ, McCoy C. Thalidomide: a review of approved and investigational uses. Clin Ther. 2003;25:342–395. doi: 10.1016/s0149-2918(03)80085-1. [DOI] [PubMed] [Google Scholar]
- 19.Bren L, Kelsey Frances Oldham. FDA medical reviewer leaves her Markon history. FDA Consum. 2001;35:24–29. [PubMed] [Google Scholar]
- 20.McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;1:552–553. doi: 10.1016/s0140-6736(63)91347-3. [DOI] [PubMed] [Google Scholar]
- 21.Curran WJ. The thalidomide tragedy in Germany: the end of a historic medicolegal trial. N Engl J Med. 1971;284:481–482. doi: 10.1056/NEJM197103042840906. [DOI] [PubMed] [Google Scholar]
- 22.Dodds EC, Goldberg L, Lawson W, Robinson R. Œstrogenic Activity of Certain Synthetic Compounds. Nature. 1938;141:247–248. [Google Scholar]
- 23.Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation. 2012;84:252–260. doi: 10.1016/j.diff.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noller KL, Fish CR. Diethylstilbestrol usage: its interesting past, important present, and questionable future. Med Clin North Am. 1974;58:793–810. doi: 10.1016/s0025-7125(16)32122-8. [DOI] [PubMed] [Google Scholar]
- 25.Smith OW, Smith GVS, Schiller S. Estrogen and progestin metabolism in pregnancy. Spontaneous and induced labor. J Clin Endocrinol Metab. 1941;1:461–469. [Google Scholar]
- 26.Smith OW, Smith G. The influence of diethylstilbestrol on the progress and outcome of pregnancy as based on a comparison of treated with untreated primigravidas. Am J Obstetrics Gynecol. 1949;58:994–1009. doi: 10.1016/0002-9378(49)90204-5. [DOI] [PubMed] [Google Scholar]
- 27.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 28.Dieckmann WJ, Davis ME, Rynkiewicz LM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol. 1953;66:1062–1081. doi: 10.1016/s0002-9378(16)38617-3. [DOI] [PubMed] [Google Scholar]
- 29.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 30.Report on Carcinogens. 13. Research Triagngle Park, NC: 2014. National Toxicology Program. http://europepmc.org/abstract/med/37351. [Google Scholar]
- 31.Center for Disease Control and Prevention, Control CFD, Prevention. [Accessed August 25, 2016];DES Update: Healthcare Providers. http://www.cdc.gov/des/hcp/pharmacology/. Published August 25, 2016.
- 32.Greenberg ER, Barnes AB, Resseguie L, et al. Breast cancer in mothers given diethylstilbestrol in pregnancy. N Engl J Med. 1984;311:1393–1398. doi: 10.1056/NEJM198411293112201. [DOI] [PubMed] [Google Scholar]
- 33.Melnick S, Cole P, Anderson D, Herbst A. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. N Engl J Med. 1987;316:514–516. doi: 10.1056/NEJM198702263160905. [DOI] [PubMed] [Google Scholar]
- 34.Verloop J, van Leeuwen FE, Helmerhorst TJM, et al. Cancer risk in DES daughters. Cancer Causes Control. 2010;21:999–1007. doi: 10.1007/s10552-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 36.Hatch EE. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA. 1998;280:630. doi: 10.1001/jama.280.7.630. [DOI] [PubMed] [Google Scholar]
- 37.Gill WB, Schumacher GF, Bibbo M, et al. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol. 1979;122:36–39. doi: 10.1016/s0022-5347(17)56240-0. [DOI] [PubMed] [Google Scholar]
- 38.Henley DV, Korach KS. Physiological effects and mechanisms of action of endocrine disrupting chemicals that alter estrogen signaling. Hormones (Athens) 2010;9:191–205. doi: 10.14310/horm.2002.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman RH, Adam E. Findings in female offspring of women exposed in utero to diethylstilbestrol. Obstet Gynecol. 2002;99:197–200. doi: 10.1016/s0029-7844(01)01682-9. [DOI] [PubMed] [Google Scholar]
- 40.Titus-Ernstoff L, Hatch EE, Hoover RN, et al. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer. 2001;84:126–133. doi: 10.1054/bjoc.2000.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonah C. Meat, Medicine and Human Health in the Twentieth Century. Routledge: 2015. [Google Scholar]
- 42.Parlee RS. Overcoming the identification burden in DES litigation: the market share liability theory. Marq L Rev. 1982:65. [Google Scholar]
- 43.Friedman MA, Woodcock J, Lumpkin MM, et al. The safety of newly approved medicines: do recent market removals mean there is a problem? JAMA. 1999;281:1728–1734. doi: 10.1001/jama.281.18.1728. [DOI] [PubMed] [Google Scholar]
- 44.Pawar RS, Grundel E. Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs) Drug Test Anal. 2016 doi: 10.1002/dta.1980. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 46.Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143:380–385. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- 47.Yen M, Ewald MB. Toxicity of weight loss agents. J Med Toxicol. 2012;8:145–152. doi: 10.1007/s13181-012-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham DJ, Green L. Further cases of valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:635. doi: 10.1056/NEJM199708283370911. [DOI] [PubMed] [Google Scholar]
- 49.Jick H, Vasilakis C, Weinrauch LA, et al. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339:719–724. doi: 10.1056/NEJM199809103391102. [DOI] [PubMed] [Google Scholar]
- 50.Studdert DM, Mello MM, Brennan TA. Medical monitoring for pharmaceutical injuries: tort law for the public’s health? JAMA. 2003;289:889–894. doi: 10.1001/jama.289.7.889. [DOI] [PubMed] [Google Scholar]
- 51.Camilleri M, Northcutt AR, Kong S, et al. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 52.Bardhan KD, Bodemar G, Geldof H, et al. A double-blind, randomized, placebo-controlled dose-ranging study to evaluate the efficacy of alosetron in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2000;14:23–34. doi: 10.1046/j.1365-2036.2000.00684.x. [DOI] [PubMed] [Google Scholar]
- 53.Gunput MD. Review article: clinical pharmacology of alosetron. Aliment Pharmacol Ther. 1999;13:70–76. doi: 10.1046/j.1365-2036.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 54.Camilleri M, Chey WY, Mayer EA, et al. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med. 2001;161:1733. doi: 10.1001/archinte.161.14.1733. [DOI] [PubMed] [Google Scholar]
- 55.Moynihan R. Alosetron: a case study in regulatory capture, or a victory for patients’ rights? BMJ. 2002;325:592–595. doi: 10.1136/bmj.325.7364.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raczkowski V, Levine P, Gallo-Torres H, Hoberman D, Piazza-Hepp T. Gastrointestinal Drugs Advisory Committee and Drug Safety and Risk Management Subcommittee Background Package. 2002 http://www.fda.gov/ohrms/dockets/ac/02/briefing/3848B1_02_FDA%20Lotronex.pdf.
- 57.Horton R. Lotronex and the FDA: a fatal erosion of integrity. Lancet. 2001;357:1544–1545. doi: 10.1016/S0140-6736(00)04776-0. [DOI] [PubMed] [Google Scholar]
- 58.Thompson CA. Novartis suspends tegaserod sales at FDA’s request. Am J Health Syst Pharm. 2007;64:1020. doi: 10.2146/news070044. [DOI] [PubMed] [Google Scholar]
- 59.Drugwatch. [Accessed October 14, 2016];Zofran: Popular Nausea Drug Pregnancy Risks, Birth Defects. https://www.drugwatch.com/zofran/. Published 2016.
- 60.Einarson A, Maltepe C, Navioz Y, et al. The safety of ondansetron for nausea and vomiting of pregnancy: a prospective comparative study. BJOG. 2004;111:940–943. doi: 10.1111/j.1471-0528.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 61.Anderka M, Mitchell AA, Louik C, et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res Part A Clin Mol Teratol. 2011;94:22–30. doi: 10.1002/bdra.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danielsson B, Wikner BN, Källén B. Use of ondansetron during pregnancy and congenital malformations in the infant. Reprod Toxicol. 2014;50:134–137. doi: 10.1016/j.reprotox.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Smith RD. Contemporary hysteroscopic methods for female sterilization. Int J Gynaecol Obstet. 2010;108:79–84. doi: 10.1016/j.ijgo.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Zipper J, Kessel E. Quinacrine sterilization: a retrospective. Int J Gynaecol Obstet. 2003;83:S7–S11. doi: 10.1016/S0020-7292(03)90084-1. [DOI] [PubMed] [Google Scholar]
- 65.Quiñones R, Alvarado A, Ley E. Hysteroscopic sterilization. Int J Gynaecol Obstet. 1976;14:27–34. doi: 10.1002/j.1879-3479.1976.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 66.Brundin J. Observations on the mode of action of an intratubal device, the P-block. Am J Obstet Gynecol. 1987;156:997–1000. doi: 10.1016/0002-9378(87)90375-9. [DOI] [PubMed] [Google Scholar]
- 67.The Center for Devices and Radiological Health. [Accessed September 2016];Essure Permanent Birth Control. 2016 http://www.fda.gov/TMedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/EssurePermanentBirthControl/default.htm.
- 68.Hurskainen R, Hovi SL, Gissler M, et al. Hysteroscopic tubal sterilization: a systematic review of the Essure system. Fertil Steril. 2010;94:16–19. doi: 10.1016/j.fertnstert.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 69.Dhruva SS, Ross JS, Gariepy AM. Revisiting Essure—toward safe and effective sterilization. N Engl J Med. 2015;373:e17. doi: 10.1056/NEJMp1510514. [DOI] [PubMed] [Google Scholar]
- 70.Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59–67. doi: 10.1016/s0029-7844(03)00373-9. [DOI] [PubMed] [Google Scholar]
- 71.Kerin JF, Cooper JM, Price T, et al. Hysteroscopic sterilization using a micro-insert device: results of a multicentre Phase II study. Hum Reprod. 2003;18:1223–1230. doi: 10.1093/humrep/deg256. [DOI] [PubMed] [Google Scholar]
- 72.Gariepy AM, Xu X, Creinin MD, et al. Hysteroscopic Essure inserts for permanent contraception: extended follow-up results of a Phase III multicenter international study. J Minim Invasive Gynecol. 2016;23:137–138. doi: 10.1016/j.jmig.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 73.Adelman MR, Dassel MW, Sharp HT. Management of complications encountered with Essure hysteroscopic sterilization: a systematic review. J Minim Invasive Gynecol. 2014;21:733–743. doi: 10.1016/j.jmig.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 74.Kamencic H, Thiel L, Karreman E, Thiel J. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries following Essure placement. J Minim Invasive Gynecol. 2016;23:1158–1162. doi: 10.1016/j.jmig.2016.08.823. [DOI] [PubMed] [Google Scholar]
- 75.Braginsky L, George ST, Locher SR. Management of perforated essure with migration into small and large bowel mesentery. J Minim Invasive Gynecol. 2015;22:504–508. doi: 10.1016/j.jmig.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Lane A, Tyson A, Thurston E. Providing Re-Essure-ance to the nickel-allergic patient considering hysteroscopic sterilization. J Minim Invasive Gynecol. 2016;23:126–129. doi: 10.1016/j.jmig.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 77.Levy B, Levie MD, Childers ME. A summary of reported pregnancies after hysteroscopic sterilization. J Minim Invasive Gynecol. 2007;14:271–274. doi: 10.1016/j.jmig.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 78.MAUDE - Manufacturer and User Facility Device Experience. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/TextSearch.cfm.
- 79.The Center for Devices and Radiological Health. [Accessed September 2016];Obstetrics and Gynecology Devices—2015 Meeting Materials of the Obstetrics and Gynecology Devices Panel. 2015 http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/ObstetricsandGynecologyDevices/ucm463457.htm.
- 80.Samuelsson EC, Arne Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180:299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- 81.Wattiez A, Nasir R, Maamari Al B, Schindler L. Laparoscopic prolapse surgery: types and evidence. Curr Opin Obstet Gynecol. 2016;28:430–434. doi: 10.1097/GCO.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 82.Lensen EJM, Withagen MIJ, Kluivers KB, et al. Surgical treatment of pelvic organ prolapse: a historical review with emphasis on the anterior compartment. Int Urogynecol J. 2013;24:1593–1602. doi: 10.1007/s00192-013-2074-2. [DOI] [PubMed] [Google Scholar]
- 83.Moore J, Armstrong JT, Willis SH. The use of tantalum mesh in cystocele with critical report of ten cases. Am J Obstet Gynecol. 1955;69:1127–1135. doi: 10.1016/0002-9378(55)90109-5. [DOI] [PubMed] [Google Scholar]
- 84.Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472–1475. doi: 10.1016/s0002-9378(96)70092-3. [DOI] [PubMed] [Google Scholar]
- 85.Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299–1304. doi: 10.1067/mob.2001.119081. discussion 1304–1306. [DOI] [PubMed] [Google Scholar]
- 86.Rice NT, Hu Y, Slaughter JC, Ward RM. Pelvic mesh complications in women before and after the 2011 FDA public health notification. Female Pelvic Med Reconstr Surg. 2013;19:333–338. doi: 10.1097/SPV.0b013e3182a330c1. [DOI] [PubMed] [Google Scholar]