Abstract
Angiostronglyus vasorum is a cardiopulmonary nematode infecting mainly canids such as dogs (Canis familiaris) and foxes (Vulpes vulpes). Natural infections have also been reported in mustelids and red pandas (Ailurus fulgens fulgens). We report the occurrence of natural A. vasorum infections in a group of captive meerkats (Suricata suricatta), housed at a university facility in Switzerland. A. vasorum first-stage larvae (L1) were initially identified in a pooled faecal sample. Individual samples, investigated with the Baermann-Wetzel technique, revealed that 41% (7/17) of the meerkats were infected, with ranges of 2–125 L1/g faeces. PCR and sequencing of part of the ITS-2 region resulted in 100% identity with A. vasorum. Infected animals did not show clinical signs. One meerkat died two days after diagnosis. Upon necropsy one adult specimen was recovered; histological examination of the lung revealed granulomatous pneumonia caused by A. vasorum larvae and eggs as well as intima and media hyperplasia and isolated arteriosclerosis of larger lung vessels. However, the cause of death was a spleen rupture with associated blood loss. All meerkats were topically treated with 10 mg imidacloprid/2.5 mg moxidectin per animal, after which they became negative in all follow up faecal examinations. Potential intermediate (gastropods) and paratenic hosts (birds) were collected from within or outside the meerkats enclosure. Gastropods were examined by PCR and bird samples by digestion. Four out of 193 (2.1%) gastropod samples were positive for A. vasorum, whereas none of the bird samples were positive. Meerkats, belonging to the Herpestidae, therefore are suitable definitive hosts for A. vasorum, with production and excretion of live L1. Meerkats kept in captivity in areas where A. vasorum is endemic and with potential contact to intermediate hosts are at risk of infection. Regular faecal examinations including Baermann-Wetzel technique should be considered.
Keywords: Angiostrongylus vasorum, Cardiopulmonary nematode, Meerkat, Suricata suricatta, New definitive host
Graphical abstract

Highlights
-
•
Meerkats are suitable definitive hosts for Angiostrongylus vasorum.
-
•
This is the first case of A. vasorum in the Herpestidae family.
-
•
A. vasorum larvae and eggs caused granulomatous pneumonia in an infected meerkat.
-
•
2% of gastropods around the meerkats enclosure were positive for A. vasorum L3.
-
•
Captive animal species in areas where A. vasorum is endemic are at risk of infection.
1. Introduction
Angiostrongylus vasorum is a cardiopulmonary nematode increasingly diagnosed across Europe. Canids such as dogs and foxes represent the most common definitive hosts and slugs and snails the most common intermediate hosts (Koch and Willesen, 2009). Frogs were reported to be intermediate and paratenic hosts (Bolt et al., 1993) and chickens paratenic hosts (Mozzer and Lima, 2015) under experimental conditions. Definitive hosts usually become infected by deliberate or accidental ingestion of intermediate hosts containing infectious third stage larvae (L3) (Guilhon and Cens, 1973, Schnyder, 2015). Ingestion of grass, food or water contaminated with secretions of infected gastropods may also lead to infection (Barçante et al., 2003, Morgan et al., 2005). Clinical manifestation in infected dogs range from mild and unspecific signs, such as exercise intolerance and inappetence, to severe respiratory signs and bleeding disorders which can lead to fatal outcomes (Staebler et al., 2005, Sigrist et al., 2017). In addition to dogs (Canis familiaris) and foxes (Vulpes vulpes) natural A. vasorum infections were observed in wolves (Canis lupus) (Segovia et al., 2001), coyotes (Canis latrans) (Bourque et al., 2005), golden jackals (Canis aureus) (Takács et al., 2013), crab-eating foxes (Cerdocyon thous) (Duarte et al., 2007), hoary foxes (Dusicyon vetulus) (Lima et al., 1994), red pandas (Ailurus fulgens fulgens) (Patterson-Kane et al., 2009, Bertelsen et al., 2010), Eurasian badgers (Meles meles) (Torres et al., 2001), stoats (Mustela erminea), a weasel (Mustela nivalis) (Simpson et al., 2016) and an otter (Lutra lutra) (Madsen et al., 1999).
Meerkats (Suricata suricatta), also known as suricates, belong to the Herpestidae family and originate from arid regions of southern Africa; they are mainly insectivores but also feed on small vertebrates (Doolan and Macdonald, 1996). Suricates are popular animals held in zoos worldwide and are frequently studied for their social behaviour (Clutton-Brock and Manser, 2016). In this study we present the first report of infections with A. vasorum in a group of captive meerkats.
2. Material and methods
2.1. Meerkats
The group of meerkats consisted of 17 individuals (9 females and 8 males) between 2.5 and 8.5 years of age. All meerkats were born in captivity and kept for behavioural studies. The enclosure is located at a facility at the University of Zurich, Switzerland, and comprised of a heated indoor area which can be fully closed off, and an outdoor area with a partial open roof and walls, consisting of concrete or wire mesh fencing. The indoor and outdoor area measure 61 m2 and 262 m2, respectively. Substrate in both areas is sandy and between 20 and 180 cm deep, containing some larger rocks and branches. General hygiene principles are applied when entering the enclosure or feeding. Meerkats are fed three meals daily with a variety of fruit, mealworm, crickets, eggs, chicks and/or pellets. Occasionally, meerkats were observed to catch and eat birds, which fly into the outdoor enclosure. The enclosure is regularly checked for slugs, snails, frogs or other intruding animals. Meerkats undergo routine faecal examination three to four times a year.
2.2. Laboratory analyses
During a routine faecal analysis of a pooled sample of the whole group performed at the Institute of Parasitology, University of Zurich, A. vasorum first stage larvae (L1) were microscopically identified (Fig. 1) based on their length and tail morphology (Guilhon and Cens, 1973, Deplazes et al., 2016). Meerkats were subsequently fed non-harmful glitter of different colours and shapes for individual differentiation of faecal matter and individual samples were collected for three consecutive days. Pooled three day faecal samples of each individual were quantitatively analysed by the Baermann-Wetzel technique (Deplazes et al., 2016) by determining the number of L1 per gram of analysed faeces. Larvae were morphologically confirmed. DNA was extracted from the isolated larvae using the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer's instructions, followed by PCR analysis for detection of part of the ribosomal internal transcribed spacer (ITS) 2-region of A. vasorum (Jefferies et al., 2011).
Fig. 1.
Angiostrongylus vasorum first stage larva isolated by the Baermann technique from a collective faecal sample of a meerkat group. Average size: 332 μm in length and 14.1 μm in width.
Two days after the individual faecal testing, one positive meerkat died. The animal was necropsied at the Institute of Veterinary Pathology, University of Zurich.
2.3. Examination of potential intermediate hosts
Potential intermediate or paratenic hosts for A. vasorum were collected and examined in order to identify potential sources of infection in the surrounding area of the meerkats enclosure.
Seven months after diagnosing A. vasorum infection in meerkats (skipping the cold winter months when gastropod collection is virtually impossible), slugs and snails were collected from the ground, field and bushes around a small periphery (maximum 20 m) from the meerkats enclosure: slugs and snails were collected monthly from May to October, except for June, at dawn on a rainy day. Slugs and snails were euthanized by freezing at −20 °C. Collected specimens were identified to the family level according to Boschi (2011). For further processing the posterior end of larger slugs was cut off with a clean scalpel and transferred to a 2 ml Eppendorf tube, smaller snails and slugs were processed entirely. Each sample was weighed and 500 μl distilled water was added. Samples were processed with the TissueLyser II (Qiagen, Hilden, Germany) with a 5 mm stainless steel bead at 30 beats per second for 10 min. Five hundred mg of 16 pooled samples, consisting of 100 μl of 9–15 gastropods each, were digested with proteinase K and lysis buffer overnight. DNA extraction was performed using the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany). Samples were directly analysed by PCR detecting part of the ITS 2-region of A. vasorum (Jefferies et al., 2011). Individual sample analysis of the positive pooled samples was subsequently performed in order to identify single positive slugs and snails.
During the time of the investigation one dead thrush (Turdus philomelos) and one bird wing (species unknown) were found in the meerkats enclosure. Tissue samples of muscle and/or organs from the animals were chopped finely and digested in 1% HCL solution with 2.4 g pepsin (800–2500 U mg, Sigma P700, Sigma-Aldrich, Missouri, USA) per litre in a 45 °C water bath rotary shaker for 1 h. Samples were washed with water and centrifuged for 3 min at 500 G and this step was repeated twice. The sediments were then examined under a stereomicroscope for A. vasorum L3.
3. Results
3.1. Detection of first stage larvae of A. vasorum and biomolecular confirmation
Seven (4 females and 3 males) of 17 meerkats were shedding live A. vasorum L1. The L1 showed the distinct dorsal spine and kink at the tip of the tail of A. vasorum (Fig. 1) (Guilhon and Cens, 1973). They measured on average 332 μm in length and 14.1 μm in width (measured on 21 larvae), which is within range of previous size descriptions for A. vasorum L1 (Deplazes et al., 2016). The number of larvae per gram faeces (LPG) ranged from 2.4 to 125 (mean: 37.7) per animal (Table 1). Sequencing analysis of the larvae confirmed the presence of A. vasorum with a 100% identity (GenBank accession nos. KF270683, GU045376, EU627598) (Fig. 2).
Table 1.
Angiostrongylus vasorum first stage larvae per gram faeces (LPG) of individuals of a meerkat group determined from individual three day samples.
| ID | sex | LPG |
|---|---|---|
| 1 | F | 0 |
| 2 | M | 0 |
| 3 | F | 0 |
| 4 | F | 66.7 |
| 5 | F | 0 |
| 6 | F | 0 |
| 7 | M | 0 |
| 8a | M | 33.3 |
| 9 | M | 0 |
| 10 | M | 125 |
| 11 | F | 2.4 |
| 12 | F | 10 |
| 13 | F | 3.8 |
| 14 | F | 0 |
| 15 | M | 0 |
| 16 | M | 0 |
| 17 | M | 22.7 |
This individual died two days after diagnosis, due to a ruptured spleen.
Fig. 2.
Sequence alignment of part of the internal transcribed spacer (ITS) 2-region (78 bp) of Angiostronglus vasorum (GenBank accession no. KF270683), of DNA isolated from A. vasorum first stage larvae obtained from meerkats and from one slug.
All meerkats, including the negative ones, were topically treated with 10 mg imidacloprid/2.5 mg moxidectin (Advocate®, Bayer Animal Health) per animal shortly after individual diagnosis. One, four and ten months after treatment faeces of all meerkats were negative for A. vasorum L1.
3.2. Necropsy and histology of one meerkat
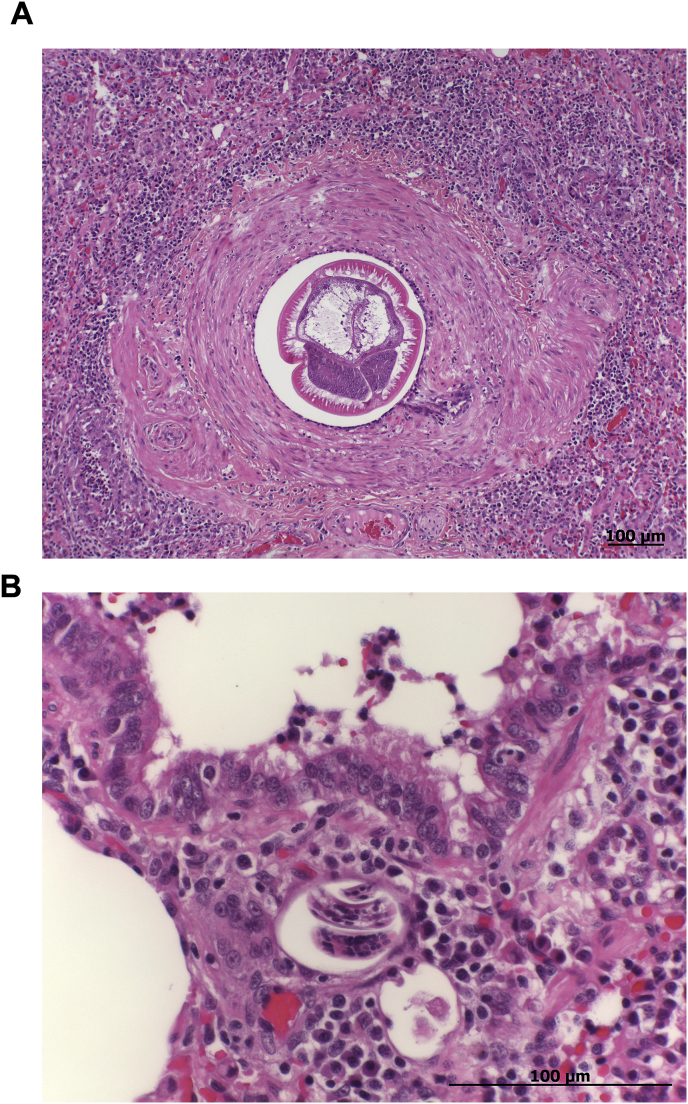
The sudden death of one meerkat was caused by a ruptured spleen with associated blood loss, most likely due to a traumatic event such as a fall. In the lung one adult A. vasorum specimen was found upon macroscopic examination after an incision into a diaphragmatic lobe. Histological examination of the lung revealed a multifocal granulomatous pneumonia caused by A. vasorum larvae and eggs as well as intima and media hyperplasia and isolated arteriosclerosis of larger lung vessels (Fig. 3).
Fig. 3.
Histological image of the lung of an Angiostrongylus vasorum infected meerkat. An adult nematode within a thickened artery (A), larvae and eggs surrounded by plasma cells, macrophages, neutrophilic granulocytes and lymphocytes (B) are visible, indicating granulomatous pneumonia as well as intima and media hyperplasia of larger lung vessels. (Hematoxylin and eosin staining, original magnification x 100 (A), x 400 (B)).
3.3. Potential intermediate and paratenic hosts
A total of 193 slugs (n = 146) and snails (n = 47) were collected (Table 2). The most commonly found gastropods belonged to the family Arionidae (62.7%, n = 121), followed by snails of the Clausiliidae family (22.8%, n = 44). Gastropods from the family Agriolimacidae (9.8%, n = 19), Limacidae (3.1%, n = 6) and Oxychilidae (0.5%, n = 1) were less common. Two small snails could not be identified due to broken shells. The number of gastropods collected each month and tested PCR positive for A. vasorum are presented in Table 2.
Table 2.
Number of slugs and snails collected between the months of May and October around the enclosure of Angiostrongylus vasorum infected meerkats.
| Month of collection | Total number of collected gastropods (n) |
A. vasorum PCR positivea (n, %) |
Families of the collected gastropod species |
|||||
|---|---|---|---|---|---|---|---|---|
| Arionidaea (n) |
Limacidae (n) |
Agriolimacidae (n) |
Clausiliidae (n) |
Oxychilidae (n) |
Unidentifiable (n) |
|||
| May | 35 | 0 (0%) | 11 | 0 | 0 | 22 | 0 | 2 |
| July | 82 | 3 (4.4%) | 68 | 0 | 13 | 1 | 0 | 0 |
| August | 39 | 0 (0%) | 34 | 4 | 1 | 0 | 0 | 0 |
| September | 33 | 1 (12.5%) | 8 | 2 | 3 | 19 | 1 | 0 |
| October | 4 | 0 (0%) | 0 | 0 | 2 | 2 | 0 | 0 |
| Total | 193 | 4 (2.1%) | 121 | 6 | 19 | 44 | 1 | 2 |
Only gastropods belonging to the Arionidae were found positive by PCR.
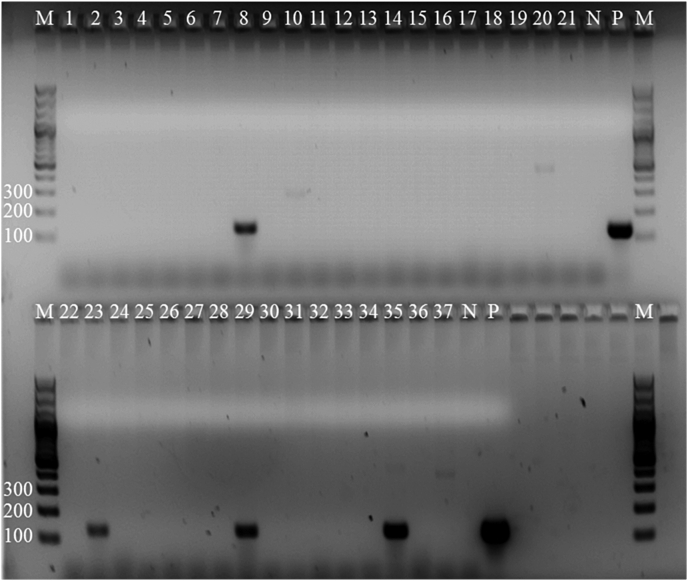
Three of the 16 pooled gastropod samples were positive for A. vasorum. These three pools consisted of 37 individual gastropod samples of which four were positive after individual testing (Fig. 4). Sequencing revealed 100% identity with A. vasorum (GenBank accession nos. KF270683, GU045376, EU627598) (Fig. 2) for all four samples based on 78 base pairs (bp). The four positive slugs were all from the Arionidae family, resulting in a prevalence of 3.3% (4/121; 95% Confidence Intervals, CI: 0.9–8.2%) for Arionidae slugs and an overall prevalence of 2.1% (CI: 0.6–5.2%, 4/193) for all collected gastropods. Three of the positive slugs were collected in July and one in September (Table 2). The thrush and the bird wing did not harbour any nematode larvae.
Fig. 4.
Agarose gel (1.5%) stained with GelRed™ showing PCR products of part of the internal transcribed spacer (ITS) 2-region of Angiostrongylus vasorum of 37 gastropod samples: samples no. 8, 23, 29 and 35 were positive. M: DNA ladder, N: negative control, P: positive control.
4. Discussion
We report the first case of A. vasorum in meerkats and within the Herpestidae family. Meerkats were shown to act as definitive hosts by harbouring an adult specimen and releasing live L1 in their faeces. Previous studies showed that A. vasorum has a low intermediate host specificity (Guilhon and Bressou, 1960, Eckert and Lämmler, 1972, Patel et al., 2014). Our findings instead, represent a further testimony that A. vasorum has also a low definitive host specificity as it not only infects canids but also mustelids (Torres et al., 2001, Simpson et al., 2016) and ailurids (Patterson-Kane et al., 2009).
At the time of diagnosis none of the infected meerkats showed clinical signs: it can be argued that the infections may have been of recent origin, since with a supposed patency of approximately seven weeks and faecal examinations routinely performed three to four times a year, the animals may have been infected for approximately two months. Alternatively, the absence of clinical signs could be attributed to their living in captivity: also foxes living in captivity did not develop clinical signs after infection with A. vasorum (Webster et al., 2017). However, it cannot be excluded that with prolonged infection the observed lung pathology could possibly induce the development of clinical signs. Accordingly, the lung pathology in the necropsied meerkat may have caused some distress and weakness which could have been the cause of a trauma by a fall of the meerkat, indirectly representing the potential cause of its death due to rupture of the spleen. Necropsy and histology of the lung revealed severe lung pathology caused by A. vasorum larvae and eggs similar to that seen in infected canid and non-canid species (Poli et al., 1991, Patterson-Kane et al., 2009, Schnyder et al., 2010, Simpson, 2010, Eleni et al., 2014, Simpson et al., 2016). However, reports of clinical signs and pathological changes due to A. vasorum in non-canids are still scarce, and diagnosis in meerkats was mostly incidental. Little is known on anthelmintic treatment of A. vasorum in non-canid species. The combination moxidectin/imidacloprid has a recognised medical indication for treatment and prophylaxis against A. vasorum in dogs (Schnyder et al., 2009) and was therefore successfully used for the meerkats.
Angiostrongylus vasorum is widespread in dogs and foxes in Switzerland and neighboring European countries (Barutzki and Schaper, 2009, Magi et al., 2009, Guardone et al., 2013, Lurati et al., 2015, Gillis-Germitsch et al., 2017, Maksimov et al., 2017). The area of Zurich, where the meerkats enclosure is located, has been shown previously to be endemic for A. vasorum in definitive hosts (Lurati et al., 2015, Gillis-Germitsch et al., 2017) and is now additionally supported by the identification of positive mollusc intermediate hosts. The meerkats likely acquired the infection by ingestion of an intermediate mollusc host harbouring infectious L3, since 2.1% of gastropods encountered within only 20 m of the meerkats enclosure were harbouring A. vasorum, a higher prevalence than one (1.6%) found in molluscs in hyperendemic areas (Patel et al., 2014). The highest number of gastropods and of A. vasorum positive specimens were collected in July. This reflects previous findings of increasing or high numbers of A. vasorum positive gastropods in summer months (Ferdushy et al., 2009, Jefferies et al., 2009).
Birds were occasionally found in the enclosure and chicken have been experimentally shown to act as potential paratenic hosts of A. vasorum (Mozzer and Lima, 2015), but for birds no evidence of the presence of A. vasorum infective stages was obtained. Similarly, frogs were found in or were sighted around the enclosure and are described as potential intermediate or paratenic hosts under experimental conditions (Bolt et al., 1993), but the meerkats were never observed to eat frogs during the observation period.
Meerkats do not naturally occur in A. vasorum endemic areas. However, they are popular and common zoo held animals worldwide. Enclosures of zoo animals are very rarely hermetically sealed and intermediate or paratenic hosts of A. vasorum may find their way into enclosures.
In a recent report from Italy Angiostrongylus dujardini was reported in meerkats (Eleni et al., 2016): two young meerkats suffering from anorexia and tachypnea died due to infection with this lungworm, a species usually infecting rodents and also transmitted by ingestion of molluscs.
It is therefore essential to regularly check enclosures of meerkats and potentially further animal species susceptible for an A. vasorum infection for intruding of intermediate or paratenic hosts. Furthermore, in susceptible animals living in A. vasorum endemic areas regular faecal examination for gastrointestinal parasites should be complemented by the Baermann technique in order to early identify a potential infection with lungworms and treat infected animals accordingly, preventing complications due to the infection.
Conflict of interest
None.
Funding
The captive meerkat population is funded by the University of Zurich. We would like to acknowledge Bayer Vital GmbH, Business Unit Animal Health, Germany, for the financial support of Nina Gillis-Germitsch in the form of a doctoral fellowship.
Ethics approval
Meerkats were facility-born at the University of Zurich. All institutional and national guidelines for the care and use of laboratory animals were followed upon approval by the Cantonal Veterinary Office of Zurich (animal permission number: 153).
Acknowledgements
The authors would like to acknowledge Francesca Gori and Isabelle Specker for their help with laboratory analyses and Lucienne Tritten and Felix Grimm for providing support with sequence interpretation. We would also like to thank Megan Wyman for gathering additional information about the meerkats and Annakatrin Häni for providing the slug identification book as well as the technical laboratory staff of the Institute of Veterinary Pathology for the preparation of the histological slides.
References
- Barçante T.A., Barçante J.M.d.P., Dias S.R.C., Lima W.d.S. Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905: emergence of third-stage larvae from infected Biomphalaria glabrata snails. Parasitol. Res. 2003;91:471–475. doi: 10.1007/s00436-003-1000-9. [DOI] [PubMed] [Google Scholar]
- Barutzki D., Schaper R. Natural infections of Angiostrongylus vasorum and Crenosoma vulpis in dogs in Germany. Parasitol. Res. 2009;105:39–48. doi: 10.1007/s00436-009-1494-x. 2007–2009. [DOI] [PubMed] [Google Scholar]
- Bertelsen M.F., Meyland-Smith F., Willesen J.L., Jefferies R., Morgan E.R., Monrad J. Diversity and prevalence of metastrongyloid nematodes infecting the red panda (Ailurus fulgens) in European zoos. Vet. Parasitol. 2010;172:299–304. doi: 10.1016/j.vetpar.2010.04.043. [DOI] [PubMed] [Google Scholar]
- Bolt G., Monrad J., Frandsen F., Henriksen P., Dietz H. The common frog (Rana temporaria) as a potential paratenic and intermediate host for Angiostrongylus vasorum. Parasitol. Res. 1993;79:428–430. doi: 10.1007/BF00931834. [DOI] [PubMed] [Google Scholar]
- Boschi C. Haupt Verlag; Bern, Switzerland: 2011. Die Schneckenfauna der Schweiz: ein umfassendes Bild- und Bestimmungsbuch. [Google Scholar]
- Bourque A., Whitney H., Conboy G. Angiostrongylus vasorum infection in a coyote (Canis latrans) from Newfoundland and Labrador, Canada. J. Wildl. Dis. 2005;41:816–819. doi: 10.7589/0090-3558-41.4.816. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T., Manser M. Meerkats: cooperative breeding in the Kalahari. In: Koenig W.D., Dickinson J.L., editors. Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution, and Behavior. Cambridge University Press; Cambridge, United Kingdom: 2016. pp. 294–317. [Google Scholar]
- Deplazes P., Eckert J., Mathis A., von Samson-Himmelstjerna G., Zahner H. Wageningen Academic Publishers; Wageningen, Netherlands: 2016. Parasitology in Veterinary Medicine. [Google Scholar]
- Doolan S.P., Macdonald D.W. Diet and foraging behaviour of group-living meerkats, Suricata suricatta, in the southern Kalahari. J. Zool. 1996;239:697–716. [Google Scholar]
- Duarte F., Vieira F., Louzada G., Bessa E., Souzalima S. Occurrence Angiostrongylus vasorum (Baillet, 1866) (Nematoda, Angiostrongylidae) in Cerdocyon thous Linnaeus, 1766 (Carnivora, Canidae) in Minas Gerais State Brazil. Arq. Bras. Med. Vet. Zoo. 2007;59:1086–1088. [Google Scholar]
- Eckert J., Lämmler G. Angiostrongylose bei Mensch und Tier. Z Parasitenk. 1972;39:303–322. doi: 10.1007/BF00329093. [DOI] [PubMed] [Google Scholar]
- Eleni C., Di Cesare A., Cavicchio P., Tonnicchia M.C., Meoli R., di Regalbono A.F., Paoletti B., Pietrobelli M., De Liberato C. Fatal Angiostrongylus dujardini infection in callitrichid monkeys and suricates in an Italian zoological garden. Parasitol. Int. 2016;65:333–335. doi: 10.1016/j.parint.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Eleni C., Grifoni G., Di Egidio A., Meoli R., De Liberato C. Pathological findings of Angiostrongylus vasorum infection in red foxes (Vulpes vulpes) from Central Italy, with the first report of a disseminated infection in this host species. Parasitol. Res. 2014;113:1247–1250. doi: 10.1007/s00436-014-3793-0. [DOI] [PubMed] [Google Scholar]
- Ferdushy T., Kapel C.M.O., Webster P., Al-Sabi M.N.S., Grønvold J. The occurrence of Angiostrongylus vasorum in terrestrial slugs from forests and parks in the Copenhagen area, Denmark. J. Helminthol. 2009;83:379. doi: 10.1017/S0022149X09377706. [DOI] [PubMed] [Google Scholar]
- Gillis-Germitsch N., Kapel C., Thamsborg S., Deplazes P., Schnyder M. Host-specific serological response to Angiostrongylus vasorum infection in red foxes (Vulpes vulpes): implications for parasite epidemiology. Parasitology. 2017:1–10. doi: 10.1017/S0031182017000427. [DOI] [PubMed] [Google Scholar]
- Guardone L., Schnyder M., Macchioni F., Deplazes P., Magi M. Serological detection of circulating Angiostrongylus vasorum antigen and specific antibodies in dogs from central and northern Italy. Vet. Parasitol. 2013;192:192–198. doi: 10.1016/j.vetpar.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Guilhon J., Bressou C. Rôle des Limacidés dans le cycle évolutif d'Angiostrongylus vasorum (Baillet, 1866) CR Acad. Sci. 1960;251:2252–2253. [PubMed] [Google Scholar]
- Guilhon J., Cens B. Angiostrongylus vasorum (Baillet, 1866) Étude biologique et morphologique. Ann. Parasit. Hum. Comp. 1973;48:567–596. [PubMed] [Google Scholar]
- Jefferies R., Morgan E.R., Helm J., Robinson M., Shaw S.E. Improved detection of canine Angiostrongylus vasorum infection using real-time PCR and indirect ELISA. Parasitol. Res. 2011;109:1577–1583. doi: 10.1007/s00436-011-2414-4. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Morgan E.R., Shaw S.E. A SYBR green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in definitive and intermediate hosts. Vet. Parasitol. 2009;166:112–118. doi: 10.1016/j.vetpar.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Koch J., Willesen J.L. Canine pulmonary angiostrongylosis: an update. Vet. J. 2009;179:348–359. doi: 10.1016/j.tvjl.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Lima W., Guimaraes M., Lemos I. Occurrence of Angiostrongylus vasorum in the lungs of the Brazilian fox Dusicyon vetulus. J. Helminthol. 1994;68:87. doi: 10.1017/s0022149x00013547. [DOI] [PubMed] [Google Scholar]
- Lurati L., Deplazes P., Hegglin D., Schnyder M. Seroepidemiological survey and spatial analysis of the occurrence of Angiostrongylus vasorum in Swiss dogs in relation to biogeographic aspects. Vet. Parasitol. 2015;212:219–226. doi: 10.1016/j.vetpar.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Madsen A., Dietz H., Henriksen P., Clausen B. Survey of Danish free living otters (Lutra lutra) - a consecutive collection and necroscopy of dead bodies. IUCN Otter Spec. Group Bull. 1999;16:65–76. [Google Scholar]
- Magi M., Guardone L., Dell'omodarme M., Prati M.C., Mignone W., Torraccia B., Monni G., Macchioni F. Angiostrongylus vasorum in red foxes (Vulpes vulpes) and badgers (Meles meles) from central and northern Italy. Hystrix. 2009;20:121–126. [Google Scholar]
- Maksimov P., Hermosilla C., Taubert A., Staubach C., Sauter-Louis C., Conraths F.J., Vrhovec M.G., Pantchev N. GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasite Vector. 2017;10:108. doi: 10.1186/s13071-017-2054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E.R., Shaw S.E., Brennan S.F., De Waal T.D., Jones B.R., Mulcahy G. Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol. 2005;21:49–51. doi: 10.1016/j.pt.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Mozzer L.R., Lima W.S. Gallus gallus domesticus: paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015;207:81–84. doi: 10.1016/j.vetpar.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Patel Z., Gill A.C., Fox M.T., Hermosilla C., Backeljau T., Breugelmans K., Keevash E., McEwan C., Aghazadeh M., Elson-Riggins J.G. Molecular identification of novel intermediate host species of Angiostrongylus vasorum in Greater London. Parasitol. Res. 2014;113:4363–4369. doi: 10.1007/s00436-014-4111-6. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane J.C., Gibbons L.M., Jefferies R., Morgan E.R., Wenzlow N., Redrobe S.P. Pneumonia from Angiostrongylus vasorum infection in a red panda (Ailurus fulgens fulgens) J. Vet. Diagn Invest. 2009;21:270–273. doi: 10.1177/104063870902100219. [DOI] [PubMed] [Google Scholar]
- Poli A., Arispici M., Mancianti F., Abramo F. Pathology of naturally acquired Angiostrongylus vasorum infection in the red fox (Vulpes vulpes) Angew. Parasitol. 1991;32:121. [PubMed] [Google Scholar]
- Schnyder M. Slugs and Angiostrongylus vasorum - how much do we know? Vet. Rec. 2015:177. doi: 10.1136/vr.h3623. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Fahrion A., Ossent P., Kohler L., Webster P., Heine J., Deplazes P. Larvicidal effect of imidacloprid/moxidectin spot-on solution in dogs experimentally inoculated with Angiostrongylus vasorum. Vet. Parasitol. 2009;166:326–332. doi: 10.1016/j.vetpar.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Fahrion A., Riond B., Ossent P., Webster P., Kranjc A., Glaus T., Deplazes P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010;107:1471–1480. doi: 10.1007/s00436-010-2021-9. [DOI] [PubMed] [Google Scholar]
- Segovia J., Torres J., Miquel J., Llaneza L., Feliu C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001;75:183–192. [PubMed] [Google Scholar]
- Sigrist N.E., Hofer-Inteeworn N., Jud Schefer R., Kuemmerle-Fraune C., Schnyder M., Kutter A.P.N. Hyperfibrinolysis and hypofibrinogenemia diagnosed with rotational thromboelastometry in dogs naturally infected with Angiostrongylus vasorum. J. Vet. Intern Med. 2017;31:1091–1099. doi: 10.1111/jvim.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson V.R. Angiostrongylus vasorum infection in a stoat. Vet. Rec. 2010;166 doi: 10.1136/vr.c666. 182–182. [DOI] [PubMed] [Google Scholar]
- Simpson V.R., Tomlinson A.J., Stevenson K., McLuckie J.A., Benavides J., Dagleish M.P. A post-mortem study of respiratory disease in small mustelids in south-west England. BMC Vet. Res. 2016;12:72. doi: 10.1186/s12917-016-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staebler S., Ochs H., Steffen F., Naegeli F., Borel N., Sieber-Ruckstuhl N., Deplazes P. Autochthone Infektionen mit Angiostrongylus vasorum bei Hunden in der Schweiz und Deutschland. Schweiz Arch. Tierh. 2005;147:121–127. doi: 10.1024/0036-7281.147.3.121. [DOI] [PubMed] [Google Scholar]
- Takács A., Szabó L., Juhász L., Lanszki J., Takács P., Heltai M. Data on the parasitological status of golden jackal (Canis aureus L., 1758) in Hungary. Acta Vet. Hung. 2013;62:33–41. doi: 10.1556/AVet.2013.058. [DOI] [PubMed] [Google Scholar]
- Torres J., Miquel J., Motjé M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol. Res. 2001;87:259–263. doi: 10.1007/s004360000316. [DOI] [PubMed] [Google Scholar]
- Webster P., Monrad J., Kapel C.M.O., Kristensen A.T., Jensen A.L., Thamsborg S.M. The effect of host age and inoculation dose on infection dynamics of Angiostrongylus vasorum in red foxes (Vulpes vulpes) Parasite Vector. 2017;10:4. doi: 10.1186/s13071-016-1940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]