Abstract
Background and purpose
Stroke survivors are often left with deficits requiring rehabilitation to recover function and yet, many are unable to access rehabilitative therapies. Mobile tablet-based therapies (MTBTs) may be a resource-efficient means of improving access to timely rehabilitation. It is unclear what MTBTs have been attempted following stroke, how they were administered, and how patients experienced the therapies. The review summarizes studies of MTBTs following stroke in terms of administrative methods and patient experiences to inform treatment feasibility.
Methods
Articles were eligible if they reported the results of an MTBT attempted with stroke participants. Six research databases were searched along with grey literature sources, trial registries, and article references. Intervention administration details and patient experiences were summarized.
Results
The search returned 903 articles of which 23 were eligible for inclusion. Most studies were small, observational, and enrolled chronic stroke patients. Interventions commonly targeted communication, cognition, or fine-motor skills. Therapies tended to be personalized based on patient deficits using commercially available applications. The complexity of therapy instructions, fine-motor requirements, and unreliability of internet or cellular connections were identified as common barriers to tablet-based care.
Conclusions
Stroke patients responded positively to MTBTs in both the inpatient and home settings. However, some support from therapists or caregivers may be required for patients to overcome barriers to care. Feasibility studies should continue to identify the administrative methods that minimize barriers to care and maximize patient adherence to prescribed therapy regiments.
Background
Rationale
Stroke survivors experience many post-stroke complications limiting their ability to function independently, including limitations to mobility [1], upper-limb function [2], communication [3], and cognition [4]. Specialized stroke rehabilitation has been shown to improve functional independence better than non-specialized care [5], and is most effective when performed early [6,7] and intensely post-stroke [8]. However, due to the growing number of stroke survivors [9] and lack of rehabilitation resources [10–13], many patients are not able to begin rehabilitative therapies early in order to maximize functional recovery. Easily accessible and resource-efficient stroke rehabilitation is needed to provide all stroke survivors with the opportunity to recover their functional abilities and improve their independence.
Mobile tablet computers with therapeutic applications could potentially offer a means of providing early and resource-efficient stroke rehabilitation. Tablet computers can be easily purchased and there exist many inexpensive or free therapy applications with scenarios analogous to those used in stroke rehabilitation. These devices are portable with fairly large, touch-responsive screens, which could be manipulated by stroke survivors depending on their post-stroke disabilities. Rehabilitation therapists can prescribe and monitor specific applications to patients based on their post-stroke difficulties.
Mobile tablet-based therapies (MTBTs) following stroke are an exciting new paradigm with many possibilities. However much remains unclear about this approach including the suitable population, the appropriate timing and setting, the necessary patient-support structure, and general therapy adherence. To better understand the feasibility of this intervention, it is critical to understand previous approaches to MTBT administration and the resulting patient experience, including treatment barriers and therapy adherence. A thorough review of the literature may provide important information for the successful conduct of large and potentially costly randomized controlled studies designed to demonstrate treatment efficacy.
Objective
The objective of this study was to summarize the administration of mobile tablet-based therapy (MTBTs) following stroke and the subsequent patient experience to inform treatment feasibility. In particular, we sought to accomplish this objective by answering three research questions:
What post-stroke deficits or complications have been targeted by MTBTs and how were the therapies administered?
What barriers to care did patients experience while engaging in MTBTs following stroke?
Were patients adherent to MTBTs following stroke?
Methods
Protocol and registration
The design of this scoping review was guided by the most current and widely known guide for scoping reviews [14], and by PRISMA-P [15] and PRISMA where applicable [16]. PROSPERO currently does not accept scoping review protocols for registration and therefore the study protocol was hosted on the University of Ottawa research depository and can be accessed here: http://hdl.handle.net/10393/35696 (S1 Protocol).
Eligibility criteria
All included articles were required to meet the following criteria: (1) the study population includes adult stroke survivors (18 years or older) of any type (ischemic/hemorrhagic) or stage (acute/chronic) in any setting, and (2) the study intervention involves stroke survivors interacting with a mobile tablet in response to a post-stroke deficit or complication. Articles were excluded if they met one or more of the following criteria: (1) the mobile tablet is primarily used by someone other than the stroke survivor, (2) the mobile tablet is more correctly described as an E-reader, and (3) the manuscript is a study protocol or conference abstract containing data otherwise available from a full study manuscript.
We defined MTBTs as patient-driven interactions with mobile tablets via various modalities for therapeutic purposes in response to a deficit, complication, or in order to prevent further health deterioration. There were no restrictions with regards to comparators, outcomes, study design and context, or settings, and conference abstracts were included if no full study manuscript could be found. However, only articles written in English were included and the search was limited to articles written from 2010 onwards as mobile tablets did not become widely popular until this time.
Information sources
Six databases were searched: MEDLINE (OVID interface), EMBASE (OVID interface), PsycINFO (OVID interface), CINAHL, Cochrane Database, and Web of Science. Additional sources were used to augment the database search for academic material: (1) a snowball search of relevant articles and reviews identified by the database search, (2) stroke research-related organizational websites, and (3) clinical trial databases were searched for completed and ongoing studies. A grey literature search was performed to find unpublished material using Google Scholar, the ProQuest Dissertation and Theses Database (Global and UK & Ireland), and the OpenGrey European grey literature database.
Search: Medline (Ovid interface) search strategy
exp Stroke/
exp cerebrovascular disorders/
(stroke* or cerebrovascular* or cerebral vascular or CVA*).tw.
((cerebr* or brain) adj3 infarct*).tw.
1 or 2 or 3 or 4
(mobile device* or mobile computer* or handheld computer* or tablet*).tw.
(ipad* or galaxy tab* or surface pro*).tw.
6 or 7
5 and 8
Restrictions: published in English between 2010-Present.
Study selection
A two-stage screening process performed by two independent reviewers was used to select included studies. In stage one, two authors (MP, DJ) screened abstracts and titles for potentially relevant articles, and in stage two, two authors (MP, DJ) read potentially relevant articles to confirm they met the inclusion criteria.
Data collection and data items
Two authors (MP, DJ) independently extracted data with the assistance of a data extraction form (S2 Appendix). Data items were selected based on the research objective and questions stated above. Items included general study information, participant characteristics, intervention details, comparator description, study outcome description and results, study setting and other contextual information.
Risk of bias in individual studies
As per current guidelines, no risk of bias assessment was performed for individual studies [14].
Analyses
The review goals and content were not appropriate for quantitative synthesis techniques commonly used in meta-analyses of systematic reviews. However, general study characteristics, participants, interventions, comparators, and patient-reported experiences were summarized narratively and using descriptive statistics where appropriate.
Results
Study selection
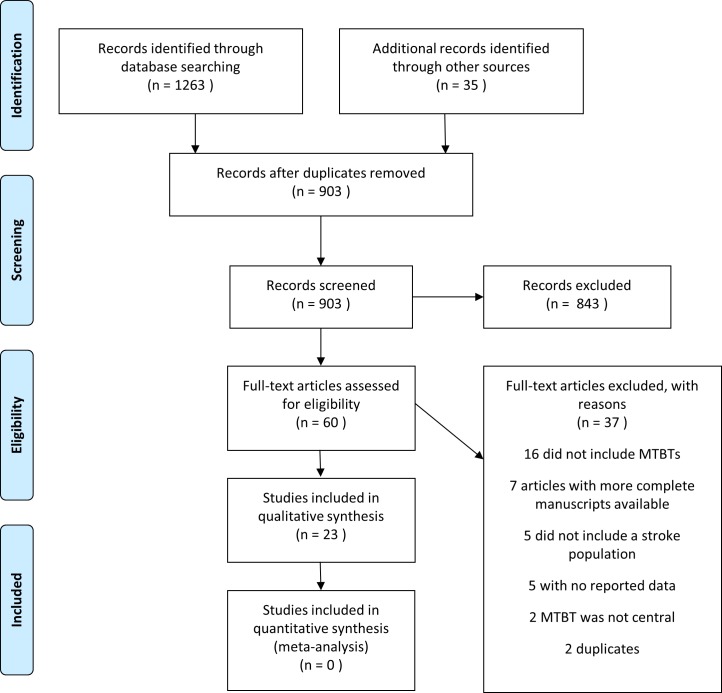
The search returned 868 articles from database searches, 8 potentially relevant articles from grey literature searches, 11 unique clinical trials from registry searches, and 16 potentially relevant articles through snowball reference searches of included studies for a total of 903 articles (Fig 1). Title and abstract screening narrowed the search down to 60 articles of which 37 were excluded leaving 23 articles for inclusion [17–39]. The eligible articles came from various search sources; 16 from database searches, 2 from grey literature, 2 from snowball searches, and included both full-texts and abstracts. Seven of the included articles were abstracts, the remaining articles were full manuscripts. None of the clinical trials identified by registry searches provided preliminary data or were completed, and therefore were not formally included.
Fig 1. PRISMA flow diagram.
Study characteristics
Most of the included studies were observational; 10 cohort studies, 3 case studies, 2 cross-sectional studies, and 1 qualitative interview (Table 1). Of the 7 experimental studies, 3 were RCTs, 2 were randomized experiments, 1 was a non-randomized experiment, and 1 used a cross-over design. Sample sizes were small ranging from 1 to 63 with an average of 18 participants. Three articles (Salaris et al. 2016 (1), Salaris et al. 2016 (2), and Janssen et al. 2016) were conference abstracts based off the same “TnT” study, although they reported different results.
Table 1. Studies of mobile tablet-based therapies following stroke.
| Article | Format | Design | Stroke Stage | Sample | Tablet Experience* |
|---|---|---|---|---|---|
| Carabeo et al. 2014 | manuscript | cohort | chronic | 3 | 1 |
| Choi et al. 2016 (1) | manuscript | cohort | chronic | 8 | 7 |
| Choi et al. 2016 (2) | manuscript | experiment | subacute | 24 | not reported |
| Crotty et al. 2014 | manuscript | cohort | not reported | 32 | not reported |
| Davis & Holzbach 2014 | abstract | case study | not reported | 1 | not reported |
| Des Roches et al. 2015 | manuscript | experiment | subacute/chronic | 51 | 22 |
| Hiyamizu et al. 2013 | abstract | experiment | unclear | 10 | not reported |
| Hoover & Carney 2014 | manuscript | cohort | chronic | 20 | 5 |
| Jang & Jang 2016 | manuscript | experiment | chronic | 21 | not reported |
| Janssen et al. 2016 | abstract | experiment | not reported | 15 | not reported |
| Katalinic et al. 2013 | manuscript | cohort | not reported | 39 | not reported |
| Kizony et al. 2016 | manuscript | experiment | subacute/chronic | 20 | not reported |
| Kurland et al. 2014 | manuscript | cohort | chronic | 5 | 1 |
| Lavoie et al. 2016 | manuscript | case study | chronic | 1 | not reported |
| Mallet et al. 2016 (1) | manuscript | cohort | acute | 30 | 21 |
| Mallet et al. 2016 (2) | abstract | cohort | acute | 12 | not reported |
| McCormick & Holmes 2016 | abstract | cohort | subacute/chronic | 13 | not reported |
| Rand et al. 2013 | manuscript | cohort | subacute/chronic | 11 | not reported |
| Routhier et al. 2016 | manuscript | case study (2) | chronic | 2 | not reported |
| Salaris et al. 2016 (1) | abstract | cross-sectional | not reported | 63 | not reported |
| Salaris et al. 2016 (2) | abstract | cross-sectional | not reported | 15 | not reported |
| Stark & Warburton 2016 | manuscript | experiment | chronic | 10 | 3 |
| White et al. 2015 | manuscript | qualitative | subacute/chronic | 11 | 0 |
*Refers to the number of participants with experience using mobile tablet computers prior to their participation in the study.
The majority of studies recruited participants in the chronic or subacute stages of stroke (14 studies), and only 2 studies included acute stroke patients. Participant stroke stage was either not reported or unclear in the remaining 7 studies. Only 8 studies explicitly reported the number of participants who had previous experience with tablets. On average, nearly half of the participants in these studies had previously used a mobile tablet.
Targets of mobile tablet-based therapy and methods of administration
Study interventions targeted a range of post-stroke deficits and complications: 11 MTBTs included an intervention for communication, 4 for fine-motor skills, 4 for quality of life (although 3 of these were from the same “TnT” study), 3 for cognitive deficits, 2 for deficits targeted by physiotherapy and 1 each for balance and upper extremity function. Therapies tended to be administered using iPad tablets to provide personalized therapy using a unique assortment of commercially available apps for individual participants (Table 2). Therapies were performed in home or inpatient settings and mostly performed independently with no reported assistance from caregivers or clinicians. However, even when therapies were independent, regular contact was often kept with patients through video-conferencing (Katalinic et al. 2013; Kurland et al. 2014), in-person visits (Lavoie et al. 2016; Routhier et al. 2016), or both (White et al. 2016). Certain MTBTs were only performed independently part of the time, with some therapist-assisted use occurring during tele-rehab sessions (Crotty et al. 2014), in-person meetings (Davis & Holzbach 2014; Des Roches et al. 2015; Janssen et al. 2016), and group therapy sessions (Hoover & Carney 2014).
Table 2. Characteristics of attempted mobile tablet-based therapies following stroke.
| Study | Target | Independent | Setting | Personalized | App(s) |
|---|---|---|---|---|---|
| Carabeo et al. 2014 | fine-motor skills | yes | inpatient | No | FINDEX |
| Choi et al. 2016 (1) | communication | yes | home | Yes | iAphasia |
| Choi et al. 2016 (2) | upper extremity | yes | inpatient | Yes | Mou-Rehab |
| Crotty et al. 2014 | communication/physiotherapy | partially | home | Yes | commercial |
| Davis & Holzbach 2014 | communication | partially | inpatient | unclear | not reported |
| Des Roches et al. 2015 | communication/cognition | partially | clinic/home | Yes | commercial |
| Hiyamizu et al. 2013 | balance | unclear | not reported | unclear | not reported |
| Hoover & Carney 2014 | communication | partially | inpatient | Yes | commercial |
| Jang & Jang 2016 | fine-motor skills | unclear | not reported | No | unnamed |
| Janssen et al. 2016 | quality of life | partially | inpatient/home | unclear | unclear |
| Katalinic et al. 2013 | communication/cognition/fine-motor skills/relaxation | yes | home | yes | commercial |
| Kizony et al. 2016 | fine-motor skills | yes | in/outpatient | No | commercial |
| Kurland et al. 2014 | communication | yes | home | yes | commercial |
| Lavoie et al. 2016 | communication | yes | home | yes | commercial |
| Mallet et al. 2016 (1) | communication | yes | acute care | yes | commercial |
| Mallet et al. 2016 (2) | communication/cognition | unclear | acute care | yes | not reported |
| McCormick & Holmes 2016 | physiotherapy | yes | not reported | unclear | SIMULATe |
| Rand et al. 2013 | fine-motor skills | yes | not reported | No | commercial |
| Routhier et al. 2016 | communication | yes | home | yes | commercial |
| Salaris et al. 2016 (1) | quality of life | partially | inpatient | Unclear | unclear |
| Salaris et al. 2016 (2) | quality of life | partially | inpatient/home | unclear | unclear |
| Stark & Warburton 2016 | communication | yes | home | yes | commercial |
| White et al. 2015 | quality of life | yes | home | yes | commercial |
Studies often personalized their MTBTs to individual patients by assigning different commercial applications based on assessments (Mallet et al. 2016 (1); Mallet et al. 2016 (2)) or perceived needs (Katalinic et al. 2013; White et al. 2015). Therapies using a single commercially available app (Des Roches et al. 2015) or single app developed by the study team (Choi et al. 2016 (1), Choi et al. 2016 (2), Jang & Jang 2016, McCormick & Holmes 2016), personalized therapies by assigning different modules based on assessments. The most frequently used commercially available were apps were Tactus Language Therapy (Hoover & Carney 2014, Katalinic et al. 2013, Mallet et al. 2016 (1); Stark & Warburton 2016; White et al. 2015), Constant Therapy (Des Roches et al. 2015; Hoover & Carney 2014; Mallet et al. 2016 (1)), and Dexteria (Katalinic et al. 2013; Kizony et al. 2016; Rand et al. 2013). Studies of communication MTBTs presented personalized visual stimuli using a variety of apps including Keynote, iBooks, and PowerPoint.
Barriers to tablet-based care following stroke
Barriers to care, methodological challenges, and patient experience were defined as separate outcomes at the protocol stage but were found to be intertwined during data collection (i.e. barriers to care are often patient reported experiences that lead to methodological challenges). Eight studies reported barriers to MTBT care, which were categorized as device, patient and system barriers (Table 3). The difficulty of the assigned tasks were identified as a device barrier in 3 studies (Carabeo et al. 2014; Choi et al. 2016 (1); Kurland et al. 2014). In each case, participants found the assigned tasks too easy to perform. Bugs in provided videoconferencing software (Katalinic et al. 2013) and the high speed of task prompts and requests (White et al. 2015) were also identified as device barriers. However, the software bug was later resolved.
Table 3. Barriers to mobile tablet-based therapy following stroke.
| Device Barriers | Patient Barriers | System Barriers |
|---|---|---|
| Task difficulty | Difficulty following complex instructions | Unreliable connections |
| Task bugs | Finger dexterity | Hospital infection control standards |
| Task speed | Accidently changing crucial settings | Hospital protocol |
| Comfort | Network security | |
| Post stroke depression | ||
| Strain | ||
| Patient’s home bandwidth | ||
| Distractibility |
The most commonly reported patient barrier was a difficulty in following complex instructions (Kurland et al. 2014; Mallet et al. 2016 (1); Routhier et al. 2016; White et al. 2015). Specifically, some participants had difficulty understanding how to use the device (Mallet et al. 2016 (1); White et al. 2015) although it was noted in both cases that participants were able to overcome their difficulties with additional familial support. In other cases, participants had difficulty navigating the device to access the therapy materials (Kurland et al. 2014; Routhier et al. 2016). The second most commonly identified patient barrier was finger dexterity (Kizony et al. 2016; Mallet et al. 2016 (1); Routhier et al. 2016). It was reported that some participant had difficulty isolating their fingers so that only one would touch the screen at a time in order for the device to respond (Kurland et al. 2014), some had difficulty manipulating the device in general, and some had difficulty opening the device case (Mallet et al. 2016 (1)). This final barrier was overcome by using a different tablet case.
Two studies reported patients feeling uncomfortable about specific aspects of their assigned MTBT (Mallet et al. 2016 (1), White et al. 2015). A participant in a MTBT for communication initially felt embarrassed speaking to their device in the hospital, however this was overcome by providing a headset (Mallet et al. 2016 (1)). In another instance, patients felt apprehensive about learning new technology and were particularly anxious about making mistakes in front of study staff (White et al. 2015). These participants felt more comfortable exploring the device at home. Various other patient barriers were reported by single studies. One participant did not use their tablet due to post-stroke depression and feelings of overwhelming post-stroke change (White et al. 2015). Participants taking part in a fine-motor intervention reported the MTBT as being strenuous when performed continually (Carabeo et al. 2014). Particular complains included muscle strain and numbness, hand numbness and jitters, and eye fatigue. Finally, one study reported that the patient’s internet bandwidth made videoconferencing software unusable (Katalinic et al. 2013) and another reported that a participant was particularly excited to use their device for non-therapeutic purposes (Kurland et al. 2014). In the latter case however, this did not appear to interfere with their therapy progress.
System barriers were mostly limited to a study conducted in the acute setting (Mallet et al. 2016 (1)), however two studies of home-based MTBTs also noted barriers in this domain (Katalinic et al. 2013; White et al. 2015). All three of these studies reported connection issues as interfering with care. Some participants lived in areas with poor 3G coverage, resulting in subpar videoconferencing quality (Katalinic et al. 2013). This was resolved by calling participants to help them connect the devices to their home internet connection. However, some participants in another home study reported finding their home internet connections to be unreliable (White et al. 2015). In addition to connection problems, Mallet et al. 2016 (1) also reported barriers related to legitimate concerns from Hospital Infection Control and Hospital IT Security and challenges related to standard of care assessment procedures. Infection control required tablets be cleaned with disinfecting wipes before being transferred to another patient to prevent the spread of disease. There were concerns about hospital network security requiring an independent security assessment before the study could continue. Finally, five eligible patients did not undergo standard of care assessments for unspecified reasons and were missed for recruitment.
Despite these barriers the overall reported patient response was very positive with many studies reporting high satisfaction and usability through either surveys (Choi et al. 2016 (2); Crotty et al. 2014; Kizony et al. 2016; Mallet et al. 2016 (1); Mallet et al. 2016 (2); McCormick & Holmes 2016; Rand et al. 2013) or qualitative interviews (Carabeo et al. 2014; Choi et al. 2016 (1); Hoover & Carney 2014; Kurland et al. 2014; Routhier et al. 2016; White et al. 2015). Specific patient reported positive aspects were the self-directed and independent aspect of tablet-based therapies (Carabeo et al. 2014; Kurland et al. 2014; Routhier et al. 2016; White et al. 2015) and the convenience of home therapy (Crotty et al. 2014; Kurland et al. 2014; Routhier et al. 2016). Patients from three studies indicated that tablet devices were useful rehab tools (Kizony et al. 2016, Rand et al. 2013) and patients from two other studies felt the device contributed to rehabilitation improvements (Carabeo et al. 2014, White et al. 2015). Three studies also reported positive responses from caregivers (Hoover & Carney 2014; Kurland et al. 2014; White et al. 2015) with Kurland et al. (2014) noting that families reported observing improvements and White et al. (2015) indicating that the device gave family and friends an opportunity to engage in therapy with the participants.
Patient adherence to tablet-based care following stroke
The majority of studies (17/23) prescribed a MTBT dose with only 6 studies not assigning specific MTBT regiments or not reporting this information (Table 4). Prescribed therapy dosages varied although most assigned regiments lasted between 2–6 weeks and assigned 3–6 MTBT sessions a week. Two studies were experiments in which participants only engaged with the MTBT for a single session (Kizony et al. 2016; Rand et al. 2013).
Table 4. Therapy dosages.
| Study | Assigned Mobile Tablet-Based Therapy Dosage | Clinician-led Sessions |
|---|---|---|
| Carabeo et al. 2014 | 9 sessions of 30 minutes over 1.5 months | standard rehab program |
| Choi et al. 2016 (1) | 4 weeks, as often/long as possible | None |
| Choi et al. 2016 (2) | 10 sessions of 30 mins over 2 weeks | 30 minutes of occupational therapy |
| Crotty et al. 2014 | 8 weeks | videoconference sessions |
| Davis & Holzbach 2014 | not reported | structured therapy sessions |
| Des Roches et al. 2015 | 6 hours a week for 10 weeks plus clinician sessions | 10 MTBT sessions (1 per week) over 10 weeks |
| Hiyamizu et al. 2013 | 9 sessions (3 per week) over 3 weeks | not reported |
| Hoover & Carney 2014 | 20 sessions (5 per week) over 4 weeks | intensive full rehab program |
| Jang & Jang 2016 | 24 sessions (6 per week) over 4 weeks | not reported |
| Janssen et al. 2016 | None, could use how they please | standard rehab program |
| Katalinic et al. 2013 | lent device for 3 months | videoconference sessions |
| Kizony et al. 2016 | single use experiment | None |
| Kurland et al. 2014 | 120–144 sessions (5–6 per week) over 6 months | 24 sessions (1 per week) over 6 months |
| Lavoie et al. 2016 | 12 sessions (4 per week) over 3 weeks | weekly non-therapeutic home meetings |
| Mallet et al. 2016 (1) | 1 hour/day during acute stay | standard acute care |
| Mallet et al. 2016 (2) | 1 hour/day during acute stay | standard acute care |
| McCormick & Holmes 2016 | 18 sessions over 18 days | not reported |
| Rand et al. 2013 | single use experiment | None |
| Routhier et al. 2016 | 20 sessions (4 per week) over 5 weeks | weekly non-therapeutic home meetings |
| Salaris et al. 2016 (1) | none, could use how they pleased | standard rehab program |
| Salaris et al. 2016 (2) | none, could use how they pleased | standard rehab program |
| Stark & Warburton 2016 | 28 sessions (everyday) for 4 weeks | not reported |
| White et al. 2015 | none, could use how they pleased | Unclear |
Tablet-based therapy usage habits were reported by a minority of studies (Table 5). Tablet usage ranged from little daily usage (Kurland et al. 2015) to daily use equal to or exceeding 1 hour (Choi et al. 2016 (1); Mallet et al. 2016 (1); Mallet et al. 2016 (2)). Specific tablet usage was not reported by a handful of studies. In particular, Stark & Warburton (2016) could not confirm usage habits using information transmitted from the tablets and relied on patient self-reported usage. White et al. (2015) qualitatively reported the usage habits of a handful of participants using quotes to communicate the variety of usage habits they observed in their participants.
Table 5. Participant mobile tablet-based therapy usage habits.
| Study | Mobile Tablet-Based Therapy Usage |
|---|---|
| Choi et al. 2016 (1) | Mean usage: 60 minutes/day |
| Des Roches et al. 2015 | Mean usage: 40 minutes/day |
| Kurland et al. 2014 | Mean usage: 18 minutes/day |
| Mallet et al. 2016 (1) | Mean usage: 150 minutes/day |
| Mallet et al. 2016 (2) | Mean usage: 85 minutes/day |
| McCormick & Holmes 2016 | Participants completed at least 30 minutes of scheduled 90 minute sessions |
| Salaris et al. 2016 (2) | Used 2–3 times/week during inpatient stay and up to 1–2 hours/days after discharge |
| Stark & Warburton 2016 | Patients reported using table at least 20 minutes every day for 4 weeks |
| White et al. 2015 | Patients reported variable tablet-usage habits |
Discussion
This systematic scoping review yielded 23 eligible articles pertaining to mobile tablet-based therapies for a variety of post-stroke deficits and complications. The majority of the identified articles reported the results of observational studies with small samples of chronic or subacute stroke patients. The goals of most of the studies were exploratory, with only a few articles reporting the results of experimental studies attempting to demonstrate treatment effectiveness. The collected body of literature suggests MTBTs following stroke may be feasible and acceptable, with many studies expressing positive experiences. However, common barriers to care were identified that may prevent certain patients from successfully engaging in therapy.
Mobile tablet-based communication, cognitive, and fine-motor therapy following stroke
The majority of the attempted MTBTs targeted communication, cognitive, and fine-motor deficits. Considering interventions for these deficits are arguably the most intuitive to implement on a tablet device, it is not entirely surprising that research has focused mainly on these interventions. Furthermore, there is already research supporting the effectiveness of computer-based speech-language therapy [4]. This research-base, coupled with the emergence of popular therapeutic apps like Constant Therapy, Tactus Therapy, and Dexteria may explain why many researchers are more interested in attempting MTBTs for these deficits over other post-stroke complications. Although the methods of administration varied among MTBTs for communication and fine-motor deficits, some trends emerged.
Most MTBTs were performed independently without clinician guidance, although in some cases caregivers or family members participated in therapy with patients. Regular contact with clinicians through videoconferencing and in-person visits were common even among independently performed therapies. The involvement of others in therapy may be beneficial for patient barriers related to therapy and device complexity could be overcome with assistance. Contact with clinicians offers an opportunity for patients to voice issues regarding tablet complexity and for therapists to re-explain complex therapy tasks or features to participants and their caregivers. However, therapists should try not to infringe upon patient independence as this was found to be one of the aspects of tablet-based therapy that patients enjoyed. Similarly, patients would likely prefer for physicians to keep contact in a manner that does not require them to leave their home as the home-based aspect of therapy was also frequently noted as a positive attribute. Videoconferencing was successfully used to contact patients, although some technical difficulties were noted and it is possible that not all patients will have access to reliable cellular or wireless internet connections.
Mobile tablet-based therapies tended to use personalized tasks tailored to patient deficits. Personalization may prevent patients from encountering therapies with inappropriate difficulty levels and encourage extended therapy engagement. However, this relationship between personalization and therapy engagement was not made clear from the collected usage data. It should be noted that even studies with personalized therapy tasks reported barriers to care involving task difficulty suggesting personalization alone is not enough. Rather, therapies should both be personalized and then adjusted as patients engage in therapy. The result is an iterative process whereby patients are assigned tasks based upon their deficits, engage in therapy, express difficulty or make progress, and then the process begins again as new tasks are assigned. This also further highlights the importance of maintaining regular contact with clinicians who are needed to make informed therapy adjustments.
Mobile tablet-based physiotherapy
Mobility and upper extremity deficits are very common post-stroke [1–2], and yet there were only four studies involving tablet-based physiotherapy activities, two of which were reported as short conference abstracts. Perhaps, the lack of physiotherapy interventions reflects the difficulty of translating traditional physiotherapy to tablets or perhaps due to interests in other promising technology such as robotics [40,41]. Although the few studies reported heterogeneous administrative methods, interesting ideas for tablet-based physiotherapy were described. One intervention for upper extremity therapy used a combination of tablet sensors with a smartphone attached to the patient upper limb to track movement in response to tablet therapy activities which was well-received by patients (Choi et al. 2016 (2)). Other studies used video capabilities to provide visual feedback on movements or provide visual demonstrations (Hiyamizu et al. 2013), and assigned patients a Fitbit to track activity levels (Crotty et al. 2014).
The ability of tablets to host video conferences with therapists could be particularly useful for providing tablet-based physiotherapy. Crotty et al. 2013 reported using a combination of video conferences to deliver therapy, and video recording to save sessions for later viewing, however the details of this administrative method with regards to physiotherapy were not made clear. Further detailed studied of similar administrative approaches and barriers to care would be helpful for determining the feasibility of tablet-based physiotherapy in the home setting. Although patients would likely enjoy the convenience of home physiotherapy instead of travelling to or staying in a rehabilitation center, the safety of home-based physiotherapy is unknown.
Mobile tablet-based depression treatment
There were no tablet-based mood interventions published, despite depression being common following stroke [42]. However, three abstracts from one study [26,36,37] and one further study [29] reported on interventions targeting quality of life, a construct related to depression. Although the results provide some promise that simply providing patients with a tablet computer could improve quality of life, and therefore mood, it remains unknown if a more structured approach to tablet-based mood therapy is feasible. Interestingly, another study reported improvements in mood and arm function after a physiotherapy intervention [33]. This suggests that providing tablet-based interventions to improve physical deficits could indirectly lead to improvements in post-stroke depression. Logically some patients would feel depressed following the sudden loss of their physical abilities and independence following stroke, and it follows that improvements in physical function could be coupled with an improvement in mood. Future studies of tablet-based intervention targeting physical abilities should consider measuring mood as a secondary outcome to further explore this hypothesis.
Designing and administering tablet-based interventions directly targeting mood is difficult, as no intervention has distinguished itself as particularly effective with stroke patients using a traditional face-to-face administrative approach. For example, although there has been considerable research on internet-based cognitive behavioral therapy in other populations [43,44], it remains unclear if this approach is effective for individuals post-stroke [45]. However, there has been promising evidence for the effectiveness of mindfulness-based therapies for improving mood post-stroke [46] and there exists the possibility of translating these interventions to tablet devices in the future.
Mobile tablet-based therapy for acute stroke patients
Early rehabilitation initiation is important to maximize recovery of function [6,7], and MTBTs could be used to provide early therapy during the significant amount of downtime experienced by patients in acute care [11]. Despite this, the only two studies attempting MTBTs in the acute stroke setting to provide early stroke rehabilitation services (Mallet et al. 2016 (1); Mallet et al. 2016 (2)) were performed by our own Ottawa Stroke Program Research Team. The technology, need, and available time exist to administer tablet-based therapy in the acute stroke setting. Further investigation into this area is warranted.
The next steps for mobile tablet-based therapies following stroke
This review provides a summary of MTBT administration methods, barriers to care, and therapy adherence following stroke. The accumulated studies suggest many stroke patients are able to successfully engage in tablet-based therapies for communication, cognitive and fine-motor deficits with minimal assistance and enjoy the independence and convenience of tablet-based therapy despite barriers to care. Attempting randomized controlled trials for therapies targeting communication, cognitive, and fine-motor deficits may not yet be appropriate despite seemingly strong support for treatment feasibility. Further studies should track therapy adherence and experiment with methods for encouraging patient adherence to therapy regiments. Successful methods for promoting therapy engagement may be simple as sending text notifications to the tablet device, setting weekly therapy goals, regular videoconferences, or perhaps a combination of strategies individually tailored to patient preferences.
Limitations
The large breadth of outcome information eligible for extraction from articles by the two independent reviewers means some relevant outcomes could have been missed. However, the data extraction process used assistive documents to help reviewers identify relevant outcomes. No risk of bias assessment was performed on the included studies as is common for scoping reviews. However, this was done in accordance with current scoping review guidelines and considering the goals of the review were to collect information on administrative methods and barriers to care, a risk of bias assessment would be unlikely to impact our findings.
Conclusions
The majority of MTBTs following stroke targeted communication, cognitive, and fine-motor deficits, and were positively received by patients despite barriers to care. The findings suggest tablet-based therapy may be feasible for certain stroke deficits although little is known about therapy adherence. Feasibility studies should continue to refine the administrative methods for frequently targeted post-stroke deficits to minimize barriers to care and maximize treatment adherence.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank Michael Boutet from the University of Ottawa Health Sciences Library for advising on the database search strategy.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a Canadian Institutes of Health Research Canadian Graduate Scholarship Master's Award (http://www.cihr-irsc.gc.ca/e/49440.html; M.P.), a University of Ottawa Ontario Graduate Scholarship (https://www.uottawa.ca/graduate-studies/students/awards/ontario-graduate-scholarship; M.P.), the University of Ottawa Brain and Mind Research Institute (https://www.uottawa.ca/brain/; D.D), the Canadian Partnership for Stroke Recovery (http://www.canadianstroke.ca/en/; D.D.), and the University of Ottawa Department of Medicine (https://med.uottawa.ca/en; D.D.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: The Copenhagen stroke study. Arch Phys Med Rehabil. 1995;76: 27–32. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: The Copenhagen stroke study. Arch Phys Med Rehabil. 1994;75(April): 394–398. [DOI] [PubMed] [Google Scholar]
- 3.Salter K, Teasell R, Foley N, Allen L. Evidence-based systematic review of stroke rehabilitation: Cognitive disorders and apraxia. Last updated July 2013. Available from: http://www.ebrsr.com/evidence-review/12-cognitive-disorders-and-apraxia.
- 4.Salter K, Teasell R, Foley N, Allen L. Evidence-based systematic review of stroke rehabilitation: Aphasia. Last updated September 2013. Available from: http://www.ebrsr.com/evidence-review/14-aphasia.
- 5.Foley N, Teasell R, Bhogal S, Speechley M, Mbbs NH. Evidence-based systematic review of stroke rehabilitation: The efficacy of stroke rehabilitation. Last updated November 2013. Available from: http://www.ebrsr.com/evidence-review/5-efficacy-stroke-rehabilitation.
- 6.Ottenbacher KJ, Jannell S. The results of clinical trials in stroke rehabilitation research. Arch Neurol. 1993;50(1): 37–44. doi: 10.1001/archneur.1993.00540010033014 [DOI] [PubMed] [Google Scholar]
- 7.Cifu D, Stewart D. Factors affecting functional outcome after stroke: a critical review of rehabilitation interventions. Arch Phys Med Rehab. 1999;80: S35–39. [DOI] [PubMed] [Google Scholar]
- 8.Teasell R, Hussein M. Evidence-based systematic review of stroke rehabilitation: Background Concepts in Stroke Rehabilitation. Last updated: September 2016. Available from: http://www.ebrsr.com/evidence-review/3-background-concepts-stroke-rehabilitation.
- 9.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383: 245–255. http://dx.doi.org/10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Heart and Stroke Foundation 2014 stroke report In: Canadian stroke best practice recommendations. The Heart and Stroke Foundation; 2014. Posted June 2014. Available from: http://www.strokebestpractices.ca/wp-content/uploads/2014/06/HSF_SMReport2014E_Final.pdf. [Google Scholar]
- 11.Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: Physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35: 1005–1009. doi: 10.1161/01.STR.0000120727.40792.40 [DOI] [PubMed] [Google Scholar]
- 12.Consensus Panel on the Stroke Rehabilitation System. Time is Function: A report from the Consensus Panel on the Stroke Rehabilitation System to the Ministry of Health and Long-Term Care. In: Consensus Panel on the Stroke Rehabilitation System. 2007. Accessed November 15th, 2016. Available from: www.tostroke.com/wp-content/uploads/…/Rehab-Consensus-Panel-Final-Report.pdf
- 13.Xie J, George M, Ayala C, McGruder H, Denny C, Croft J. Outpatient rehabilitation among stroke survivors—21 States and the District of Columbia, 2005. Morbity Mortal Wkly Rep. 2007;56(20): 504–507. [PubMed] [Google Scholar]
- 14.Peters MDJ, Godfrey CM, Khalil H, Mcinerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13: 141–146. doi: 10.1097/XEB.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1): 1 doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7): e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carabeo CGG, Dalida CMM, Padilla EMZ, Rodrigo MMT. Stroke patient rehabilitation: A pilot study of an android-based game. Simul Gaming. 2014;45(2): 151–166. doi: 10.1177/1046878114531102 [Google Scholar]
- 18.Choi Y, Park HK, Paik N, National S, Hospital B. A telerehabilitation approach for chronic aphasia following stroke. Telemed e-Health. 2016;May: 434–440. doi: 10.1089/tmj.2015.0138 [DOI] [PubMed] [Google Scholar]
- 19.Choi Y-H, Ku J, Lim H, Kim YH, Paik N-J. Mobile game-based virtual reality rehabilitation program for upper limb dysfunction after ischemic stroke. Restor Neurol Neurosci. 2016;34(3): 455–463. doi: 10.3233/RNN-150626 [DOI] [PubMed] [Google Scholar]
- 20.Crotty M, Killington M, van den Berg M, Morris C, Taylor A, Carati C. Telerehabilitation for older people using off-the-shelf applications: acceptability and feasibility. J Telemed Telecare. 2014;20(7): 370–376. doi: 10.1177/1357633X14552382 [DOI] [PubMed] [Google Scholar]
- 21.Davis B, Holzbach A. The impact of tablet use on motivation and language outcomes in the inpatient rehabilitation setting: A case study. Int Brain Inj Assoc. Conference(Online):1 https://ibia.conference-services.net/reports/template/onetextabstract.xml?xsl=template/onetextabstract.xsl&conferenceID=3754&abstractID=787511. [Google Scholar]
- 22.Des Roches CA, Balachandran I, Ascenso EM, Tripodis Y, Kiran S. Effectiveness of an impairment-based individualized rehabilitation program using an iPad-based software platform. Front Hum Neurosci. 2015;8(Article): 1–29. doi: 10.3389/fnhum.2014.01015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiyamizu M, Kasahara N, H M, Matsuo A, Morioka S. Effects of video-feedback on balance learning in stroke patients. Cerebrovasc Dis. 2013;35(Suppl 3): 778 https://doi.org/10.1159/000353129 May 27, 2013. [Google Scholar]
- 24.Hoover EL, Carney A. Integrating the iPad into an intensive, comprehensive aphasia program. Semin Speech Lang. 2014;35(1): 25–37. doi: 10.1055/s-0033-1362990 [DOI] [PubMed] [Google Scholar]
- 25.Jang SH, Jang WH. The effect of a finger training application using a tablet PC in chronic hemiparetic stroke patients. Somatosens Mot Res. 2016;33(2): 124–129. doi: 10.1080/08990220.2016.1197117 [DOI] [PubMed] [Google Scholar]
- 26.Janssen H, Salaris M, Quinn R, Jordan L-A, Galvin R, Veitch K, et al. Tablet computers may contribute to better stroke survivor quality of life one month after discharge from inpatient rehabilitation. Int J Stroke. 2016;11(1S): 15 doi: 10.1177/1747493016661644 [Google Scholar]
- 27.Katalinic O, Young A, Doolan D. Case study: the interact home telehealth project. J Telemed Telecare. 2013;19(7): 418–424. doi: 10.1177/1357633X13506513 [DOI] [PubMed] [Google Scholar]
- 28.Kizony R, Zeilig G, Dudkiewicz I, Schejter-Margalit T, Rand D. Tablet apps and dexterity: Comparison between 3 age groups and proof of concept for stroke rehabilitation. J Neurol Phys Ther. 2016;40(1): 31–39. doi: 10.1097/NPT.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 29.Kurland J, Wilkins AR, Stokes P. iPractice: Piloting the effectiveness of a tablet-based home practice program in aphasia treatment. Semin Speech Lang. 2014;35(1): 51–63. doi: 10.1055/s-0033-1362991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavoie M, Routhier S, Légaré A, Macoir J. Treatment of verb anomia in aphasia: efficacy of self-administered therapy using a smart tablet. Neurocase. 2016;22(1): 109–118. doi: 10.1080/13554794.2015.1051055 [DOI] [PubMed] [Google Scholar]
- 31.(1) Mallet KH, Shamloul RM, Corbett D, Finestone HM, Hatcher S, Lumsden J, et al. RecoverNow: Feasibility of a mobile tablet-based rehabilitation intervention to treat post-stroke communication deficits in the acute care setting. PLoS ONE. 2016:11(12): e0167950 doi: 10.1371/journal.pone.0167950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(2) Mallet K, Shamloul R, Pugliese M, Corbett D, Hatcher S, Power E, et al. The Recovernow patient perspective: A patient engagement survey for a mobile device delivered stroke rehabilitation in the acute care setting. Int J Stroke. 2016;11(2S): 73 doi: 10.1177/1747493016659793 [DOI] [PubMed] [Google Scholar]
- 33.McCormick SA, Holmes PS. See, Imagine, Move—Upper Limb Action Therapy (SIMULATe): iPad-based mental and physical motor (re)learning for stroke recovery. Stroke. 2016;47(ATP157). [Google Scholar]
- 34.Rand D, Schejter-Margalit T, Dudkiewicz I, Kizony R, Zeilig G. The use of the iPad for poststroke hand rehabilitation: a pilot study. IEEE Conf Pap. 2013: 109–113. doi: 10.1109/ICVR.2013.6662068 [Google Scholar]
- 35.Routhier S, Bier N, Macoir J. Smart tablet for smart self-administered treatment of verb anomia: Two single-case studies in aphasia. Aphasiology. 2016;30(2–3): 269–289. doi: 10.1080/02687038.2014.973361 [Google Scholar]
- 36.(1) Salaris M, Quinn R, Jordan L-A, Galvin R, Veitch K, Young A, et al. Don’t forget to take your tablet! Using tablet computers to increase self-directed therapy during inpatient stroke rehabilitation. Int J Stroke. 2016;11(1S): 13. [Google Scholar]
- 37.(2) Salaris M, Quinn R, Jordan L-A, Galvin R, Veitch K, Young A, et al. Tablets are not hard to swallow after stroke! Tablet computers are used frequently for self-directed therapy and leisure activities despite limited pre-stroke exposure. Int J Stroke. 2016;11(1S): 21. [Google Scholar]
- 38.Stark BC, Warburton EA. Improved language in chronic aphasia after self- delivered iPad speech therapy. Neuropsychol Rehabil. 2016;Online: 1–14. doi: 10.1080/09602011.2016.1146150 [DOI] [PubMed] [Google Scholar]
- 39.White J, Janssen H, Jordan L, Pollack M. Tablet technology during stroke recovery: A survivor’s perspective. Disab. 2015;37(13): 1186–1192. doi: 10.3109/09638288.2014.958620 [DOI] [PubMed] [Google Scholar]
- 40.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Sys Rev. 2015;11: CD006876 doi: 10.1002/14651858.CD006876.pub4.www.cochranelibrary.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database Sys Rev. 2013;7: CD006185 doi: 10.1002/14651858.CD006185.pub3 www.cochranelibrary.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salter K, Mehta S, Bhogal S, Cotoi A, Teasell R, Foley N, et al. Evidence-based systematic review of stroke rehabilitation: Post stroke depression. Last updated August 2013. Available from: http://www.ebrsr.com/evidence-review/18-post-stroke-depression.
- 43.Andrews G, Cuijpers P, Craske MG, Mcevoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: A meta-analysis. PLoS One. 2010;5(10): e13169 doi: 10.1371/journal.pone.0013169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams AD, Andrews G. The effectiveness of internet cognitive behavioural therapy (iCBT) for depression in primary care: A quality assurance study. PLoS One. 2013;8(2): 57447 doi: 10.1371/journal.pone.0057447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Sys Rev. 2008;4: CD003437 http://www.thecochranelibrary.com. doi: 10.1002/14651858.CD003437.pub3 [DOI] [PubMed] [Google Scholar]
- 46.Lawrence M, Booth J, Mercer S, Crawford E. A systematic review of the benefits of mindfulness-based interventions following transient ischemic attack and stroke. Int J Stroke. 2013;8(August): 465–474. doi: 10.1111/ijs.12135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.