Abstract
Quantifying the ability of a compound to modulate cell migration rate is a crucial part of many studies including those on chemotaxis, wound healing and cancer metastasis. Existing migration assays all have their strengths and weaknesses. The “scratch” assay is the most widely used because it seems appealingly simple and inexpensive. However, the scratch assay has some important limitations, as the tool introducing the “wound” might injure/stress the boundary cells and/or harm underlying matrix coatings, which in both cases will affect cell migration. This described method is a Cell Exclusion Zone Assay, in which cell-free areas are created by growing cells around removable silicone stoppers. Upon appropriate staining with fluorescent dyes and microscopically visualizing the monolayers, the migration rate is then quantified by counting the cells (nuclei) intruding the void area left by the silicone insert. In the current study human small intestine epithelial cells were seeded on a physiological substrate matrix to produce collectively migrating monolayers. Different substrates were tested to determine the optimal surface for enterocyte adherence and migration and morphological changes monitored. Recombinant human epidermal growth factor and osteopontin purified from urine were tested to see if the established migration assay produces accurate and reliable migration data with human small intestine cells. The obtained data accurately confirmed that the two bioactive proteins modulate cellular migration in a dose-dependent manner. The presented assay can likely be converted for use with other adherent cell lines or substrate matrices and allows for high throughput, while cost is kept low and versatility high. Co-staining can be applied in order to assay for cell death, different cell types, cell stress and others allowing intricate analysis of migration rate of mixed populations and correction for cell viability.
Abbreviations: (ECM), Extracellular matrix; (Caco-2), human epithelial colorectal adenocarcinoma cells; (FHs-74 int), non-malignant human fetal small intestine cells; (EGF), Recombinant human epidermal growth factor; (ROI), region of interest; (OPN), osteopontin; (BME), Basal membrane extract; (DMEM), Dulbecco's modified Eagle medium; (FBS), fetal bovine serum; (FRET), Förster resonance energy transfer
Keywords: Migration assay, Wound healing, Collective migration, Epithelium, Small intestine cells, Bioactive
Highlights
-
•
We demonstrate an accurate and cheap migration assay as alternative to “scratching”.
-
•
Provides clear distinction between migration, inflammation, proliferation and apoptosis.
-
•
Optimization for collective migration and normal morphology of small intestine cells.
-
•
The assay exhibits wide compatibility with cell types, substrates and equipment.
-
•
Cost is low, but content and accuracy high and independent of user.
1. Introduction
Quick resurfacing is essential for intestinal wound repair in order to regain the ability to function as a protective barrier preventing invasion by pathogenic microorganisms. This is achieved by proliferation and directional migration of enterocytes from the edges of the wound. The quantification of cell migration rate is an important tool to determine the modulatory potential of extrinsic factors on epithelial healing, chronic wounds, villi restitution, cancer cell migration, angiogenesis, embryogenesis and similar types of research (McCormack et al., 1993, Ng et al., 2001, Nobes and Hall, 1999, Stojadinovic et al., 2005, Wang et al., 2008). Different techniques have been employed to quantify cell migration like scratch assays, Boyden chambers and silicone inserts all with separate benefits and drawbacks. The scratch assay is an animal free mimic of wound healing, but consumes many cells and a substantial amount of compound when screening in 6 or 12-well plates. These restrictions make it less ideal for studying primary cultures or patient samples as well as discerning the direct mode of action (molecule-cell, inflammation based migration, paracrine signalling, etc.) (Liang et al., 2007). The Boyden chamber assay (i.e. Transwell assay) is a two chamber assay also used for migration studies. It should be noted that the Boyden chamber, especially when coupled with basal membrane extract coating, is technically an invasion assay and commonly used for screening the invasiveness of cancer cells and their expression of metalloproteases necessary to penetrate the extracellular matrix (Marshall, 2011).
The scratch assay is most widely used when quantifying migration rate, as it provides a simple and economical set up in the hands of experienced users. Over time the versatility of the traditional scratch assay has been discussed and several attempts has been made to optimize the assay to accommodate higher throughput, better consistency and enhanced statistics using automation and advanced imaging software (Vogel et al., 2010, Yarrow et al., 2004). Among the disadvantages linked to the scratch assay are that small differences in the protocols for creating scratches convey large inter-lab variance. Scratching can damage the underlying cell-substrate coating and results can be compromised by the release of factors from cells damaged during the formation of the wound by the pin tool (Kam et al., 2008, Staton et al., 2009, Vogt, 2010). Scratching can also be impractical with limited cell or compound resources as previously mentioned.
In order to get precise estimates of cell migration one might wish to reduce adversely contributing factors like cell proliferation, eicosanoid production via cytoplasmic phospholipases, substrate matrix effects, and protein adsorption. Proliferation is commonly negated by starvation in serum free media or by addition of Mitomycin C or similar compounds which arrest cell mitosis irreversibly by DNA crosslinking (SZYBALSKI and IYER, 1964, Tomasz, 1995). As many proteins adsorb to plastic surfaces to various extents (Andrade and Hlady, 1986), excluding or accounting for the effect of coating the polystyrene with the protein or peptide of interest should be done. Substrate optimization is important for physiological relevance and cell type specificity and should be considered.
It might also be advantageous to minimize phospholipase activation, as this could propagate an inflammatory response that through release of intracellular signal molecules may have impact on cell proliferation and migration (Dennis et al., 1991, Palombella and Vilcek, 1989). Eicosanoid production and release of secretory vesicles is minimized by decreasing cell damage and monolayer disruption which keeps phospholipases deactivated (Arun et al., 2013). Accordingly, as inflammation and wound healing are closely intertwined responses, isolation of the effect of a certain compound on either action is important to disclose the relevant bioactive mechanism in a satisfactory manner (Shaw and Martin, 2009).
The present work was initiated in order to establish a cheap, unbiased and accurate method of quantifying non-malignant human enterocyte migration on biologically relevant extracellular matrix (ECM) as compared to traditional scratch assays. For comparison, scratch assays were performed with human epithelial colorectal adenocarcinoma cells (Caco-2) and non-malignant human fetal small intestine cells (FHs-74 int). The presented assay is based on FHs-74 int and optimized for observing modulatory effects of bioactive proteins on gastrointestinal cell migration rate. We show that treatment with 5 ng/ml epidermal growth factor (EGF) induces migration more than two-fold and show how osteopontin (OPN) induces migration to a similar extent at 5 µg/ml.
2. Materials and methods
2.1. Reagents, cells and software
Fetal small intestine cells (FHs-74 int – ATCC# CCL-241) and tumorigenic colon epithelia cells (Caco-2-DSMZ# ACC 169) were obtained from LGC Standards AB (Boras, Sweden) and Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and maintained as per manufacturer's instructions. Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin, recombinant human EGF, SYTO-24® DNA stain (cat# S7559) and CellMask Orange (cat# C10045) were from Life technologies, Denmark. Recombinant human insulin, gelatine from swine skin (cat# G2500), bovine plasma fibronectin (cat# F1141) and propidium iodide (cat# 81845) were from Sigma-Aldrich, Denmark. Basal membrane extract (BME) was obtained from R&D systems (growth factor reduced Cultrex™, R&D systems, Denmark). The Oris migration system was used as donor for our optimized migration assay (Platypus technologies, WI, USA). Clear bottom, 96-well plates were from Nunc, Denmark (cat# 165305). Human OPN was purified from human urine as previously described (Christensen et al., 2008). All chemicals and solutions were analytical grade and endotoxin free when applicable. Images were acquired using Olympus Cell^B version 3.3 (build 2108) software. Image processing and nuclei counting was done in ImageJ v1.43u using maximum resolution (4140×3096 pixel) TIFF files. Microsoft Excel 2010 and Graphpad Prism v5 was used for data handling and statistical analysis.
2.2. Cell culture
FHs-74 int cells were cultured using DMEM+10% v/v FBS+1% v/v penicillin/streptomycin+10 ng/ml recombinant human insulin at 37 °C 5% CO2 and sub-cultured three times a week at 1:20. The FHs-74 int cells were monitored for morphological changes and experiments carried out between passage three and 15. Caco-2 cells were cultured using DMEM+10% v/v FBS+1% v/v penicillin/streptomycin at 37 °C 5% CO2 and sub-cultured three times a week at 1:10. Experiments were carried out between passage three and 25.
The migration rate was quantified using a modified assay by Platypus technologies. The silicone inserts were rinsed in 70% ethanol, evaporated dry, washed in growth media and mounted in a clear bottom, 96-well plate coated with 50 µg/ml BME protein as per the “thin gel” manufacturer specification. The outermost rows and columns were avoided due to rim effects. 100,000 cells were seeded per well around the inserts to reach rapid confluence. After a 24 h incubation period, the inserts were removed and cell debris gently removed by washing once with pre-warmed, serum- and phenol-red-free DMEM. EGF and OPN were dissolved in DMEM+1% FBS v/v+1% v/v penicillin/streptomycin and gently added by dispensing down the well wall. The FBS concentration in the starvation media was found by titrating FBS concentration versus cellular morphology changes and proliferation/apoptosis in the assay (Supplemental Fig. 1A and B). Upon another 24 h incubation the plate was washed in DMEM without phenol red and stained with 2 µM SYTO-24® DNA stain and 3 µM propidium iodide for 30 min at 37 °C. Experiments focusing on morphology were done without propidium iodide, but with 5 µg/ml CellMask orange added at the last 5 min of the incubation as recommended by the manufacturer. Following incubation the wells were washed once in pre-warmed, serum- and phenol-red free DMEM before adding 100 µl pre-warmed, serum and phenol-red free DMEM+25 mM HEPES to stabilize the monolayer while acquiring images.
2.3. Fluorescence microscopy
Light intensity was reduced as low as possible to avoid photocytotoxicity, fluorophore bleaching and burning of the cells. On our setup, 10% light at 4× magnification and 500 ms exposure time was sufficient. Bright field and phase contrast white light images were acquired to study ECM and experimental parameter changes on cell morphology. These were supplemented with differential interference contrast images using the same microscope and light source with white light, differential interference contrast polarization filters and Nomarski-Wollaston prisms in place. Förster resonance energy transfer (FRET) between SYTO-24 and propidium iodide was utilized to efficiently separate healthy from dying cells and to quantify nuclei. The overall monolayer was visualized using CellMask Orange plasma membrane stain, however destabilization of the plasma membrane was observed at greater than 30 min acquisition time, hence CellMask images were acquired in a timeframe less than 30 min. Image acquisition was done on a Leica DMI 3000B coupled to an Olympus DP72 image sensor with a Leica H/PLAN, 4×/0.10 objective. 470/40:525/50 excitation and emission filters were used for SYTO-24® fluorescence image acquisition and 546/12:605/75 excitation and emission filter for CellMask® Orange acquisition. Healthy versus dying images were acquired utilizing SYTO-24 and propidium iodide FRET using the 488 nm laser line and a 470/40:500LP filter. Microscopes not equipped with RGB sensors should use appropriate emission filters for two channel acquisition.
2.4. Image analysis and migration rate quantification
Migration rate was quantified by counting emerged nuclei in the void area left by the silicone insert. A global scale is set from a reference micrometre/micro-ruler image at the relevant magnification (Analyze→Set scale). Nuclei of same or different colours (FRET) are isolated from background by use of threshold on black background (Image→Adjust→Threshold). Separation of adjoined nuclei is done after conversion to an 8 bit image (Image→Type→8 bit) by the watershed algorithm (process→binary→watershed). To exclude the cells outside the original 2 mm wound, a circular selection of 2 mm diameter is defined and placed on the wound edge (Edit→Selection→Specify). A particle count of the nuclei confined within the selection is performed using 30 µm2-infinity size restraints and exclusion of nuclei contacting the boundary edge (Analyze→Analyze particles, “Exclude on edges”). Masks are collected as well as results and summary files (“Display results”, “Summarize”).
Restraints can be altered to exclude polynuclear or lobed cells (circularity) or cell types based on nucleus size or cell size (size restraint). Restraining by cell size requires addition of a cell permeant cytoplasm stain or plasma membrane dye. Quantification of different cell types in mixed populations can be done by processing the complete dataset containing parameters for each particle counted. Utilizing different stains would enable the user to count different cells accurately by a number of parameters using cell size, cell morphology, nuclear morphology or plasma membrane proteins via specific antibodies etc.
Data processing was done in Microsoft Excel 2010 and Graphpad Prism v5 and statistical significance calculated using a two-tailed Welch's t-test.
3. Results
3.1. Scratch assay and optimization using human intestine cells
In order to select an appropriate migration assay, multiple scratch assay tests were performed using the tumorigenic pseudo-small intestine cell line Caco-2 and human fetal small FHs-74 int cells. A pipette tip was initially used to inflict the scratch, however the tightness of the Caco-2 monolayer increased monolayer lifting causing apoptosis/necrosis driven negative migration rates. Cell death at the wound edge was mirrored in FHs-74 int cells (Supplemental Fig. 1). To create reproducible, high quality wounds we experimented with miniature razorblades (Fig. 1A). Razorblades greatly enhance the quality of the wound edge, but sacrifices ECM in the process. Creating fixed wound distance proved a challenge due to physical restraints in the well and the best results were obtained by cutting and scraping the cells off completely. The effect of paracrine signalling from the adjacent wound edge is lost on this account hence experiments performed in this manner should be cautiously compared to classical wounds. As the cell monolayer does not migrate evenly, large differences in wound distance were seen using Caco-2 cells on tissue culture treated polystyrene (Fig. 1A versus B). Employing scratch assays requires vigorous testing to obtain usable standard deviation and extensive consideration into minimizing user bias. Whole wound quantification like wound closure timing could be used to remove some of these intrinsic errors (Yuki et al., 2006), however, less adherent cells loosen and disperse in an uneven pattern making final wound closure determination difficult (Fig. 2).
Fig. 1.
Scratch wounds and lobed migration. Representative images of Caco-2 cells cut by a razor blade from more than four replicate 6-well plates. Creating wounds using razor blades create high quality wounds as seen in (A). Lobed migration patterns (arrow) were observed after 18 h incubation in starvation media as seen in (B).
Fig. 2.
Creating consistent wounds and maintaining ECM. Representative images of FHs-74 int monolayers on tissue culture treated polystyrene. Fixed distance voids were created using Ibidi silicone inserts (A). Separation and individual migration occurs with less adherent enterocytes as well as lobing (B). Inducing migration using 5 ng/ml EGF introduces aberrant spiky morphology and extensive monolayer disintegration is observed (C).
3.2. Improving biological relevance, monolayer homogeneity and cell morphology
To decrease variation in the obtained datasets, we employed Ibidi silicone inserts with fixed 500 µm wound width, however tightly adhering epithelial cells like Caco-2 would stick to the silicone or tear upon removal of the insert making this insert type less ideal. Despite maintaining the ECM coating the Ibidi inserts did not mitigate the issues of uneven migration along the wound, finding the same scratch location over time and similar issues shared with scratching. We also considered choosing a physiologically relevant intestinal epithelial line important. Caco-2 cells differentiate into small intestine-like cells over time, however as Caco-2 cells are a heterogeneous, tumorigenic colon cell line used as an approximation for small intestine cells and display different EGF receptor expression, transepithelial resistance and respond differently depending on passage, media composition and many other factors (reviewed in Sambuy et al. (2005)), they are not optimal as a model for the small intestine (Kuwada et al., 1999). Previous studies have also shown different responses between FHs-74 int and Caco-2 to digested peptides of Enprocal, a supplementary food for frail elderly consisting of 80% whey protein concentrate, warranting consideration of the physiologically relevant cell line to be used (Kanwar and Kanwar, 2009). To make the assay more physiologically relevant, we employed FHs-74 int cells which are a primary human fetal small intestine cell line previously used to study the effect of growth factors and conditioned media on proliferation (Fig. 2A) (Wagner et al., 1998, Hirai et al., 2002).
Utilizing the FHs-74 int cells with the Ibidi insert improved the scratch consistency dramatically and the wound had a fixed width and no surface or cell damage was observed. An equidistant void implies paracellular signalling is consistent, however lobing and disintegrated monolayers were still observed (Fig. 2B). Treating FHs-74 int cells with 5 ng/ml EGF resulted in exaggerated polarized morphology and substantial loss of monolayer integrity (Fig. 2C).
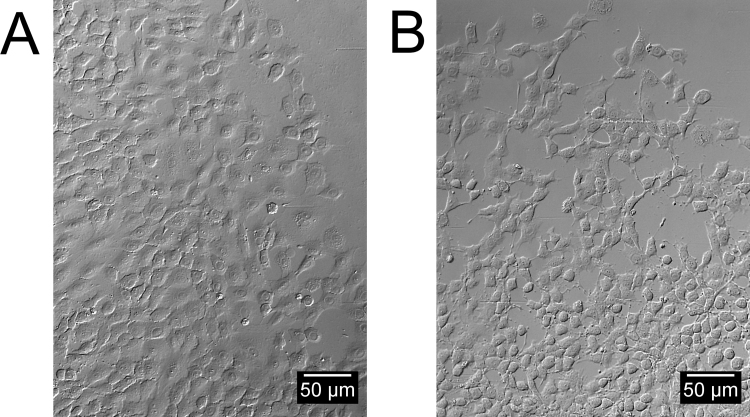
To solve the problems of reproducible wounds, intact ECM, higher throughput and unbiased quantification we optimized an existing assay by Platypus Technologies. Morphological concerns were addressed by growing and running EGF stimulated experiments on tissue culture treated polystyrene, fibronectin, gelatine and BME matrix using standard protocols (not shown). FHs-74 int cells should be grown on tissue culture treated polystyrene according to the manufacturer however we found that growing FHs-74 int cells on in vivo-like substrate induced dramatic morphological changes. The most homogeneous monolayers where obtained using BME with a softer, fried egg morphology in untreated as well as EGF treated monolayers (Fig. 3A). The wound edge appeared more uniform with a more coherent, collectively migrating monolayer edge. This was radically different to the polarized and jagged morphology seen with cells on tissue culture treated polystyrene where monolayers rapidly became irregular with cells migrating less coherent and uniform (Fig. 3B). On the basis of these observations, the FHs-74 int cells were grown on BME hence-forth.
Fig. 3.
Optimizing parameters for correct morphology and migration type. FHs-74 int cells stimulated with 5 ng/ml EGF migrating into void area on BME coated polystyrene (A) or tissue culture treated polystyrene (B). Cells migrating on BME display soft edges and a higher degree of monolayer integrity versus cells growing on tissue culture treated polystyrene.
3.3. Experimental setup and quantification
Seeding density, serum percentage in the starvation media, buffer type and experimental time frame were obtained using SYTO-24 and propidium iodide nuclear stains as described in materials and methods (Supplemental Fig. 1). These stains were chosen due to the high degree of FRET allowing easy quantification of proliferation and apoptosis rate using a 470/40:500LP filter. As the proliferation and apoptosis rates depend on the same parameters, we optimized seeding density, FBS content and buffer type to keep proliferation and apoptosis at a minimum. As seen in Fig. 4A, SYTO-24 and propidium iodide are highly efficient for live/dead staining due to a large overlap of emission:excitation spectra. Following optimization, less than 1.3% cells of the total monolayer stained for apoptosis (Fig. 4B and C, N=12,249 and N=158, respectively) and less than 0.8% in the wound (circular region of interest not shown). Mitotic nuclei were easily detectable and close to non-existent (not shown) hence compounding effects of proliferation were deemed negligible. The benefit of using silicone inserts is apparent as uniform, consistent wounds are produced as seen in Fig. 5A. Upon migration, images are obtained of the wounds and quantification done using the known wound diameter of 2 mm. Acquiring images of the complete wound area have inherent benefits of negating the irregular migration distance (Fig. 5B). After applying the selection region of interest (ROI), nuclei are counted and the value output including a mask for visual inspection (Fig. 5C). This process is consistent, relatively easy and involves very little user bias as compared to the traditional scratch assay. For comparison, masks are shown of control versus 5 ng/ml EGF treated cells (Fig. 5C and D).
Fig. 4.
Using FRET to minimize apoptosis and proliferation. The large degree of FRET between SYTO-24 and propidium iodide allows for efficient determination of live versus dead counts. As seen in (A), these DNA stains provide a binary indicator with only positive (green) or negative (red) nuclei. Dying cells are highlighted with arrows. Determining the degree of apoptosis is done by thresholding to the respective wavelengths and quantifying live (B) and dying (C) nuclei, in these frames live=12,249 and dead=158 respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
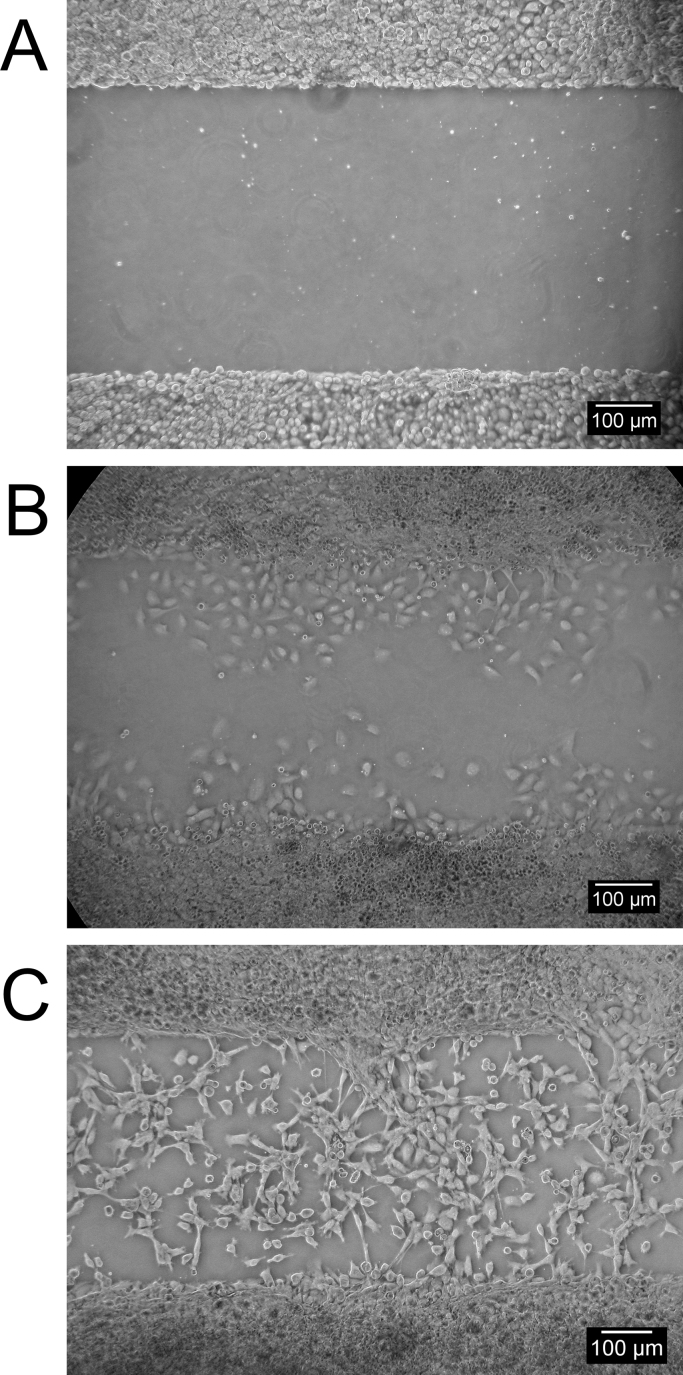
Cell exclusion zone assay overview. Upon confluence of the FHs-74 int cells, the silicone insert is removed exposing a well-defined void with no ECM damage (A). Cell nuclei are stained using SYTO-24 or a similar double strand DNA specific dye and images acquired. Using ImageJ, a 2 µm circular region of interest is drawn (B) and cells migrated within the insert border were counted using the particle counting algorithm to quantify nuclei in the area. The resulting mask and nuclei count after 24 h incubation in starvation medium is shown (C). For comparison, a mask of 24 h incubation in starvation medium with 5 ng/ml EGF is shown in (D). The resulting nuclei count is N=1567 and N=3393 for (C) and (D) respectively, i.e. a ~two fold increase in cells migrated into the wound void when induced by 5 ng/ml EGF.
To verify our setup, FHs-74 int cells were grown on tissue culture treated polystyrene or BME matrix and allowed to migrate for 24 h in starvation media or starvation media with 5 ng/ml EGF. CellMask Orange was used to visualize the monolayer. As evident from Fig. 6, both treated and untreated cells exhibit a marked polarized morphology on polystyrene (Fig. 6A and B) as compared to BME matrix (Fig. 6C and D). This is particularly evident when monolayer migration is induced using 5 ng/ml EGF and affects the monolayer on a global scale. Collective migration is prominent on BME matrix (Fig. 6D) whereas monolayer disintegration is seen on polystyrene (Fig. 6B). Having observed the behaviour of FHs-74 int cells on a BME matrix stimulated or non-stimulated with EGF, we feel confident to equate nuclei count with wound closure and migration rate.
Fig. 6.
Wound comparison of FHs-74 int cells on tissue culture treated polystyrene or BME substrate. Shown are representative CellMask Orange stained monolayers from more than four biological replicates. The monolayers were grown on polystyrene or BME matrix in DMEM+1% FBS v/v medium with or without 5 ng/ml EGF. Images A and C show a direct comparison of untreated FHs-74 int cell on polystyrene or BME matrix, respectively. With 5 ng/ml EGF added a dramatic morphology change is seen in cells on polystyrene (B) versus cells growing on BME matrix (D). Caco-2 cells were similarly grown on polystyrene and BME and exhibited less substrate dependency, but more extensive lobing (not shown).
3.4. Validation of the assay
OPN is a multifunctional phosphorylated protein containing an integrin binding Arg-Gly-Asp (RGD)-sequence, and OPN has been shown to modulate migration and invasion of many cell types (Sørensen and Petersen, 1993, Weber et al., 2002, Standal et al., 2004). The modulation of migration and invasion has previously been shown in both scratch and Boyden chamber assays (Furger et al., 2003, Li et al., 2015, Minai-Tehrani et al., 2013).
As the ability of OPN to promote migration of epithelial cells has been well documented it was used to validate the assay (Tuck et al., 2002). OPN was added to FHs-74 int monolayer voids, incubated, imaged and processed as described in materials and methods. As seen in Fig. 7, 5 µg/ml OPN induced migration of FHs-74 int cells in a potent fashion with comparable migration to 5 ng/ml EGF at p<0.001. This data was acquired from sextuplicates.
Fig. 7.
Osteopontin induces migration. OPN was purified from human urine as described (Christensen et al., 2008) and added to FHs-74 monolayers grown and seeded as per presented protocol. OPN was added as a gradient from 0.5 µg/ml to 25 µg/ml and 5 ng/ml EGF used as a positive control. Images of the Cell Exclusion Zone were acquired and nuclei migrated into the void quantified as described. OPN was shown to induce FHs-74 int cell migration potently with 5 µg/ml OPN exhibiting similar effects as 5 ng/ml EGF. * denotes P<0.05 and ** denotes P<0.001 when tested against controls. Standard deviation displayed.
4. Discussion
The scratch assay is a commonly used method to measure migration as it is low cost and easily applied. Inherent to the method are several drawbacks which can be mitigated by the method described in this paper. The inability to avoid ECM damage and irregular wound width due to tearing of the monolayer are the most dominant drawbacks. As wound healing is partly mediated by juxtacrine as well as paracrine signalling, fixed wound distance is crucial for repeatable results (Schultz et al., 1991, Werner and Grose, 2003). Quantification of migration rate in scratch assays presents another challenge. Obtaining low standard deviation of wound distance is inherently difficult due to extensive lobing experienced in many cell lines or due to monolayer disintegration as observed using FHs-74 int. While lobing is only an issue in relation to quantification, disintegration of the enterocyte monolayer makes the wound physiologically irrelevant as collective cell migration is imperative for intestinal barrier integrity during wound closure (Defranco et al., 2008, Friedl and Gilmour, 2009).
For quantification, plate readers have been proposed as read-out, but in our hands gave varying results due to differing stain quality, floating apoptotic cells and other factors. As quantification by plate reader yields total wound fluorescence, it is easily influenced by many variables affecting staining including apoptosis level, pipetting error and photobleaching. These variables make accurate quantification of migration inhibitors and inter-batch comparisons difficult.
In summation, quantifying cell migration consistently is a general problem in wound studies, relying heavily on user expertise and strict protocols. Cell monolayers furthermore tend to migrate in lobed patches or separate completely into distinct, single cell entities making wound distance measurements difficult. Inherent bias to the prevalent scratch assay makes comparison difficult between data obtained by different users and laboratories.
4.1. Conclusion
The current work presents an optimized alternative to the traditional scratch assay employing a non-tumorigenic small intestine cell line growing on a physiological relevant substrate. This method was successfully used to quantify the effects of two bioactive proteins on the modulation of small intestine cell migration rate. Moreover, the method provides several features including high throughput, high reproducibility, unbiased quantification and wide compatibility with existing equipment and fluorophores. Furthermore, the assay is compatible with user specific ECM coating and cell lines, which enhance the versatility and allows for intricate analysis of cell-cell as well as cell-substrate interactions.
Authors’ contributions
SN developed the method, conducted the experiments and wrote the manuscript. BC purified OPN and proofread the manuscript. JTR supervised and contributed in writing and proofreading of the manuscript.
Author disclosure statement
No competing financial interests exist.
Acknowledgements
The authors are grateful to Arla Foods (amba) (Grant no. 438307-Kra09), Aarhus University and The Danish Council for Independent Research – Natural Sciences (FNU) (Grant no. 645-08-0600) for financial support.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.meteno.2016.03.002.
Contributor Information
Steffen Nyegaard, Email: nyegaard@gmail.com.
Brian Christensen, Email: bc@mbg.au.dk.
Jan Trige Rasmussen, Email: jatr@mbg.au.dk.
Appendix A. Supplementary material
Supplementary material. Supplemental figure 1. Optimization of media and scratch comparison FHs-74 int cells were seeded and incubated for 24 h in a gradient of serum including 0%, 1%, 2.5% and 5%. The monolayers were stained with SYTO-24 and propidium iodide and imaged. Microscopic analysis showed substantial and undesirable proliferation at higher serum concentrations (not shown). At 1% the monolayer displayed negligible proliferation and cell death (A) compared to 0 % serum which exhibited substantial cell death (B). Small wounds were made in FHs-74 int monolayers using a pipette tip and stained at 8 h, 16 h and 24 h post scratching. At 8 h (C) SYTO24:PI FRET appeared at the wound edge with a progression towards red (D and E) over time indicating progressive apoptosis/necrosis. Pronounced cell death was seen at the wound edge at 24 h (E), a phenomenon not observed with silicone inserts.
References
- Andrade J.D., Hlady V. Biopolymers/Non-Exclusion HPLC, Advances in Polymer Science. Springer Berlin Heidelberg; Berlin, Heidelberg: 1986. Protein adsorption and materials biocompatibility: a tutorial review and suggested hypotheses; pp. 1–63. [Google Scholar]
- Arun S.N., Xie D., Howard A.C., Zhong Q., Zhong X., McNeil P.L., Bollag W.B. Cell wounding activates phospholipase D in primary mouse keratinocytes. J. Lipid Res. 2013;54:581–591. doi: 10.1194/jlr.M027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B., Petersen T.E., Sørensen E.S. Post-translational modification and proteolytic processing of urinary osteopontin. Biochem. J. 2008;411:53–61. doi: 10.1042/BJ20071021. [DOI] [PubMed] [Google Scholar]
- Defranco B.H., Nickel B.M., Baty C.J., Martinez J.S., Gay V.L., Sandulache V.C., Hackam D.J., Murray S.A. Migrating cells retain gap junction plaque structure and function. Cell Commun. Adhes. 2008;15:273–288. doi: 10.1080/15419060802198298. [DOI] [PubMed] [Google Scholar]
- Dennis E.A., Rhee S.G., Billah M.M., Hannun Y.A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. Publ. Fed. Am. Soc. Exp. Biol. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Friedl P., Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Furger K.A., Allan A.L., Wilson S.M., Hota C., Vantyghem S.A., Postenka C.O., Al-Katib W., Chambers A.F., Tuck A.B. β3 integrin expression increases breast carcinoma cell responsiveness to the malignancy-enhancing effects of osteopontin. Mol. Cancer Res. 2003;1:810–819. [PubMed] [Google Scholar]
- Hirai C., Ichiba H., Saito M., Shintaku H., Yamano T., Kusuda S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J. Pediatr. Gastroenterol. Nutr. 2002;34:524–528. doi: 10.1097/00005176-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Kam Y., Guess C., Estrada L., Weidow B., Quaranta V. A novel circular invasion assay mimics in vivo invasive behavior of cancer cell lines and distinguishes single-cell motility in vitro. BMC Cancer. 2008;8:198. doi: 10.1186/1471-2407-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar J.R., Kanwar R.K. Gut health immunomodulatory and anti-inflammatory functions of gut enzyme digested high protein micro-nutrient dietary supplement-Enprocal. BMC Immunol. 2009;10:7. doi: 10.1186/1471-2172-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S.K., Li X.F., Damstrup L., Dempsey P.J., Coffey R.J., Wiley H.S. The dynamic expression of the epidermal growth factor receptor and epidermal growth factor ligand family in a differentiating intestinal epithelial cell line. Growth Factors Chur Switz. 1999;17:139–153. doi: 10.3109/08977199909103522. [DOI] [PubMed] [Google Scholar]
- Li Y., Xie Y., Cui D., Ma Y., Sui L., Zhu C., Kong H., Kong Y. Osteopontin promotes invasion, migration and epithelial-mesenchymal transition of human endometrial carcinoma cell HEC-1A through AKT and ERK1/2 signaling. Cell. Physiol. Biochem. 2015;37:1503–1512. doi: 10.1159/000438518. [DOI] [PubMed] [Google Scholar]
- Liang C.-C., Park A.Y., Guan J.-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Marshall J. Transwell® invasion assays. In: Wells C.M., Parsons M., editors. Cell Migration. Humana Press; Totowa, NJ: 2011. pp. 97–110. [Google Scholar]
- McCormack S.A., Viar M.J., Johnson L.R. Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am. J. Physiol. 1993;264:G367–G374. doi: 10.1152/ajpgi.1993.264.2.G367. [DOI] [PubMed] [Google Scholar]
- Minai-Tehrani A., Chang S.-H., Park S.B., Cho M.-H. The O-glycosylation mutant osteopontin alters lung cancer cell growth and migration in vitro and in vivo. Int. J. Mol. Med. 2013;32:1137–1149. doi: 10.3892/ijmm.2013.1483. [DOI] [PubMed] [Google Scholar]
- Ng T., Parsons M., Hughes W.E., Monypenny J., Zicha D., Gautreau A., Arpin M., Gschmeissner S., Verveer P.J., Bastiaens P.I.H., Parker P.J. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombella V.J., Vilcek J. Mitogenic and cytotoxic actions of tumor necrosis factor in BALB/c 3T3 cells. Role of phospholipase activation. J. Biol. Chem. 1989;264:18128–18136. [PubMed] [Google Scholar]
- Sørensen E.S., Petersen T.E. Purification and characterization of three proteins isolated from the proteose peptone fraction of bovine milk. J. Dairy Res. 1993;60:189–197. doi: 10.1017/s0022029900027503. [DOI] [PubMed] [Google Scholar]
- Sambuy Y., Angelis I., Ranaldi G., Scarino M.L., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- Schultz G., Rotatori D.S., Clark W. EGF and TGF-alpha in wound healing and repair. J. Cell. Biochem. 1991;45:346–352. doi: 10.1002/jcb.240450407. [DOI] [PubMed] [Google Scholar]
- Shaw T.J., Martin P. Wound repair at a glance. J. Cell. Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standal T., Borset M., Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp. Oncol. 2004;26:179–184. [PubMed] [Google Scholar]
- Staton C.A., Reed M.W.R., Brown N.J. A critical analysis of current in vitro and in vivo angiogenesis assays. Int. J. Exp. Pathol. 2009;90:195–221. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic O., Brem H., Vouthounis C., Lee B., Fallon J., Stallcup M., Merchant A., Galiano R.D., Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of β-Catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 2005;167:59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski W., Iyer V.N. Crosslinking of dna by enzymatically or chemically activated mitomycins and porfiromycins, bifunctionally “alkylating” antibiotics. Fed. Proc. 1964;23:946–957. [PubMed] [Google Scholar]
- Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem. Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Tuck A.B., Hota C., Wilson S.M., Chambers A.F. Osteopontin-induced migration of human mammary epithelial cells involves activation of EGF receptor and multiple signal transduction pathways. Oncogene. 2002;22:1198–1205. doi: 10.1038/sj.onc.1206209. [DOI] [PubMed] [Google Scholar]
- Vogel S., Wottawa M., Farhat K., Zieseniss A., Schnelle M., Le-Huu S., von Ahlen M., Malz C., Camenisch G., Katschinski D.M. Prolyl hydroxylase domain (PHD) 2 affects cell migration and F-actin formation via RhoA/rho-associated kinase-dependent cofilin phosphorylation. J. Biol. Chem. 2010;285:33756–33763. doi: 10.1074/jbc.M110.132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt A. Advances in two-dimensional cell migration assay technologies. Eur. Pharm. Rev. 2010;5:26–29. [Google Scholar]
- Wagner C.L., Forsythe D.W., Wagner M.T. The effect of recombinant TGFalpha, human milk, and human milk macrophage media on gut epithelial proliferation is decreased in the presence of a neutralizing TGFalpha antibody. Biol. Neonate. 1998;74:363–371. doi: 10.1159/000014054. [DOI] [PubMed] [Google Scholar]
- Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G.F., Zawaideh S., Hikita S., Kumar V.A., Cantor H., Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 2002;72:752–761. [PubMed] [Google Scholar]
- Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Yarrow J.C., Perlman Z.E., Westwood N.J., Mitchison T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki T., Ishihara S., Rumi M.A.K., Ortega-Cava C.F., Kadowaki Y., Kazumori H., Ishimura N., Amano Y., Moriyama N., Kinoshita Y. Increased expression of midkine in the rat colon during healing of experimental colitis. Am. J Physiol. Gastrointest. Liver Physiol. 2006;291:G735–G743. doi: 10.1152/ajpgi.00388.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Supplemental figure 1. Optimization of media and scratch comparison FHs-74 int cells were seeded and incubated for 24 h in a gradient of serum including 0%, 1%, 2.5% and 5%. The monolayers were stained with SYTO-24 and propidium iodide and imaged. Microscopic analysis showed substantial and undesirable proliferation at higher serum concentrations (not shown). At 1% the monolayer displayed negligible proliferation and cell death (A) compared to 0 % serum which exhibited substantial cell death (B). Small wounds were made in FHs-74 int monolayers using a pipette tip and stained at 8 h, 16 h and 24 h post scratching. At 8 h (C) SYTO24:PI FRET appeared at the wound edge with a progression towards red (D and E) over time indicating progressive apoptosis/necrosis. Pronounced cell death was seen at the wound edge at 24 h (E), a phenomenon not observed with silicone inserts.