Abstract
There have no universally accepted criteria and have been established for classification of underactive bladder (UAB) at present. Thus, the study described the comprehensive clinical and urodynamic characteristics of UAB in patients with lower urinary tract symptoms.
A total of 1726 patients (1259 men and 467 women; 6–88 years old) who were admitted to our center with a diagnosis of UAB were included in this retrospective study. It was due to the type of rehabilitation hospital, so higher percentage of neurological patients were included. The demographics, clinical characteristics, and urodynamic recordings were reviewed. The clinical characteristics and urodynamic findings of UAB were further classified.
For the etiologic analysis, UAB with aging and without clear causes accounted for 11.5% of cases (199/1726), UAB with bladder outflow obstruction accounted for 2.6% (45/1726), and UAB acting on the nerve pathway of the voiding reflex accounted for 84.6% (1460/1726). There were a number of cases (1.3% [22/1726]) which had >2 factors assigned. For studies involving urodynamic findings and clinical symptoms, the percentage of patients with detrusor hyperreflexia with impaired contractility (DHIC), detrusor underactivity (DU), and acontractile detrusor (AcD) was 0.7%, 5.6%, and 93.7%, respectively.
UAB can be classified into 4 types based on possible etiologic mechanisms (idiopathic, myogenic, neurogenic, and integrative). Based on urodynamic findings and symptoms, UAB can be classified into 3 types (DU, AcD, and DHIC). The classification of UAB can provide a reasonable basis for the future research.
Keywords: detrusor underactivity, etiology, underactive bladder, urodynamics
1. Introduction
Detrusor underactivity (DU) is a form of common lower urinary tract dysfunction (LUTD) that occurs in approximately 20% to 25% of all patients with lower urinary tract symptoms (LUTS).[1] Although the International Continence Society (ICS) standardization report defines DU as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or failure to achieve complete bladder emptying within a normal time span,”[2] it is a urodynamic definition not based on symptoms. Underactive bladder (UAB) focuses on symptoms that patients find bothersome and raises the profile of the important clinical condition.[3] Chapple et al proposed that a definition of a symptom complex for UAB analogous to the ICS definition of overactive bladder (OAB) syndrome would be of potential clinical value: UAB is a symptom complex suggestive of DU and is usually characterized by a prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and a slow stream.[4]
Because the underlying pathophysiology of UAB is multifactorial, a single medication that focuses on a single mechanism could not be effective for others. Therefore, it is necessary to be clear on its classification based on possible etiologic mechanisms induced by various risk factors; however, one question is that no universally accepted criteria have been established for classification of UAB at present. Urodynamic studies of UAB can help us better understand the nature of UAB, another question is whether or not UAB presents invariable urodynamic modality is unknown. Recently a review described the possible etiology and made a initial classification of UAB.[5] Based on this, the purpose of this study was to analyze the etiology, urodynamic findings, and clinical symptoms in patients with UAB from our database as detailed as possible, make a reasonable classification of UAB, and clarify the aforementioned questions.
2. Methods
2.1. Patient selection
We retrospectively reviewed the medical records of 4538 consecutive patients who had LUTS and underwent video-urodynamic examinations between December 2004 and January 2016 at our institution. The study was approved by the Institutional Review Board at the China Rehabilitation Research Centre (No. 2015-K-066). All of the patients underwent detailed physical examinations, comprehensive history taking, and invasive video-urodynamic examinations. Clinical urodynamic practice was performed according to the ICS Good Urodynamic Practice standards.[6] All measurements were obtained using a Triton urodynamic analyzer (LABORIE, Mississauga, ON, Canada). A double-lumen transurethral catheter (7F, Cook, Bloomington, IN) was used for filling and to record vesical pressure. Abdominal pressure was measured with a balloon catheter (12F, Cook). The residual urine in the bladder was drained before cystometry. Saline with contrast medium at room temperature was infused at 10 to 30 mL/min. The type of detrusor dysfunction was evaluated during the urodynamic examination and X-ray images of the bladder were also obtained. A pressure-flow study (PFS) was performed if the patient could void. Each patient underwent the urodynamic test once.
2.2. Patient evaluation
UAB was diagnosed according to symptoms.[4] The inclusion criteria for this study were as follows: prolonged urination time with or without a sensation of incomplete bladder emptying, hesitancy, reduced sensation on filling, and a slow stream; decreased sensation of the need to urinate, impaired or absent bladder sensation, or high postvoid residual volume; and emptying bladder by catheterization or voiding with straining or increased abdominal pressure. The exclusion criteria were as follows: acute urinary tract infection; bladder tumor or stone before operations; spinal cord injury (SCI) in spinal shock period; urethral stricture; therapies for UAB in order to artificially increase bladder capacity including augmentation cystoplasty, recent (in 6 months) botulinum toxin injection, sacral neuromodulation, and so on; and pure bladder outflow obstruction (BOO).
In this study, the Schaefer nomogram was used to grade obstruction and detrusor contractility.[7] Detrusor contraction strength below W+ according to the Schaefer nomogram is regarded as weak detrusor contractility if the patient has urine flow during the PFS. If there is no urine flow during the PFS, DU is defined as detrusor pressure (Pdet) ≤ 40 cm H2O.[8,9] BOO combined with DU was diagnosed by PFS, urinary pressure profile, and cystoscopy. We reviewed and analyzed the urodynamic findings for the included patients according to the above-mentioned standards. We also reviewed the medical histories of the included patients in detail and recorded the possible etiologies leading to UAB. UAB was classified according to diverse etiologies with different pathogenic mechanisms. LUTD resulting in UAB was clarified and classified by urodynamic findings.
2.3. Statistical analysis
Data are expressed as percentage (%).
3. Results
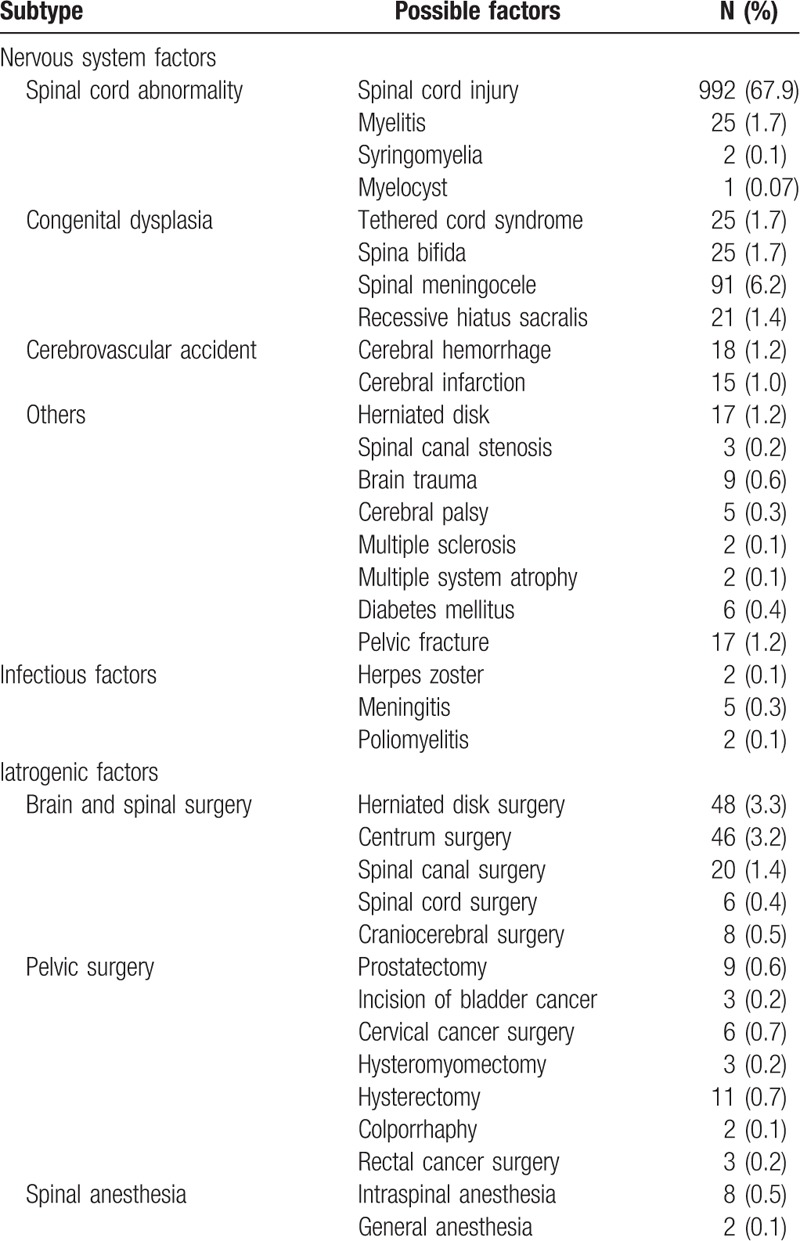
During the period from December 2004 to January 2016, there were 4538 consecutive patients with LUTS who sought evaluation at the urodynamic center in our department. A total of 1726 patients with UAB (1259 men and 467 women; 6–88 years old) were included into the study cohort. By analyzing the related etiology, many risk factors causing UAB were found (Table 1). In some patients, the causes of UAB were not been identified, thus UAB may be classified as idiopathic due to the lack of an apparent etiology. It accounted for 11.5% (199/1726) of the cases. Patients with BOO leading to UAB had myogenic UAB, which accounted for 2.6% (45/1726) of cases. Patients in whom risk factors leading to UAB acted on the neurologic etiology had neurogenic UAB, which accounted for 84.6% (1460/1726) of cases. This type of UAB was further divided into 3 subtypes, as follows: nervous system factors (87.4% [1276/1460]); infectious factors involving the nervous system (0.5% [7/1460]); and iatrogenic factors (12.1% [177/1460]) (Table 2). The nervous system factors contributed to the majority of UAB cases. UAB patients with >2 factors were referred to as integrative, which occurred 1.3% (22/1726) of cases.
Table 1.
Etiological classification of underactive bladder in 1726 patients.
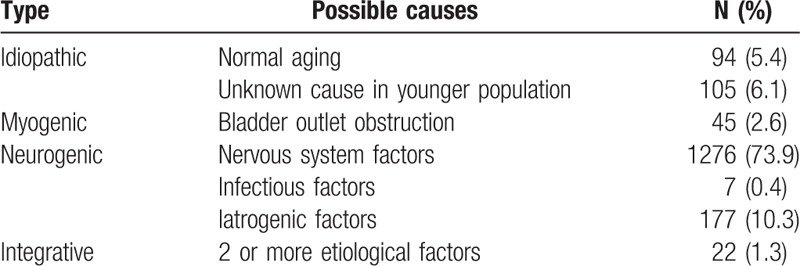
Table 2.
Factors leading to neurogenic underactive bladder in 1460 patients.
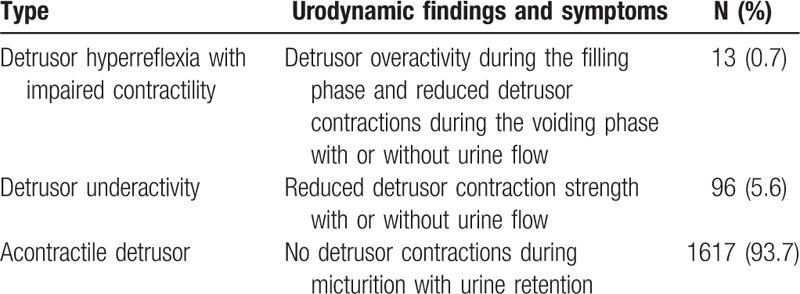
The urodynamic findings included reduced detrusor contractility (with or without detrusor overactivity [DO] during the filling phase) or absent detrusor contraction. The patients might have urine flow or complete urinary retention (Table 3). Among 1726 UAB patients, the percentage of detrusor hyperreflexia with impaired contractility (DHIC), DU, and acontractile detrusor (AcD) was 0.7%, 5.6%, and 93.7%, respectively. Among the 3 groups of patients, the symptom presentations were different, as follows: DU with typical symptoms of the above-mentioned UAB; DHIC with UAB and OAB symptoms; and AcD with urinary retention and emptying bladder by catheterization or voiding with straining or increased abdominal pressure.
Table 3.
Urodynamic classification of underactive bladder in 1726 patients.
4. Discussion
Few studies have focused on the etiologic classification of UAB. Osman et al proposed a classification based on etiologic factors and classified UAB into 4 types (idiopathic, neurogenic, myogenic, and iatrogenic).[10] But iatrogenic factors often resulted in injury to the peripheral pelvic plexus and it should be classified as neurogenic. Meanwhile, some patients could have 2 or more etiologies. This might be labeled as integrative. Tyagi et al[11] classified UAB into 3 types based on the mechanisms underlying UAB (neurogenic, myogenic, and integrative). But they neglected many aged patients with the symptoms of UAB who had no inapparent causes. This might be labeled as idiopathic. We proposed a similar but distinct classification based on the possible causes (idiopathic, myogenic, neurogenic, and integrative) (Table 1). van Koeveringe et al[12] defined idiopathic UAB as an age-related decrease in detrusor contractility or other inapparent causes, but BOO with age was not excluded. Kuo[13] defined idiopathic UAB as no evident neuropathy, no functional or anatomic bladder outlet obstruction, low or no Pdet combined with a maximum flow rate, and a large postvoid residual volume or urinary retention. We thought those individuals in whom the main cause was an age-related decrease in detrusor contractility (excluding BOO) or other inapparent causes and with symptoms of UAB might be labeled as idiopathic. Cucchi et al[14] thought that UAB was most often idiopathic, although it might be caused by other causes. However, we found that only 11.5% (199/1726) of patients were idiopathic in the current study. The characteristics of the rehabilitation hospital from which the data were derived may have contributed to the findings.
Long-term BOO could not only cause the structural changes of detrusor muscle cells, but also a reduction in detrusor blood flow.[15] Chronic overexpansion causes injury to postsynaptic parasympathetic ganglia in bladder wall, and induces muscle fiber damage, which leads to decompensation of detrusor function and progresses to DU. Lee et al[16] reported that 37% to 47% of patients with benign prostate hyperplasia had impaired detrusor contractility. In the current study, we labeled UAB subjective to BOO as myogenic. Myogenic UAB may result from altered excitation–contraction coupling mechanisms of detrusor muscle cells and result in reduced autonomous activity of the bladder. [12]
Neurogenic UAB may result from direct changes in the efferent limb of the micturition reflex, the afferent signals initiating the reflex, and the integrative control.[12] In the current study, we summarized the possible factors leading to neurogenic UAB (Table 2), which included nervous system factors, infectious factors involving the nervous system, and iatrogenic factors. Neurogenic UAB represented the largest proportion of this study cohort (84.6% [1460/1726]); a number of etiologic factors could contribute to neurogenic UAB. Neurogenic causes must be included in the definition of UAB because there are many patients with neurogenic bladder and UAB symptoms in clinical practice. SCI was an important factor which accounted for 67.9% (992/1460) of the Neurogenic UAB. The injury of thoracic segments took a more proportion (48.9%, 485/992) of the SCI. Complete injury accounted for a more proportion (47.8%, 473/992) the SCI. Most patients showed AcD and had urinary retention. The emptying bladder was mainly performed by catheterization in order to avoid the damage of the upper urinary tract. Although other neurologic problems, such as Parkinson disease, AIDS, neurosyphilis, and Guillain–Barre syndrome, were not listed by the study, other neurologic problems could also be contributing factors to UAB.[17]
Although there was a relatively small percentage (1.3% [22/1726]), we found that some patients who had a diagnosis of UAB could have 2 or more etiologies. Because it was unknown which etiology led to UAB and whether or not UAB resulted from a combined effect, this type could be considered integrative UAB. Indeed, integrative UAB could be the combination of myogenic and neurogenic factors or a combination of 2 or more neurogenic factors.
Because the etiologies leading to UAB are not understood completely at present, we also propose a classification based on urodynamic findings and symptoms and classify UAB into 3 types (DU, AcD, and DHIC) (Table 3). DU means that detrusor contraction function is impaired. Patients with DU are able to void (emptying completely or incompletely) or no urine flow (retention) during the urodynamic examination. AcD indicates that there are no detrusor contractions during urodynamic studies. The logical assumption is that AcD is the extreme of DU during progression.[10] Patients with AcD have urinary retention, and the emptying bladder is performed by straining, increased abdominal pressure, or catheterization. Most patients (93.7%) in this study had characteristics of AcD. The possible reasons may be the following: our center is affiliated with a rehabilitation hospital and most of the patients have different degrees of nervous system lesions, thus the neurogenic bladder population is considerable; and our center is a well-known center in neuro-urology, and most of the patients have serious conditions and visit us in an advanced stage. DHIC is defined as DO during the filling phase and underactive detrusor contractions during the voiding phase, thus a weak detrusor.[18] DHIC presents as a combination of DU and DO (UAB and OAB symptoms). We found that 0.7% (13/1726) of patients with UAB demonstrated urodynamic manifestations of DHIC. It is important to identify DHIC with urodynamic testing because treating patients with anticholinergic drugs may worsen bladder emptying leading to urine retention and/or urinary tract infection.[19]
There have been no studies on the relevant issues with large patient numbers. We propose classification based on etiology, urodynamic findings, and symptoms. This study had some limitations. The study was a single center, retrospective data analysis. The data were derived from the rehabilitation hospital and had bias. Factors, including patient position, unfamiliar environment, and intraurethral catheter, can influence the urodynamic results and may result in more AcD. A multicenter, community-dwelling population, prospective study should be performed to validate our classification of UAB.
5. Conclusion
UAB has raised considerable controversy in many areas at present. Due to its complexity of clinical categorization, we face a new challenge in the field of functional urology. Based on the large sample data, we classified UAB into 4 types based on etiology, and into 3 types based on symptoms and urodynamic findings in this retrospective study. The classification can provide a reasonable basis for future research.
Footnotes
Abbreviations: AcD = acontractile detrusor, BOO = bladder outflow obstruction, DO = detrusor overactivity, DU = detrusor underactivity, DHIC = detrusor hyperreflexia with impaired contractility, ICS = International Continence Society, LUTD = lower urinary tract dysfunction, LUTS = lower urinary tract symptoms, OAB = overactive bladder, PFS = pressure-flow study, Pdet = detrusor pressure, SCI = spinal cord injury, UAB = underactive bladder.
This study is supported by the National Natural Science Foundation of China (81570688).
The authors have no conflicts of interest to disclose.
References
- [1].Seki N, Kai N, Seguchi H, et al. Predictive regarding outcome after transurethral resection for prostatic adenoma associated with detrusor underactivity. Urology 2006;67:306–10. [DOI] [PubMed] [Google Scholar]
- [2].Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116–26. [DOI] [PubMed] [Google Scholar]
- [3].Chancellor MB, Kaufman J. Case for pharmacotherapy development for underactive bladder. Urology 2008;72:966–7. [DOI] [PubMed] [Google Scholar]
- [4].Chapple CR, Osman NI, Birder L, et al. The underactive bladder: a new clinical concept? Eur Urol 2015;68:351–3. [DOI] [PubMed] [Google Scholar]
- [5].Li X, Liao L. Updates of underactive bladder: a review of the recent literature. Int Urol Nephrol 2016;48:919–30. [DOI] [PubMed] [Google Scholar]
- [6].Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 2002;21:261–74. [DOI] [PubMed] [Google Scholar]
- [7].Schaefer W. Basic principles and clinical application of advanced analysis of bladder voiding function. Urol Clin N Am 1990;17:553–66. [PubMed] [Google Scholar]
- [8].Jeong SJ, Kim HJ, Lee YJ, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: a comparison between men and women. Korean J Urol 2012;53:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int 2004;93:745–50. [DOI] [PubMed] [Google Scholar]
- [10].Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol 2014;65:389–98. [DOI] [PubMed] [Google Scholar]
- [11].Tyagi P, Smith PP, Kuchel GA, et al. Pathophysiology and animal modeling of underactive bladder. Int Urol Nephrol 2014;46(suppl 1):S11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Koeveringe GA, Vahabi B, Andersson KE, et al. Detrusor underactivity: a plea for new approaches to a common bladder dysfunction. Neurourol Urodyn 2011;30:723–8. [DOI] [PubMed] [Google Scholar]
- [13].Kuo HC. Recovery of detrusor function after urethral botulinum A toxin injection in patients with idiopathic low detrusor contractility and voiding dysfunction. Urology 2007;69:57–61. [DOI] [PubMed] [Google Scholar]
- [14].Cucchi A, Quaglini S, Rovereto B. Development of idiopathic detrusor underactivity in women: from isolated decrease in contraction velocity to obvious impairment of voiding function. Urology 2008;71:844–8. [DOI] [PubMed] [Google Scholar]
- [15].Belenky A, Abarbanel Y, Cohen M, et al. Detrusor resistive index evaluated by Doppler ultrasonography as a potential indicator of bladder outlet obstruction. Urology 2003;62:647–50. [DOI] [PubMed] [Google Scholar]
- [16].Lee JG, Shim KS, Koh SK. Incidence of detrusor underactivity in men with prostatism older than 50 years. Korean J Urol 1999;40:347–52. [Google Scholar]
- [17].Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol 2013;15:11–22. [PMC free article] [PubMed] [Google Scholar]
- [18].Stav K, Shilo Y, Zisman A, et al. Comparison of lower urinary tract symptoms between women with detrusor overactivity and impaired contractility, and detrusor overactivity and preserved contractility. J Urol 2013;189:2175–8. [DOI] [PubMed] [Google Scholar]
- [19].Kitta T, Mitsui T, Kanno Y, et al. Postoperative detrusor contractility temporarily decreases in patients undergoing pelvic organ prolapse surgery. Int J Urol 2015;22:201–5. [DOI] [PubMed] [Google Scholar]


