Abstract
Background:
Proton pump inhibitors (PPIs) are usually prescribed to protect against gastrointestinal bleeding in patients on dual antiplatelet therapy. This meta-analysis reviewed clinical outcomes in patients taking aspirin and clopidogrel, with and without concomitant PPIs to address concerns of adverse reactions.
Methods:
We searched PubMed, Embase, and the Cochrane Library for articles published between January 1, 2010 and April 11, 2017. The primary end points were major adverse cardiovascular events and gastrointestinal bleeding. Secondary end points were myocardial infarction, stent thrombosis, revascularization, cardiogenic death, and all-cause mortality.
Results:
The meta-analysis included 33,492 patients in 4 randomized controlled trials and 8 controlled observational studies. Overall, patients taking PPIs had statistical differences in major adverse cardiovascular events [odds ratio (OR) 1.17 (95% confidence interval [CI] 1.07–1.28); P = .001; I2 = 28.3%], gastrointestinal bleeding [OR 0.58 (95% CI 0.36–0.92); P = .022; I2 = 80.6%], stent thrombosis [OR 1.30 (95% CI 1.01–1.68); P = .041; I2 = 0%], and revascularization [OR 1.20 (95% CI 1.04–1.38); P = .011; I2 = 5.1%], compared those not taking PPIs. There were no significant differences in myocardial infarction [OR 1.03 (95% CI 0.87–1.22); P = .742; I2 = 0%], cardiogenic death [OR 1.09 (95% CI 0.83–1.43); P = .526; I2 = 0%], or all-cause mortality [OR 1.08 (95% CI 0.93–1.25); P = .329; I2 = 0%).
Conclusions:
Among the patients taking aspirin and clopidogrel, the results indicated that the combined use of PPIs increased the rates of major adverse cardiovascular events, stent thrombosis, and revascularization.
Keywords: aspirin, clopidogrel, coronary heart disease, coronary stenting, percutaneous coronary intervention, proton pump inhibitors
1. Introduction
Dual antiplatelet therapy (DAPT), especially combination therapy with aspirin and clopidogrel, is recommended for the treatment of acute coronary syndrome (ACS) and patients with percutaneous coronary intervention (PCI).[1,2]Antiplatelet drugs significantly reduce the risk of cardiovascular events, but also have side effects involving the gastrointestinal (GI) mucous membrane such as GI bleeding, peptic ulcer, perforated peptic ulcer, and digestive tract obstruction.[3,4] Proton pump inhibitors (PPIs) are usually prescribed for patients on DAPT to attenuate the side effects,[5–7] but there are concerns of adverse interactions of antiplatelet drugs and PPIs.[8,9] Current guidelines do not recommend the routine use of PPIs by patients with a low risk of GI bleeding[1] and advise that combination therapy with DAPT and PPIs has the potential of adverse drug interactions.[7–9]
Aspirin absorption is dependent on gastric pH, and the secretion of gastric acid may be decreased by PPIs. Consequently, long-term use of PPIs might reduce the antiplatelet activity of aspirin.[10,11] Clopidogrel is a prodrug that needs undergo metabolic transformation to generate its active metabolite. The transformation depends on the hepatic cytochrome P-450 (CYP) enzyme system, which is also responsible for the metabolism of PPIs. The competitive inhibition and adverse interactions of clopidogrel and PPIs have been confirmed by pharmacokinetics and platelet aggregation studies.[12–15] Few meta-analyses of the impact of PPIs on aspirin or clopidogrel monotherapy have been published,[16–19] but GI adverse events are more common in people on DAPT, and PPIs are more often prescribed for people on DAPT than for those on aspirin or clopidogrel monotherapy. Reports of the clinical outcomes of patients undergoing combination therapy with DAPT and PPIs have been published, but the results are either inconsistent,[5–9] or the eligibility was not restricted to patients with coronary heart disease. This meta-analysis evaluated the clinical outcomes of patients on combination therapy with aspirin and clopidogrel with or without PPIs.
2. Methods
This meta-analysis was performed following the PRISMA checklist. Ethical approval and patient consent were not required because our study was retrieved from previous published studies.
2.1. Search strategy and selection criteria
We searched PubMed, Embase, and the Cochrane Library for articles published between January 1, 2010 and April 11, 2017 using the terms “proton pump inhibitors,” “aspirin,” “clopidogrel,” “acute coronary syndrome,” “percutaneous coronary intervention,” and “coronary stenting.” Eligible studies were those that included ACS, PCI, or coronary stenting patients given combination therapy with aspirin and clopidogrel, compared a PPI with a placebo group, reported a primary end point of major adverse cardiovascular events (MACE), and had at least 3 months of follow-up. Nonclinical studies (e.g., editorials or letters to the editor), reports of case series, different reports of the same trial, reports of incomplete, interim, or duplicate data, or trials with patient samples of 100 or fewer were excluded.
2.2. Data extraction and quality assessment
Data extraction was performed independently by 2 investigators (W.H. and J.T.) following the predefined inclusion and exclusion criteria. Differences in opinion were resolved by discussion, with a final decision by a third investigator if necessary. The baseline patient characteristics included first author, year of publication, study population, sex, proportion of patients with diabetes mellitus, hypertension and ACS, the mean follow-up period, type of PPIs, and study sample.
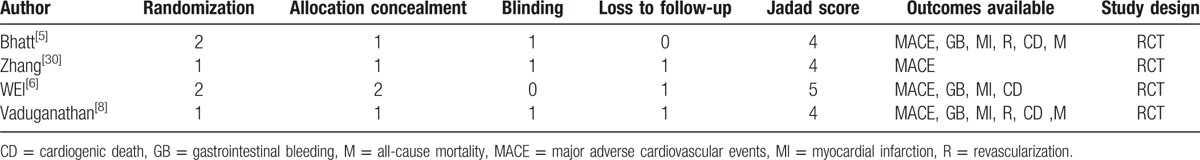
The quality of each controlled observational study was assessed using the Newcastle–Ottawa Scale, which includes patient selection, comparability of groups, and evaluation of outcomes. A maximum of 4 stars were possible for patient selection, 2 for group comparability, and 3 for evaluation of outcomes.[20] The star scores of the controlled observational studies are shown in Table 1. High-quality studies had 6 or more stars.[21] The quality of each randomized controlled trial (RCT) was assessed by the modified Jadad scale, which includes randomization, allocation concealment, blinding, and loss to follow-up.[22] High-quality studies had 4 or more scores.[23] The scores of the RCTs are shown in Table 2.
Table 1.
The quality outcomes of assessed cohort studies based on the Newcastle-Ottawa Scale.

Table 2.
The quality outcomes of assessed randomized controlled trials based on the modified Jadad scale.
2.3. End points
The primary end points were MACE (defined as ACS, stent thrombosis, revascularization, stroke, and all-cause mortality) and GI bleeding (overt and occult). Secondary end points were myocardial infarction (MI), stent thrombosis, revascularization, cardiogenic death, and all-cause mortality. Stent thrombosis included both definite and probable. If revascularization was not reported, then target vessel revascularization was used as the most preferred alternative; target lesion revascularization was the least preferred. If different reports of the same trial were retrieved, then the publication with the most complete data was abstracted.
2.4. Statistical analysis
The results were analyzed with Stata 12.0 and fixed-effects (Mantel–Haenszel) or random-effects (M-H heterogeneity) models. We computed the pooled odds ratios (ORs) for dichotomous end points with 95% confidence intervals (CIs). The level of statistical significance was α = 0.05. A fixed-effects model was used to analyze studies with an acceptable heterogeneity of P > .1 or I2 < 50%. If substantial heterogeneity (P < .1 or I2 > 50%) was indicated, then a random-effects model was used, and the possible sources of heterogeneity were analyzed by a statistician.[24] Publication bias was estimated using a funnel plot by checking whether the trials were symmetrically distributed.
3. Results
3.1. Search results
A total of 295 articles were initially retrieved from PubMed, 501 from Embase, and 107 from the Cochrane Library; 576 duplicates were excluded, leaving 327 potentially relevant articles. An additional 302 articles were excluded after reading the titles and abstracts. Of the 25 remaining articles, 12 studies including 33,492 patients were selected for analysis. A flowchart of the trial selection process is shown in Figure 1.
Figure 1.
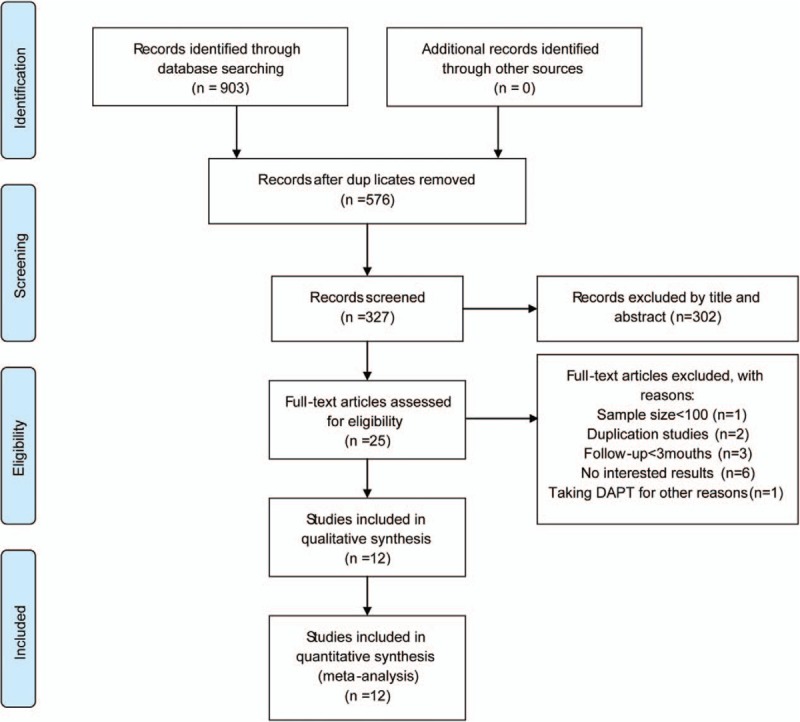
A flowchart of the trial selection. DAPT = dual antiplatelet therapy.
3.2. Baseline characteristics
The baseline characteristics of the patients in the 4 RCTs[5,6,8,30] and 8 controlled observational studies[7,9,13,25–29] are shown in Table 3. The study sample sizes ranged from 104 to 8582 patients. PPIs were used by 16,845 of the 33,492 patients (50.29%); 16,647 patients did not use PPIs. The mean age of the patients enrolled in the 12 studies ranged from 58 to 69 years, the majority were men, and the majority had hypertension. The proportion of patients with diabetes mellitus ranged from 18% to 39%, but 1 trial[6] did not report the number of patients with diabetes. The proportion of patients with ACS varied widely; mean follow-up duration ranged from 3.5 to 24 months.
Table 3.
The baseline characteristics of the included studies.

3.3. Primary endpoints
All 12 articles[5–9,13,25–30] reported MACE as a primary endpoint. It occurred in 1580 of the 16,839 patients (9.38%) in the PPI group, and 1281 of 16,632 patients (7.70%) in the placebo group. As a forest plot showed acceptable heterogeneity (I2 = 28.3%; P = .167), a fixed-effects model was used to evaluate the occurrence of MACE. Compared with placebo, PPIs increased the occurrence of MACE [OR 1.17 (95% CI 1.07–1.28); P = .001; Fig. 2]. Because MACE is of key clinical significance and was reported in all the included trials, publication bias was estimated in the funnel plot (Fig. 3), which showed a symmetrical distribution of the trials indicating that publication bias was not likely to have influenced the analysis.
Figure 2.

Risk estimates of major adverse cardiovascular events (MACE). CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
Figure 3.

Funnel plot indicating major adverse cardiovascular events (MACE) among patients taking aspirin and clopidogrel with or without proton pump inhibitors (PPIs).
GI bleeding was reported in 8 articles,[5–8,13,25,28,29] and was reported in 315 of 9038 patients (3.49%) in the PPI group, and 608 of 14,898 patients (4.08%) in the placebo group. As significant heterogeneity was found for the articles reporting GI bleeding (I2 = 80.6%; P < .001), a random-effects model was used to evaluate its occurrence. The rate of GI bleeding was lower in the PPI group than in the placebo group [OR 0.58 (95% CI 0.36–0.92); P = .022; Fig. 4]. The possible sources of heterogeneity are discussed below.
Figure 4.
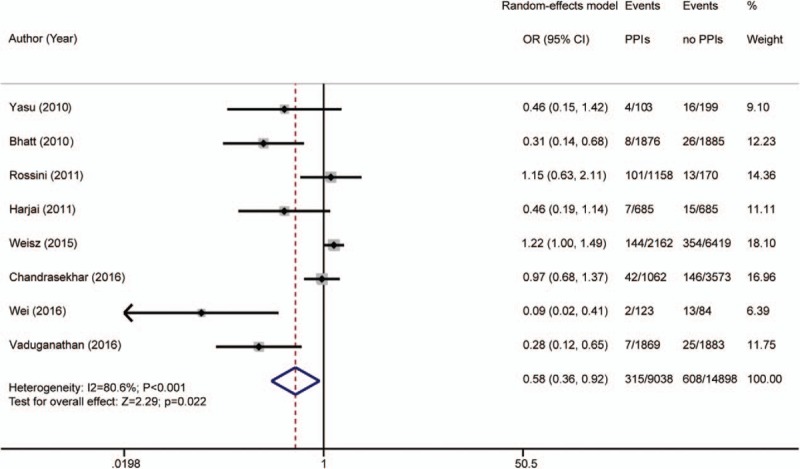
Risk estimates of gastrointestinal (GI) bleeding. CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
3.4. Secondary endpoints
The secondary endpoints were MI, stent thrombosis, revascularization, cardiogenic death, and all-cause mortality. Heterogeneity of the 8 articles[5–9,13,26,29] reporting MI was not detected (I2 = 0%; P = .804); a fixed-effects model was used for analysis. A total of 265 of 14,816 patients (1.79%) in the PPI group, and 391 of 15,684 patients (2.49%) in the placebo group experienced an MI. The rates of MI in the PPI and placebo groups were not significantly different [OR 1.03 (95% CI 0.87–1.22); P = .724; Fig. 5].
Figure 5.
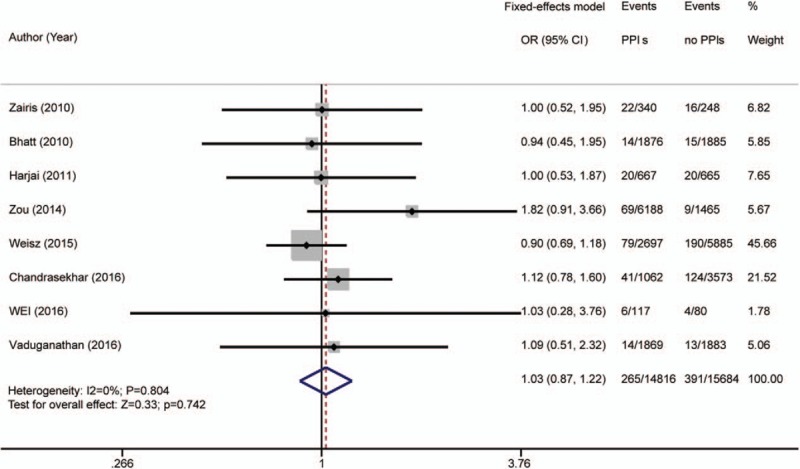
Risk estimates of myocardial infarction (MI). CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
Stent thrombosis was reported in 8 articles.[7,9,13,25–29] It occurred in 182 of 12,924 patients (1.41%) in the PPI group, and 145 of 12,733 patients (1.14%) in the placebo group. No heterogeneity was found (I2 = 0%; P = .561); a fixed-effects model was used. The occurrence of stent thrombosis was higher in the PPI group than in the placebo group [OR 1.30 (95% CI 1.01–1.68); P = .041; Fig. 6].
Figure 6.

Risk estimates of stent thrombosis. CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
Eight articles[5,7–9,13,25,26,29] reported revascularization. There were 662 events in the 14,820 PPI patients (4.47%) and 455 events in the 15,812 placebo patients (2.88%). Heterogeneity was acceptable (I2 = 5.1%; P = .391); a fixed-effects model was used. The occurrence of revascularization [OR 1.20 (95% CI 1.04–1.38); P = .011; Fig. 7] was higher in the PPI than the placebo group.
Figure 7.
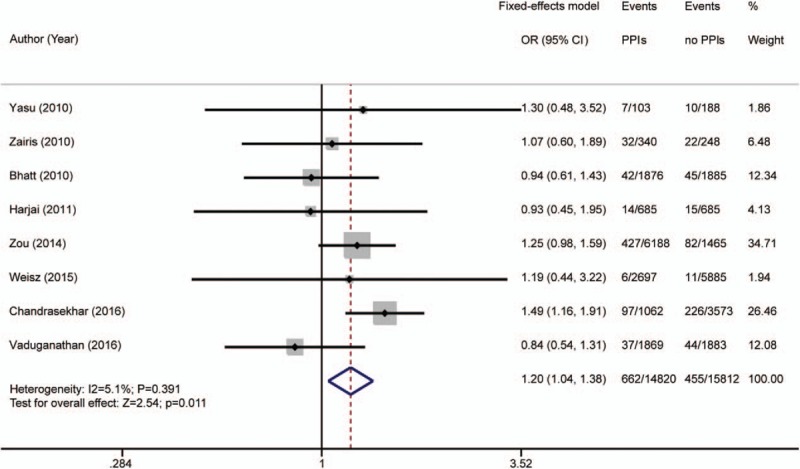
Risk estimates of revascularization. CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
Seven articles[5,6,8,13,25–27] reported cardiogenic death. It occurred in 91 of 7693 patients (1.18%) in the PPI group and 139 of 10,688 patients (1.30%) in the placebo group. Study heterogeneity was not detected (I2 = 0%; P = .823); a fixed-effects model was used. The occurrence of cardiogenic death in the PPI group was not significantly different from that in the placebo group [OR 1.09 (95% CI 0.83–1.43); P = .526; Fig. 8].
Figure 8.
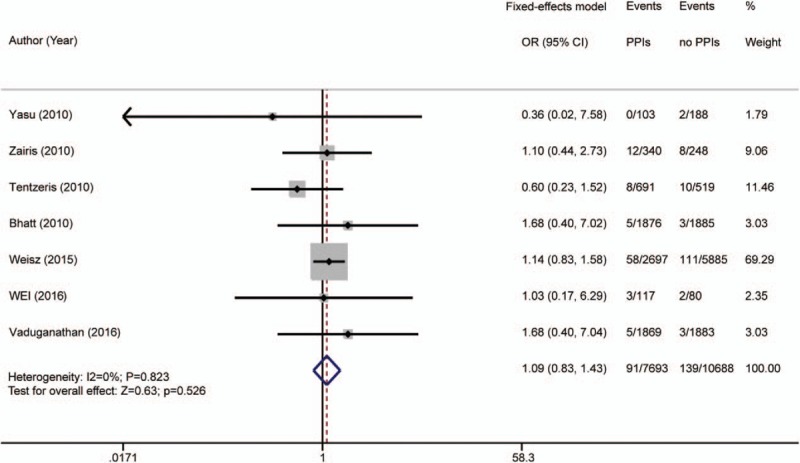
Risk estimates of cardiogenic death. CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
All-cause mortality was reported in 8 articles.[5,7–9,13,27–29] It occurred in 459 of 16,226 patients (2.83%) in the PPI group and 463 of 16,065 patients (2.88%) in the placebo group. Study heterogeneity was not detected (I2 = 0%; P = .433); a fixed-effects model was used. The difference in all-cause mortality in the PPI and placebo groups was not significant [OR 1.08 (95% CI 0.93–1.25); P = .329; Fig. 9).
Figure 9.

Risk estimates of all-cause mortality. CI = confidence interval, OR = odds ratio, PPI = proton pump inhibitor.
4. Discussion
Few meta-analyses have assessed the impact of PPIs on antiplatelet drugs, but they selected studies that included patients on aspirin or clopidogrel monotherapy or studies that did not restricted eligibility to patients with coronary heart disease.[16–19] Patients with a high risk of MACE are often given aspirin and clopidogrel combination therapy. The concomitant use of PPIs by those patients may multiply their occurrence of MACE compared with patients on aspirin or clopidogrel monotherapy and PPIs. However, meta-analyses of the outcomes of aspirin, clopidogrel, and PPIs combination therapy are lacking. This analysis found that the concomitant use of aspirin, clopidogrel, and PPIs decreased the rate of GI bleeding but increased the rates of MACE, stent thrombosis, and revascularization. There were no differences in the risks of MI, cardiogenic death, and all-cause mortality.
In some studies, PPIs reduced the occurrence of GI bleeding in patients on DAPT,[5,6,8] but other results were contradictory.[7,13,25,28,29] Many studies did not stratify the participants by risk of GI bleeding, with the use of PPIs was determined by clinicians. In many studies, the PPI users had a higher risk of GI events than nonusers. Even though the difference in GI bleeding in the PPI and placebo groups was not significant, that may have attributed to the protective effect of PPIs on the GI mucous membrane. This may have contributed to study heterogeneity.[7]
Previous studies found that when DAPT and PPIs were used in combination, the risks of MACE, stent thrombosis, and revascularization were increased.[7,9] PPIs inhibit gastric acid secretion, which increases the gastric pH and can alter the pharmacokinetics of aspirin. This might account for a decline in bioavailability and therapeutic effects of combination therapy with aspirin and PPIs.[10,11]
Another underlying mechanism involves interaction of metabolic pathways that requires CYP enzymes. Clopidogrel is a prodrug that undergoes CYP-dependent metabolic transformation to generate its active metabolite.[12–15] CYP2C19 is the predominant enzyme in this conversion, but CYP3A4/5, CYP2B6, and CYP1A2/1 may be involved. PPIs are also metabolized by the CYP enzyme system, but different PPIs require different isoenzymes. Omeprazole/esomeprazole is metabolized by CYP2C19 to 5-hydroxyomeprazole, which is converted to 5-hydroxyomeprazole sulfone by CYP3A4. Lansoprazole is metabolized by CYP2C19 and CYP3A4 to 5-hydroxy lansoprazole, lansoprazole sulfone, or lansoprazole sulfide. Pantoprazole is metabolized by CYP2C19 and CYP3A4 to hydroxy pantoprazole or pantoprazole sulfone. Rabeprazole is primarily metabolized nonenzymatically, and a portion is metabolized by CYP2C19.[31,32] The interaction of clopidogrel and PPIs thus involves competitive inhibition.[12–15] In patients carrying hypofunctional CYP2C19 alleles, the active metabolites of clopidogrel and platelet inhibition are significantly decreased,[33] and combination therapy might not effectively reduce the occurrence of MACE. Patients with genetic polymorphisms involving hypofunctional CYP2C19 alleles might benefit more from DAPT–PPI combination therapy with clopidogrel and rabeprazole rather than one of the other PPIs,[31] or from ticagrelor, a potent antiplatelet drug that is not affected by CYP2C19 polymorphisms.[34]
Aspirin directly damages the GI mucous membrane, and clopidogrel delays the healing of peptic ulcers. Antiplatelet drugs thus have side effects such as acute GI hemorrhage, and perforated peptic ulcer, and these stressful events may increase the occurrence of cardiovascular events. A 2008 ACCF/ACG/AHA expert consensus panel recommended routine use of PPIs for patients on DAPT.[35] Subsequent studies reported that patients on combination therapy with DAPT and PPIs increased the incidence of MACE, compared those not taking PPIs,[7,9] and led to revisions of the treatment recommendations. The 2016 American College of Cardiology and American Heart Association guidelines recommend PPIs for patients with a history of prior GI bleeding or the potential for GI bleeding. The routine use of PPIs is currently not suggested patients at low risk of GI bleeding,[1] and it is important to identify patients at risk of GI events. This includes those older than 65 years of age, a history of prior GI bleeding, or peptic ulcer, with Helicobacter pylori infection, concomitant use of anticoagulants, steroids, or nonsteroidal anti-inflammatory drugs.[35] Patients with a history of prior GI bleeding should be treated with caution. For prompt detection of bleeding, patients should check their stool color, and fecal occult blood testing and hemoglobin testing should be done at routine evaluation visits. The 13C-urea breath test can be used to screen for Helicobacter infection in high-risk patients, and those taking DAPT should be given anti-Helicobacter therapy if positive.[36] PPIs are often given to patients at high risk of GI events, but long-term use of PPIs is discouraged because inhibition of gastric acid secretion and loss of pepsin activity can lead to development of GI disorders.[37] It may be more reasonable to prescribe PPIs for patients at high risk of GI events in the first 3 months after ACS or PCI. PPIs can then be replaced by H2-receptor antagonists or gastric mucosa protective agents. The time of peak risk of DAPT-induced digestive tract bleeding could be used to guide the timing and duration of PPI use, but published recommendations are lacking. On the contrary, prevention should precede treatment. Carotid artery wall motion helps to diagnose atherosclerosis at a preclinical stage, and can be assessed by nonlinear state-space models constructed from ultrasound sequences[38] or elasticity-based state-space models.[39] The recovery of myocardial motor function could be used to evaluate the impact of PPIs on cardiovascular events.[40] Hemodynamics analysis of narrowed coronary arteries[41] and visualization based on 3D printed models[42] provide noninvasive assessments of coronary conditions that can aid in the medical decision-making process.
The limitations of this meta-analysis included the selection of non-RCTs, which are subject to selection bias, confounding bias, and baseline differences of the experimental and control groups. Moreover, PPIs differ in the CYP isoenzymes required for metabolism[31,32] and have different levels of impact on clopidogrel activity.[13–15] But subgroup analyses of PPI–DAPT were not possible because of limited patient data. Consequently, which of the available PPIs is safer when combined with aspirin and clopidogrel could not have been determined.
5. Conclusion
Combination therapy with aspirin, clopidogrel, and PPIs decreased GI bleeding and potentially increased MACE. The GI benefits should be weighed against the MACE risks when prescribing PPIs to patients taking aspirin and clopidogrel. The meta-analysis included nonrandomized controlled studies, which are subject to selection bias or baseline study group differences. The results should be interpreted with caution.
Acknowledgments
Grammar consulting and writing assistance were kindly provided by Ying Liu and Xinhui Mao. Statistical consultation was kindly provided by Yun Yang, PhD.
Footnotes
Abbreviations: ACS = acute coronary syndrome, CI = confidence interval, CYP = hepatic cytochrome P-450, DAPT = dual antiplatelet therapy, GI = gastrointestinal, MACE = major adverse cardiovascular events, MI = myocardial infarction, OR = odds ratio, PPIs = proton pump inhibitors, PCI = percutaneous coronary intervention, RCT = randomized controlled trial.
WH and JT are co-first authors on this work.
The authors report no conflicts of interest.
References
- [1].Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082–115. [DOI] [PubMed] [Google Scholar]
- [2].O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78–140. [DOI] [PubMed] [Google Scholar]
- [3].Han Y, Xu B, Xu K, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv 2016;9:e003145. [DOI] [PubMed] [Google Scholar]
- [4].Costa F, Vranckx P, Leonardi S, et al. Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. Eur Heart J 2015;36:1242–51. [DOI] [PubMed] [Google Scholar]
- [5].Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. [DOI] [PubMed] [Google Scholar]
- [6].Wei P, Zhang YG, Ling L, et al. Effects of the short-term application of pantoprazole combined with aspirin and clopidogrel in the treatment of acute STEMI. Exp Ther Med 2016;12:2861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chandrasekhar J, Bansilal S, Baber U, et al. Impact of proton pump inhibitors and dual antiplatelet therapy cessation on outcomes following percutaneous coronary intervention: results from the PARIS Registry. Catheter Cardiovasc Interv 2016;89:E217–25. [DOI] [PubMed] [Google Scholar]
- [8].Vaduganathan M, Bhatt DL, Cryer BL, et al. Proton-pump inhibitors reduce gastrointestinal events regardless of aspirin dose in patients requiring dual antiplatelet therapy. J Am Coll Cardiol 2016;67:1661–71. [DOI] [PubMed] [Google Scholar]
- [9].Zou JJ, Chen SL, Tan J, et al. Increased risk for developing major adverse cardiovascular events in stented Chinese patients treated with dual antiplatelet therapy after concomitant use of the proton pump inhibitor. PLoS One 2014;9:e84985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Giraud MN, Sanduja SK, Felder TB, et al. Effect of omeprazole on the bioavailability of unmodified and phospholipid-complexed aspirin in rats. Aliment Pharmacol Ther 1997;11:899–906. [DOI] [PubMed] [Google Scholar]
- [11].Wurtz M, Grove EL, Kristensen SD, et al. The antiplatelet effect of aspirin is reduced by proton pump inhibitors in patients with coronary artery disease. Heart 2010;96:368–71. [DOI] [PubMed] [Google Scholar]
- [12].Angiolillo DJ, Gibson CM, Cheng S, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther 2011;89:65–74. [DOI] [PubMed] [Google Scholar]
- [13].Weisz G, Smilowitz NR, Kirtane AJ, et al. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: the ADAPT-DES study. Circ Cardiovasc Interv 2015;8:e001952. [DOI] [PubMed] [Google Scholar]
- [14].Fernando H, Bassler N, Habersberger J, et al. Randomized double-blind placebo-controlled crossover study to determine the effects of esomeprazole on inhibition of platelet function by clopidogrel. J Thromb Haemost 2011;9:1582–9. [DOI] [PubMed] [Google Scholar]
- [15].Parri MS, Gianetti J, Dushpanova A, et al. Pantoprazole significantly interferes with antiplatelet effect of clopidogrel: results of a pilot randomized trial. Int J Cardiol 2013;167:2177–81. [DOI] [PubMed] [Google Scholar]
- [16].Mo C, Sun G, Lu ML, et al. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J Gastroenterol 2015;21:5382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tran-Duy A, Vanmolkot FH, Joore MA, et al. Should patients prescribed long-term low-dose aspirin receive proton pump inhibitors? A systematic review and meta-analysis. Int J Clin Pract 2015;69:1088–111. [DOI] [PubMed] [Google Scholar]
- [18].Bundhun PK, Teeluck AR, Bhurtu A, et al. Is the concomitant use of clopidogrel and proton pump inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta-analysis of recently published studies (2012–2016). BMC Cardiovasc Disord 2017;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Niu Q, Wang Z, Zhang Y, et al. Combination use of clopidogrel and proton pump inhibitors increases major adverse cardiovascular events in patients with coronary artery disease: a meta-analysis. J Cardiovasc Pharmacol Ther 2016;22:142–52. [DOI] [PubMed] [Google Scholar]
- [20].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [21].Shabanzadeh DM, Sorensen LT. Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis. Ann Surg 2012;256:934–45. [DOI] [PubMed] [Google Scholar]
- [22].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [23].Xie C, Tang Y, Wang Y, et al. Efficacy and safety of antidepressants for the treatment of irritable bowel syndrome: a meta-analysis. PLoS One 2015;10:e0127815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yasu T, Ikee R, Miyasaka Y, et al. Efficacy and safety of concomitant use of rabeprazole during dual-antiplatelet therapy with clopidogrel and aspirin after drug-eluting stent implantation: a retrospective cohort study. Yakugaku Zasshi 2010;130:1743–50. [DOI] [PubMed] [Google Scholar]
- [26].Zairis MN, Tsiaousis GZ, Patsourakos NG, et al. The impact of treatment with omeprazole on the effectiveness of clopidogrel drug therapy during the first year after successful coronary stenting. Can J Cardiol 2010;26:e54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tentzeris I, Jarai R, Farhan S, et al. Impact of concomitant treatment with proton pump inhibitors and clopidogrel on clinical outcome in patients after coronary stent implantation. Thromb Haemost 2010;104:1211–8. [DOI] [PubMed] [Google Scholar]
- [28].Rossini R, Capodanno D, Musumeci G, et al. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron Artery Dis 2011;22:199–205. [DOI] [PubMed] [Google Scholar]
- [29].Harjai KJ, Shenoy C, Orshaw P, et al. Clinical outcomes in patients with the concomitant use of clopidogrel and proton pump inhibitors after percutaneous coronary intervention: an analysis from the Guthrie Health Off-Label Stent (GHOST) investigators. Circ Cardiovasc Interv 2011;4:162–70. [DOI] [PubMed] [Google Scholar]
- [30].Zhang JR, Wang DQ, Du J, et al. Efficacy of clopidogrel and clinical outcome when clopidogrel is coadministered with atorvastatin and lansoprazole: a prospective, randomized, controlled trial. Medicine (Baltimore) 2015;94:e2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hagymasi K, Mullner K, Herszenyi L, et al. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2011;12:873–88. [DOI] [PubMed] [Google Scholar]
- [32].Liu LP, Wang Y, Si R, et al. Esomeprazole and rabeprazole did not reduce antiplatelet effects of aspirin/clopidogrel dual therapy in patients undergoing percutaneous coronary intervention: a prospective, randomized, case-control study. Expert Opin Pharmacother 2016;17:7–16. [DOI] [PubMed] [Google Scholar]
- [33].Nakata T, Miyahara M, Nakatani K, et al. Relationship between CYP2C19 loss-of-function polymorphism and platelet reactivities with clopidogrel treatment in Japanese patients undergoing coronary stent implantation. Circ J 2013;77:1436–44. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Zhao Y, Pang M, et al. High-dose clopidogrel versus ticagrelor for treatment of acute coronary syndromes after percutaneous coronary intervention in CYP2C19 intermediate or poor metabolizers: a prospective, randomized, open-label, single-centre trial. Acta Cardiol 2016;71:309–16. [DOI] [PubMed] [Google Scholar]
- [35].Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008;118:1894–909. [DOI] [PubMed] [Google Scholar]
- [36].Winiarski M, Bielanski W, Plonka M, et al. The usefulness of capsulated 13C-urea breath test in diagnosis of Helicobacter pylori infection in patients with upper gastrointestinal bleeding. J Clin Gastroenterol 2003;37:34–8. [DOI] [PubMed] [Google Scholar]
- [37].Fashner J, Gitu AC. Common gastrointestinal symptoms: risks of long-term proton pump inhibitor therapy. FP Essent 2013;413:29–39. [PubMed] [Google Scholar]
- [38].Gao Z, Li Y, Sun Y, et al. Motion tracking of the carotid artery wall from ultrasound image sequences: a nonlinear state-space approach. IEEE Trans Med Imaging 2018;37:273–83. [DOI] [PubMed] [Google Scholar]
- [39].Gao Z, Xiong H, Liu X, et al. Robust estimation of carotid artery wall motion using the elasticity-based state-space approach. Med Image Anal 2017;37:1–21. [DOI] [PubMed] [Google Scholar]
- [40].Zhang H, Li B, Young AA, et al. Recovery of myocardial kinematic function without the time history of external loads. EURASIP J Adv Signal Process 2010;1:e310473. [Google Scholar]
- [41].Liu X, Peng C, Xia Y, et al. Hemodynamics analysis of the serial stenotic coronary arteries. Biomed Eng Online 2017;16:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yang Y, Liu X, Xia Y, et al. Impact of spatial characteristics in the left stenotic coronary artery on the hemodynamics and visualization of 3D replica models. Sci Rep 2017;7:15452. [DOI] [PMC free article] [PubMed] [Google Scholar]


