Abstract
Several studies have reported that elevated red blood cell distribution width (RDW) was associated with the poor prognosis of different kinds of cancers. The aim of this study was to investigate the prognostic role of RDW in patients undergoing resection for nonmetastatic rectal cancer.
We retrospectively reviewed a database of 625 consecutive patients who underwent curative resection for nonmetastatic rectal cancer at our institution from January 2009 to December 2014. The cutoff value of RDW was calculated by receiver-operating characteristic curve.
The results demonstrated that patients in high RDW-cv group had a lower overall survival (OS) (P = .018) and disease-free survival (P = .004). We also observed that patients in high RDW-sd group were associated with significantly lower OS (P = .033), whereas the disease-free survival (DFS) was not significantly different (P = .179).
In multivariate analysis, we found elevated RDW-cv was associated poor DFS (hazard ratio [HR] = 1.56, P = .010) and RDW-sd can predict a worse OS (HR = 1.70, P = .009).
We confirmed that elevated RDW can be an independently prognostic factor in patients undergoing resection for nonmetastatic rectal cancer. So more intervention or surveillance might be paid to the patients with nonmetastatic rectal cancer and elevated RDW values in the future.
Keywords: nonmetastatic, prognosis, rectal cancer, red blood cell distribution width, resection
1. Introduction
Rectal cancer has a high incidence and death rate in China.[1] In the past decades, the treatment for rectal cancer has made great progress with the help of multidisciplinary cooperation.[2] The acceptance of total mesorectal excision in rectal surgery has greatly reduced the local recurrence after resection. However, even undergoing radical resection, a large number patients still have poor prognosis for the local recurrence or distal metastatic postoperatively.[3–5] Up to now, we still cannot accurately predict the prognosis of different patients. In oncology surgery, 5-year survival is a crucial parameter to evaluate prognosis because overwhelming majority recurrence or metastasis will happen in 5 years after surgery.[6,7] So it is important to find some parameters to help us assess the prognosis.
Traditionally, the treatment strategy and prognosis assessment of rectal cancer mainly base on the tumor node metastasis (TNM) stage. But this is limited and not enough. Sometime even early-stage patients can have a bad prognosis. And it is still controversial about the adjuvant therapy for stage II patients.[8] With the development of Precision Medicine, we need to reference more parameters to make individualized strategy. In recent years, more and more prognostic biomarkers have been reported in different cancers, including red blood cell distribution width (RDW).[9]
RDW is a hematological parameter, which is reported in blood routine examination including RDW-cv and RDW-sd.[10] It can reflex the heterogeneity of red blood cell,[10,11] and also has some associations with systemic inflammation.[10,12,13] What is more, in recent years, many studies have reported that RDW, including RDW-cv and RDW-sd, can predict the prognosis in different kinds of cancers, such as lung cancer, liver cancer, and gastric cancer.[9,14–16] Nevertheless, to our best knowledge, specific data about the prognostic role of RDW in rectal cancer are still rare. If this inexpensive and convenience parameter can be used in rectal cancer for prognosis, both doctors and patients can benefit from it. So we conduct this study to assess whether RDW can predict the prognosis in nonmetastatic rectal cancer within 5 years after surgery.
2. Materials and methods
2.1. Patients
We retrospectively reviewed the patients with rectal cancer and undergoing radical surgery in the Department of Gastrointestinal surgery, West China Hospital, Sichuan University from January 2009 to December 2014. The inclusion criteria were: rectal cancer confirmed by historical biopsy; undergoing radical resection; checked blood test in 2 weeks before surgery and RDW can be got. The exclusion criteria were: received neoadjuvant therapy; distal metastasis; presence of infection; beyond 85 years’ old. Ethical approval was not necessary because this study was a retrospective study.
We collected patients’ sex, age, pretreatment carcinoembryonie antigen (CEA), tumor location, tumor size, differentiation, TNM stage, vascular invasion, perineural invasion, adjuvant therapy, RDW-cv, and RDW-sd. TNM stage was assessed according to the American Joint Committee on Cancer TNM staging standard, 7th edition.
2.2. Follow-up
Follow-up was performed every 3 months intervals for the first 2 years, every 6 months intervals in the next 3 years, and every 12 months intervals after 5 years after surgery. We set up the terminal time of follow-up as 5 years after surgery. The examinations included physical examination, blood test, CEA levels, computed tomography (CT) of chest, and CT or magnetic resonance imaging (MRI) of abdominal and pelvic (every 6 months within the first 2 years and every 12 months after 2 years after surgery) and colonoscopy (every 2 years). Local recurrence was defined as the recurrent disease in the pelvis or at the incision, whereas the distant recurrence was defined as the recurrence beyond the above parts. Both of them were confirmed by biopsy, CT, or MRI. Follow-up data of all enrolled patients were available.
2.3. Statistical analysis
The primary endpoint and the secondary endpoint of this study were OS and DFS in 5 years after surgery, respectively. OS was the time of surgery to the date of death from any causes or the date of follow-up, whereas DFS was calculated from the surgery to the recurrence or end of follow-up.
The optima cutoff value of RDW was calculated by ROC curve. The χ2 test or Fisher exact test was used to analyze the association between clinicopathologic characteristics and RDW. The OS and DFS were analyzed and compared by using the Kaplan–Meier method and the log-rank test. Multivariate analysis was performed using Cox proportional hazards regression. Data analyses were all carried out using SPSS software (version 22.0; SPSS Inc, Chicago, IL). A P value <.05 was recognized as statistically significant.
3. Results
3.1. Patient characteristics
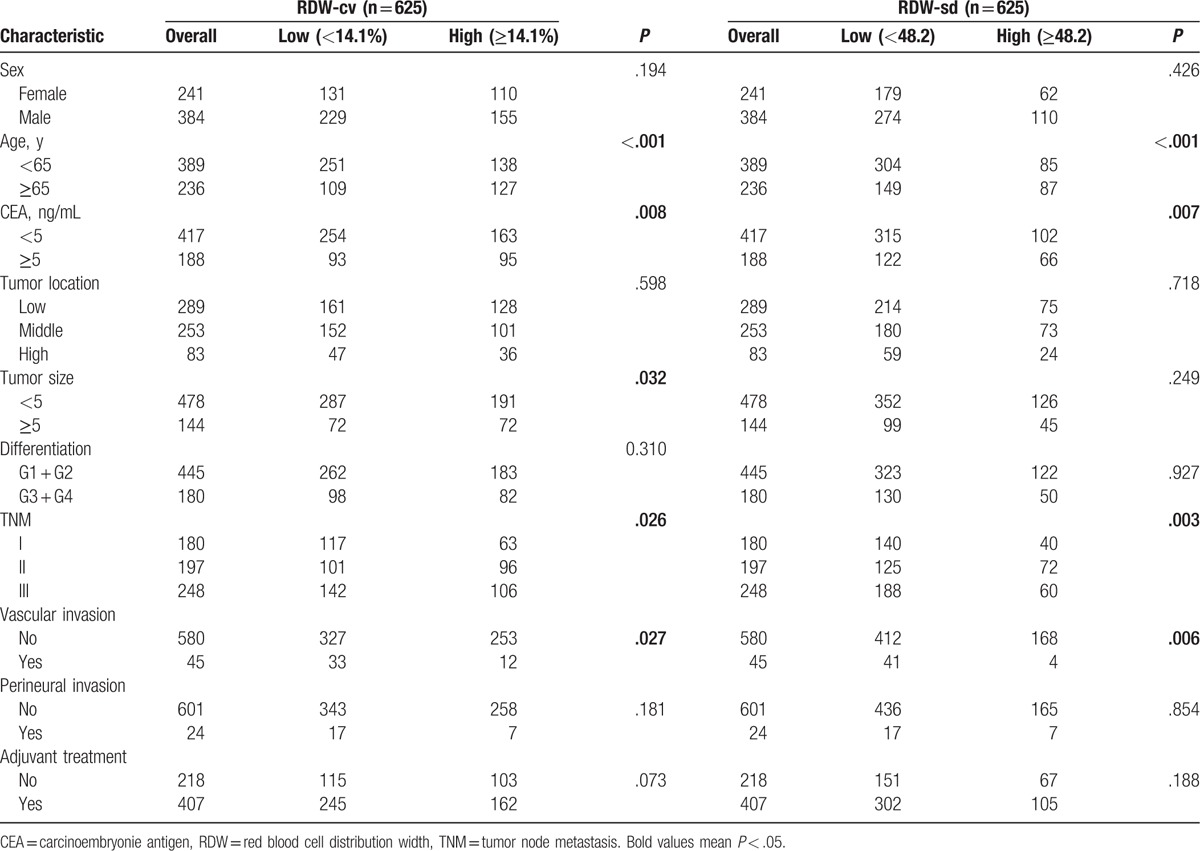
A total of 625 nonmetastatic rectal cancer patients without neoadjuvant therapy and undergoing radical resection were enrolled in this study. The last follow-up time was August 15, 2017. The median follow-up time was 60 (range 3–60) months. The cutoff values for RDW-cv and RDW-sd were 14.1% and 48.2, respectively. According to the cutoff value, patients were divided into low group and high group.
In the groups divided by RDW-cv, we found significant difference in term of age (P < .01), CEA (P = .008), tumor size (P = .032), TNM stage (P = .026), and vascular invasion (P = .027).Similarly, between the groups based on RDW-sd, there existed significant difference in age (P < .001), CEA (P = .007), TNM stage (P = .003), and vascular invasion (P = .854). (Table 1).
Table 1.
Patient clinicopathological characteristics.
3.2. Survival outcomes
The results demonstrated that patients in high RDW-cv group had a lower overall survival (OS) (P = .018) and disease-free survival (DFS) (P = .004). (Fig. 1) We also observed that patients in high RDW-sd group were associated with significantly lower OS (P = 0.033) while the DFS was not significantly different (P = .179) (Fig. 2).
Figure 1.
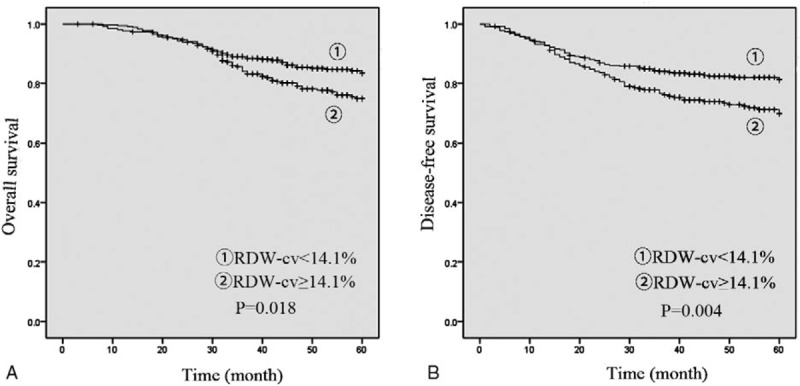
Preoperative RDW-cv for OS (A) and DFS (B). DFS = disease free survival, OS = overall survival, RDW = red blood cell distribution width.
Figure 2.
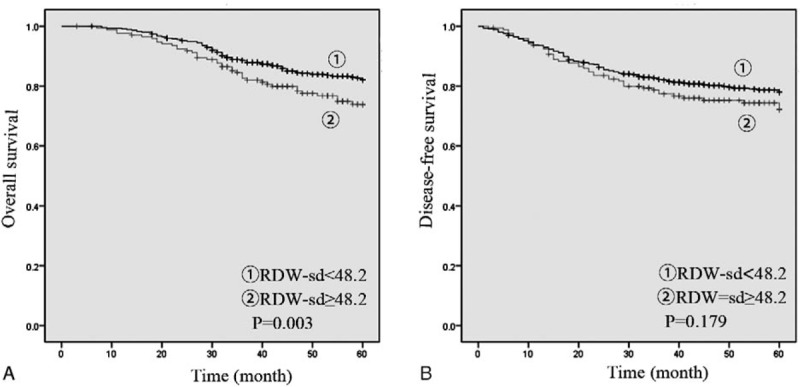
Preoperative RDW-sd for OS (A) and DFS (B). DFS = disease free survival, OS = overall survival, RDW = red blood cell distribution width.
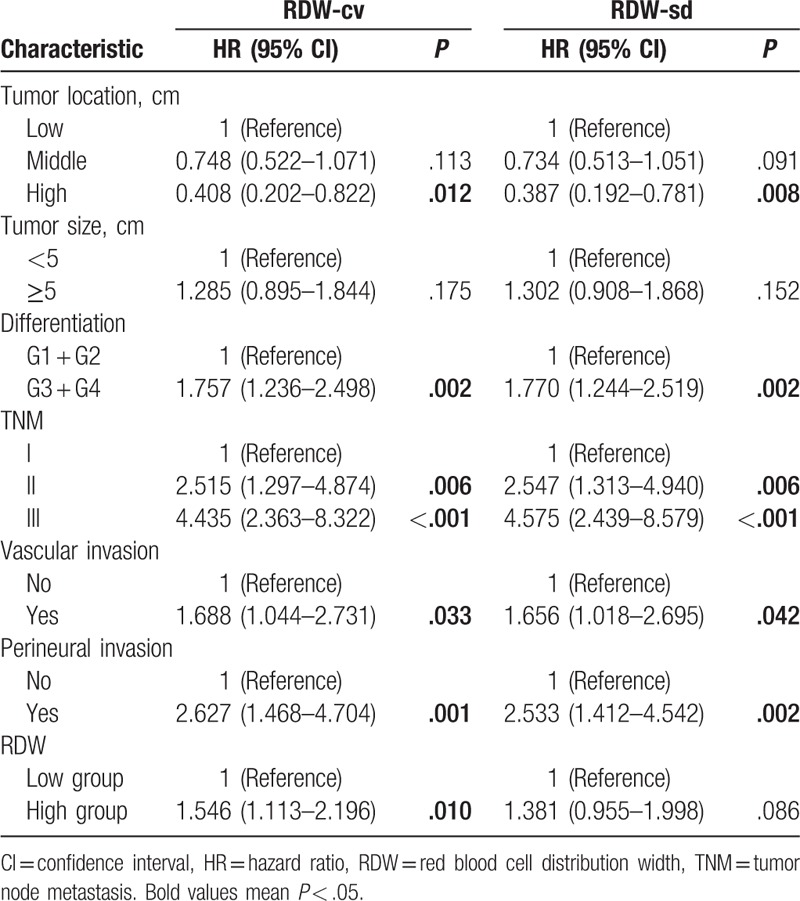
On multivariate analysis, preoperative RDW-cv level was an independent prognostic factor of poor DFS (hazard ratio [HR] = 1.56, P = .010) and RDW-sd was associated with poor OS (HR = 1.70, P = .009) (Tables 2 and 3).
Table 2.
Multivariate analyses of RDW for OS in patients with nonmetastatic rectal cancer.
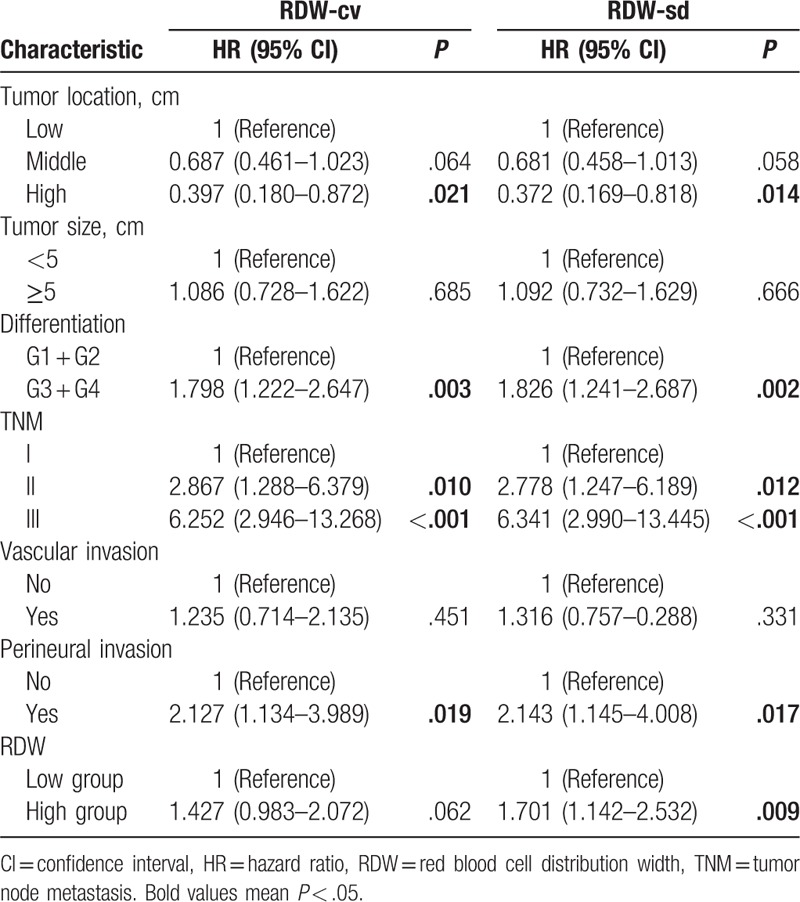
Table 3.
Multivariate analyses of RDW for DFS in patients with nonmetastatic rectal cancer.
4. Discussion
RDW has obtained increasing attention in recent years, particularly in tumor field.[17–20] In our study, we evaluated the prognostic role of RDW in patients undergoing curative resection for nonmetastic rectal cancer. Based on the multivariate analysis, we found that elevated RDW-cv is associated with poor DFS (HR = 1.56, P = .010) and elevated RDW-sd can predict poor OS (HR = 1.70, P = .009).
RDW can indicate the variability of erythrocytes size In the past; the major role of RDW was to diagnose anemia.[10] Besides, RDW is also associated with several diseases, such as heart disease, pulmonary disease, and even tramua.[11,21] What's more, RDW is also related with some inflammatory disease including pancreatitis, hepatitis, and regarded as an indicator of the overall inflammatory, although the potential mechanism has not been demonstrated clearly.[22,23]
Several studies have demonstrated that RDW was related to poor prognosis of different cancer.[9] Most articles explained it with inflammation response in tumorgenesis. The relationship between tumor and inflammation was firstly reported in 1863 by Virchow.[24] After that, several studies have demonstrated that inflammation in microenvironment can promote the development of cancer.[25,26] Colorectal cancer is a disease, which has close association with inflammation.[25] Colorectal cancer can develop from inflammatory bowel diseases and inflammation polys.[27–29] So inflammation might play a crucial role in the development of colorectal cancer. Besides, there also are some articles indicated that inflammation can play dual role in tumorgenesis. That's to say sometimes inflammation can fight with cancer. But we think in the whole process of tumor development, the effect of promotion might be the leadership.
In addition, many other hematological parameters have been reported in colorectal cancer for the prognosis role, including neutrophil-lymphcyte ratio,[30] lymphcyte-monocyte ratio,[31] C-reactive protein,[32] interleukin-6,[33] albumin,[34,35] platelet, and hemoglobin.[36] These parameters are also closely related with inflammatory response and anemia.
We have to mention that RDW is also a marker of anemia.[37] In fact, anemia is a common symptom in patients with colorectal cancer[38,39] and some articles demonstrated that anemia could increase postoperative morbi-mortality.[39] Besides, several studies also indicated that anemia can predict the poor prognosis of colorectal cancer.[40] And anemia can also be caused by a systemic inflammatory response to the tumor in colorectal cancer.[41] So anemia is also related with the survival outcomes of colorectal cancer.
Above all, we have enough reason to believe that RDW is associated with the prognosis of nonmetastatic rectal cancer.
One previous study[42]reported that elevated RDW was associated with poor prognosis in patients with colorectal cancer, especially II stage disease. However, it just included 90 patients and did not present the data of rectal cancer or colon cancer independently. Besides, one previous meta-analysis[9] demonstrated that RDW might be a potential prognostic marker in patients with cancer. Nonetheless, that article included different types of tumors and number of studies dealing with each type of cancers was ≤5. In addition, there was no colorectal cancer in that article and the cutoff value of RDW was not unified. Our study suggested that RDW was associated with both OS and DFS in rectal cancer patients. Although we did not invest the specific molecular mechnishm, we thought inflammation factors could have an influence on rectal cancer survival. This is the first large-scale cohort study demonstrating that there exists association between RDW and either OS or DFS in rectal cancer. Unfortunately, this study was a retrospective study. Still, a prospective cohort study is needed to evaluate the association between RDW and OS and DFS in rectal cancer patients.
In conclusion, this study represents the first analysis of the value of preoperative RDW level in patients with nonmetastatic rectal cancer to the best of our knowledge. The analysis of our data demonstrates that preoperative RDW levels correlate with leading prognostic factors in patients undergoing surgery for rectal cancer. The predicting role of RDW is confirmed in multivariate analysis. So it seems reasonable to suggest that evaluation of the preoperative RDW level is helpful for predicting the prognosis of patients with nonmetastatic rectal cancer.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CI = confidence intervals, DFS = disease-free survival, HR = hazard ratio, OS = overall survival, RDW = red blood cell distribution width, ROC = receiver-operating characteristic, TNM = tumor node metastasis.
XZ and QW contributed equally to this work.
Source of support: This work was supported by the Science and Technology Support Program of the Science & Technology Department of Sichuan Province (Grant numbers: 2016SZ0043).
This work was supported by the Science and Technology Support Program of the Science & Technology Department of Sichuan Province (Grant numbers: 2016SZ0043).
The authors report no conflicts of interest.
References
- [1].Zheng R, Zeng H, Zhang S, et al. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 2017;36:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bochis OV, Fekete Z, Vlad C, et al. The importance of a multidisciplinary team in rectal cancer management. Clujul Med 2017;90:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822–8. [DOI] [PubMed] [Google Scholar]
- [4].Ding P, Liska D, Tang P, et al. Pulmonary recurrence predominates after combined modality therapy for rectal cancer: an original retrospective study. Ann Surg 2012;256:111–6. [DOI] [PubMed] [Google Scholar]
- [5].Chang CY, Kim HC, Park YS, et al. The effect of postoperative pelvic irradiation after complete resection of metastatic rectal cancer. J Surg Oncol 2012;105:244–8. [DOI] [PubMed] [Google Scholar]
- [6].Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (London, England) 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- [7].Aguero F, Murta-Nascimento C, Gallen M, et al. Colorectal cancer survival: results from a hospital-based cancer registry. Rev Esp Enferm Dig 2012;104:572–7. [DOI] [PubMed] [Google Scholar]
- [8].Wei B, Zheng XM, Lei PR, et al. Predictive models of adjuvant chemotherapy for patients with stage ii colorectal cancer: A retrospective study. Chi Med J 2017;130:2069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu L, Li M, Ding Y, et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget 2017;8:16027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. [DOI] [PubMed] [Google Scholar]
- [11].Miyamoto K, Inai K, Takeuchi D, et al. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ J 2015;79:1100–6. [DOI] [PubMed] [Google Scholar]
- [12].Liu Q, Dang AM, Chen BW, et al. The association of red blood cell distribution width with anemia and inflammation in patients with Takayasu arteritis. Clin Chim Acta 2015;438:205–9. [DOI] [PubMed] [Google Scholar]
- [13].Dogan S, Celikbilek M, Zararsiz G, et al. Red blood cell distribution width as a non-invasive marker for the assessment of inflammation in non-alcoholic steatohepatitis. Hepatogastroenterology 2015;62:393–8. [PubMed] [Google Scholar]
- [14].Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013;8:e80240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark 2016;16:507–12. [DOI] [PubMed] [Google Scholar]
- [16].Yazici P, Demir U, Bozkurt E, et al. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark 2017;18:19–25. [DOI] [PubMed] [Google Scholar]
- [17].Wei TT, Wang LL, Yin JR, et al. Relationship between red blood cell distribution width, bilirubin, and clinical characteristics of patients with gastric cancer. Int J Lab Hematol 2017;39:497–501. [DOI] [PubMed] [Google Scholar]
- [18].Wang J, Xie X, Cheng F, et al. Evaluation of pretreatment red cell distribution width in patients with multiple myeloma. Cancer Biomark 2017;20:267–72. [DOI] [PubMed] [Google Scholar]
- [19].Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017;23:4879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goyal H, Hu ZD. Prognostic value of red blood cell distribution width in hepatocellular carcinoma. Ann Transl Med 2017;5:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yesil A, Senates E, Bayoglu IV, et al. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver 2011;5:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fu Y, Mao Y, Chen S, et al. A novel inflammation- and nutrition-based prognostic system for patients with laryngeal squamous cell carcinoma: combination of red blood cell distribution width and body mass index (COR-BMI). PLoS One 2016;11:e0163282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Z, Hong N, Robertson M, et al. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci RepV 7 2017;43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu QB, Wang M, Hu T, et al. Prognostic role of the lymphocyte-to-monocyte ratio in patients undergoing resection for nonmetastatic rectal cancer. Medicine 2016;95:e4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol 2016;17:230–40. [DOI] [PubMed] [Google Scholar]
- [26].Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353:2654–66. [DOI] [PubMed] [Google Scholar]
- [27].Bopanna S, Kedia S, Das P, et al. Long-term follow-up reveals high incidence of colorectal cancer in Indian patients with inflammatory bowel disease. United European Gastroenterol J 2017;5:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loberg M, Kalager M, Holme O, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
- [30].Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014;134:2403–13. [DOI] [PubMed] [Google Scholar]
- [31].Song W, Wang K, Zhang RJ, et al. Prognostic value of the lymphocyte monocyte ratio in patients with colorectal cancer: a meta-analysis. Medicine 2016;95:e5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Goyal A, Terry MB, Jin Z, et al. C-reactive protein and colorectal cancer mortality in U.S. adults. Cancer Epidemiol Biomarkers Prev 2014;23:1609–18. [DOI] [PubMed] [Google Scholar]
- [33].Zhou B, Shu B, Yang J, et al. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control 2014;25:1397–405. [DOI] [PubMed] [Google Scholar]
- [34].Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis 2013;28:1629–36. [DOI] [PubMed] [Google Scholar]
- [35].Mocellin MC, Pastore e Silva Jde A, Camargo Cde Q, et al. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids 2013;48:879–88. [DOI] [PubMed] [Google Scholar]
- [36].Al-Saeed EF, Tunio MA, Al-Obaid O, et al. Correlation of pretreatment hemoglobin and platelet counts with clinicopathological features in colorectal cancer in Saudi population. Saudi J Gastroenterol 2014;20:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Auezova R, Ryskeldiev N, Doskaliyev A, et al. Association of preoperative levels of selected blood inflammatory markers with prognosis in gliomas. Onco Targets Ther 2016;9:6111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qiu L, Wang DR, Zhang XY, et al. Impact of perioperative blood transfusion on immune function and prognosis in colorectal cancer patients. Transfus Apher Sci 2016;54:235–41. [DOI] [PubMed] [Google Scholar]
- [39].Leichtle SW, Mouawad NJ, Lampman R, et al. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg 2011;212:187–94. [DOI] [PubMed] [Google Scholar]
- [40].Morner ME, Edgren G, Martling A, et al. Preoperative anaemia and perioperative red blood cell transfusion as prognostic factors for recurrence and mortality in colorectal cancer-a Swedish cohort study. Int J Colorectal Dis 2017;32:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Munoz M, Gomez-Ramirez S, Martin-Montanez E, et al. Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol 2014;20:1972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kust D, Lucijanic M, Urch K, et al. Clinical and prognostic significance of anisocytosis measured as a red cell distribution width in patients with colorectal cancer. QJM 2017;110:361–7. [DOI] [PubMed] [Google Scholar]


