Abstract
Rationale:
Longitudinally extensive transverse myelitis (LETM) is characterized by contiguous inflammatory lesions of spinal cord extending to ≥3 vertebral segments. The etiology of LETM is complicated, including various infection, autoimmune disease, and so on. Neuromyelitis optic spectrum disorder (NMOSD) is the most common cause of LETM. Several case reports have suggested the associations between NMOSD and pulmonary tuberculosis (PTB).
Patient concerns:
Patient 1, a 20-year-old woman who had a past history of PTB, presented with weakness, numbness, and pain in the limbs. The serum anti-aquaporin-4 antibody (AQP4-Ab) was strongly positive, and the magnetic resonance imaging (MRI) scan of cervical and thoracic spinal cord after admission to the hospital revealed hyperintensity lesions extending from C3 to T8 on T2-weighted (T2W) image, T1-weighted (T1W) image, and fluid-attenuated inversion recovery (FLAIR) image. Patient 2, a 21-year-old woman who had a past medical history of PTB without receiving any treatment, presented for numbness in bilateral lower limbs and in the chest. The anti-AQP4-Ab was negative both in the serum and in the cerebral spinal fluid (CSF) of the patient. The MRI scan during hospitalization of cervical and thoracic spinal cord revealed diffuse hyperintense signal extending C3 to T11 on T2W and FLAIR images and hypointense signal on T1W image.
Diagnosis:
The first patient was diagnosed with anti-AQP4-Ab positive NMOSD, while the second case was an anti-AQP4-Ab negative LETM patient.
Interventions:
Both of the patients received a combination of corticosteroid and anti-tuberculosis (isonicotinyl hydrazide 0.3 g/d, rifampin 0.45 g/d, pyrazinamide 1 g/d, and ethambutol 1 g/d) treatment.
Outcomes:
The patients were followed up for up to 1 year. The Expanded Disability Status Scale (EDSS) of both patients were decreased and the lesion size in the spinal cord was significantly reduced at the time point of the follow-up.
Lessons:
Combination of anti-tuberculosis and corticosteroid treatment may have better prognosis for patient of LETM with PTB.
Keywords: longitudinally extensive transverse myelitis, neuromyelitis optica spectrum disorder, pulmonary tuberculosis
1. Introduction
Longitudinally extensive transverse myelitis (LETM) is characterized by contiguous inflammatory lesions of spinal cord extending to ≥3 vertebral segments.[1] The causes of LETM including various infections, neoplastic reason, and autoimmune disease.[2] Neuromyelitis optica spectrum disorder (NMOSD) is the most common cause of LETM.
In recent years, more and more cases of pulmonary tuberculosis (PTB) patients with optic myelitis symptoms were published,[3–5] suggesting the potential link between mycobacterium tuberculosis infection and NMOSD.[6,7] A retrospective case-control study conducted by Zatjirua et al[8] in South Africa, showed the odds ratio for the presence of active PTB in the neuromyelitis optica (NMO) group versus the control group was 4.6. Moreover, a positive correlation between the activity of PTB and the occurrence of NMO has been found.
We report 2 cases of LETM concurrent with PTB. The first case was an anti-aquaporin-4 antibody (AQP4-Ab) positive NMOSD patient with a past history of PTB. The second case was anti-AQP4-Ab negative LETM patient with active PTB.
2. Case presentation
2.1. Case 1
A 20-year-old woman was admitted to our hospital for weakness, numbness, and pain in the limbs for 6 months and worsened for 3 days. The patient felt persistent pain in the neck, left shoulder, and the upper left arm 6 months before, and the situation became worse and both upper extremities were affected. The patient visited the local hospital and was diagnosed as myelitis and she received gamma globulin (20 g for 5 days) and dexamethasone (5 mg for 15 days). Her symptoms were improved gradually. However, 1 month later, the weakness, persistent numbness, and pain in both lower extremities occurred again and gradually worsened with time. Therefore, the patient returned to the local hospital, and received vitamin B12 treatment, her symptom became slightly better but not completely recover. Six days before the admission to our hospital, the patient had symptoms of sneezing nose. Three days later, the patient had severe weakness and numbness in the lower extremities. Her past medical history was otherwise negative except for PTB for 6 years. At that time, the patient received systematic anti-tuberculosis treatments and her symptoms were completely disappeared and chest computed tomography (CT) showed chronic pulmonary tuberculosis.
Neurological examination revealed decreased muscle strength of both lower extremities (1/5 of the right extremity and 3/5 of the left extremity according to the Medical Research Council [MRC] grade) with bilateral hyperreflexia. Bilateral Babinski sign were positive. Hyperalgesia was found below the level of T8, deep sensation of the right lower limb was absent. The Expanded Disability Status Scale (EDSS) of the patient during hospitalization was 6.5. The magnetic resonance imaging (MRI) scan of the cervical spine (6 months before admission) revealed cervical spinal cord lesions without gadolinium enhancement (Fig. 1). The serum anti-AQP4-Ab was strongly positive, and the Immunoglobulin G (IgG) oligoclonal bands were negative both in the serum and in the cerebrospinal fluid (CSF). CSF routine test showed elevated protein levels (0.39 g/L, normal range from 0.15 to 0.45 g/L) and increased cell numbers (leukocyte count 25 × 106/L, normal range from 0 to 8 × 106/L), of which 92% were monocytes. Autoimmune diseases related test including anti-nuclear antibodies, anti-dsDNA, anti-streptolysin, rheumatoid factor, thyroid function tests, erythrocyte sedimentation rate (ESR), and hypersensitive C-reactive protein were all negative. T cell spot tuberculosis (TB) testing was positive. No tubercula bacilli were found in sputum TB smear. Anti-TB antibodies of Lipoarabinomannan-IgG and 38 KD/IgG in the serum were positive. Quantitative detection of Brucella polymerase chain reaction was negative in the CSF. Brain MRI after admission demonstrated multiple patchy abnormal signal in pons, the right thalamus, the left basal ganglia, corona radiate and corpus callosum on fluid-attenuated inversion recovery (FLAIR) imaging, diffusion-weighted imaging (DWI), and T2-weighted (T2W) imaging (Fig. 2). The second MRI scan of cervical and thoracic spinal cord after admission to the hospital revealed hyperintensity lesions extending from C3 to T8 on T2W image, T1-weighted (T1W) image, and FLAIR image (Fig. 3A–C). Chest CT during hospitalization showed left lung upper lobe bronchiectasis and multiple calcifications of upper lobe in both sides (Fig. 3D). The patient was treated with methylprednisolone of 500 mg for 3 days, afterwards, the dose of methylprednisolone was decreased to half every 3 days, meanwhile she also received gamma globulin and anti-tuberculosis drugs (isonicotinyl hydrazide 0.3 g/d, rifampin 0.45 g/d, pyrazinamide 1 g/d, and ethambutol 1 g/d). The patient discharged from hospital after 1 months’ treatment. The EDSS was decreased to 0 at the time point of follow up 12 months later. The spinal cord MRI showed the lesion size in the cervical and thoracic spine were significantly reduced (Fig. 4).
Figure 1.
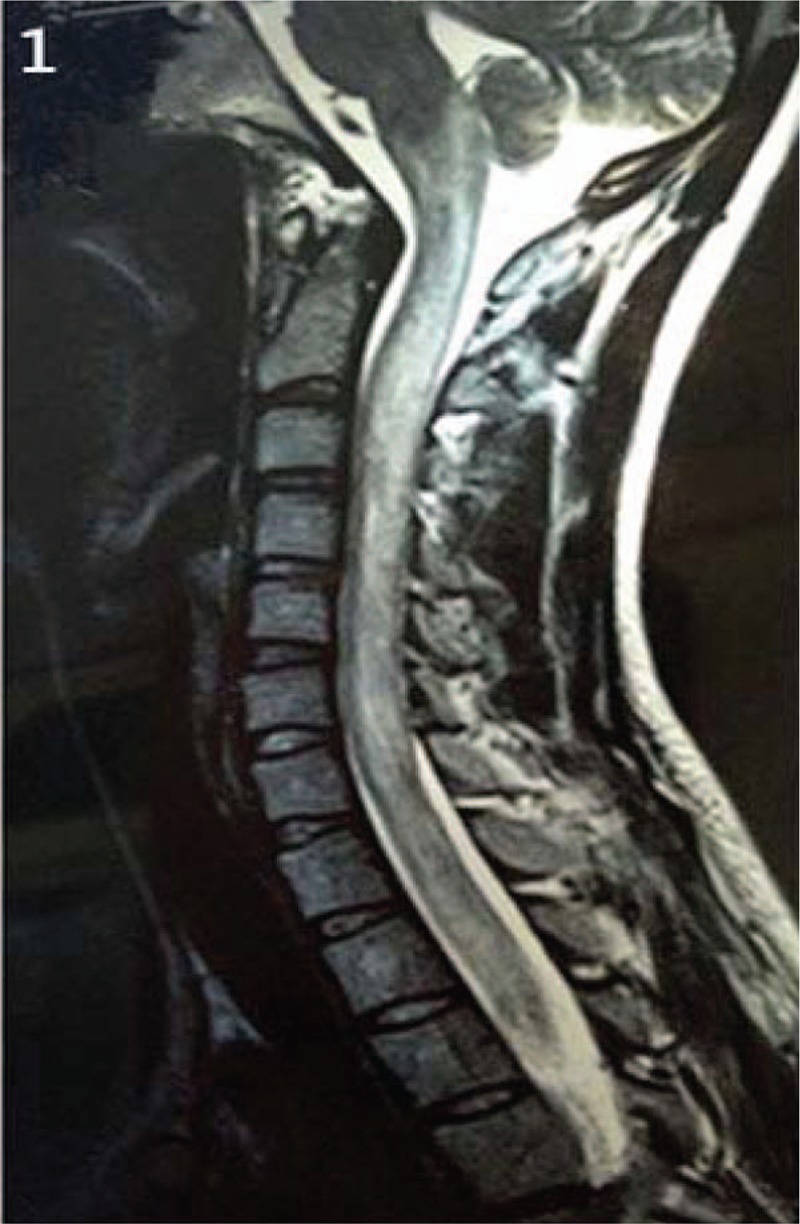
MRI scans of case 1 (6 months before admission) showed hyperintensity lesions in the spinal cord from C1 to C7 on T2 sequence. MRI = magnetic resonance imaging.
Figure 2.
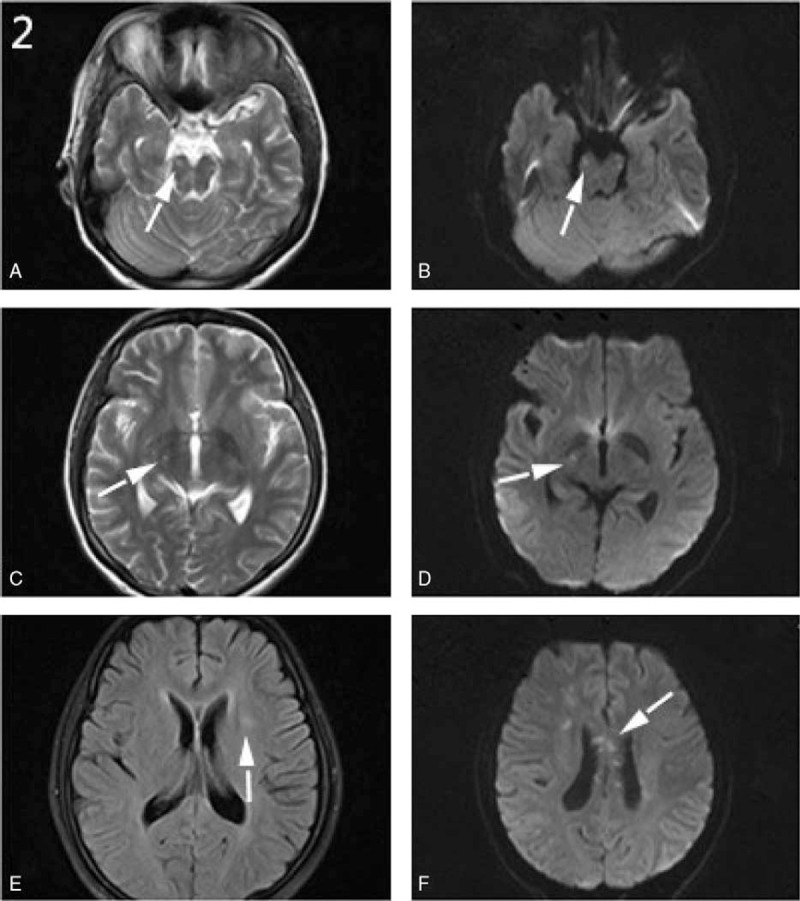
A–F, Brain MRI scans of case 1 showed multiple hyperintensity lesions (A–D) in the right thalamus and pons on the T2 and DWI sequences. High-intensity lesions were also found in the left basal ganglia on the FLAIR sequence (E) as well as in corona radiate and corpus callosum on the DWI sequence (F). DWI = diffusion-weighted imaging, FLAIR = fluid-attenuated inversion recovery, MRI = magnetic resonance imaging.
Figure 3.
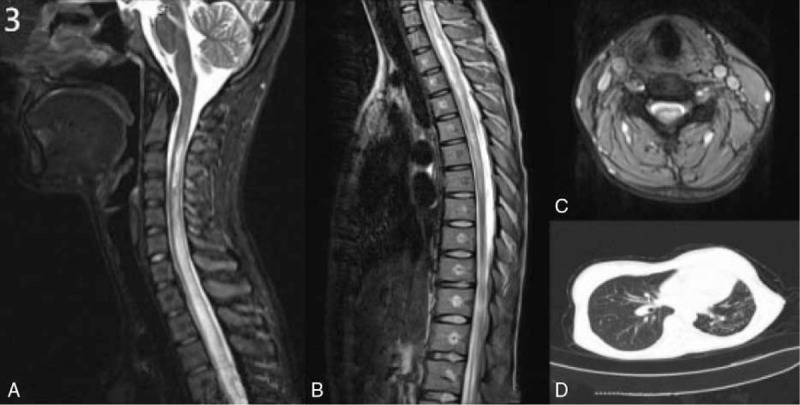
A–D, MRI scans of case 1 before treatment showed hyperintensity lesions in the spinal cord from C3 to T8 on T2 sequence (A–C). Chest CT scan showed bronchitis and pneumonia in both sides, bronchiectasis in the left upper lobe and multiple calcifications of in both upper lobe (D). CT = computed tomography, MRI = magnetic resonance imaging.
Figure 4.
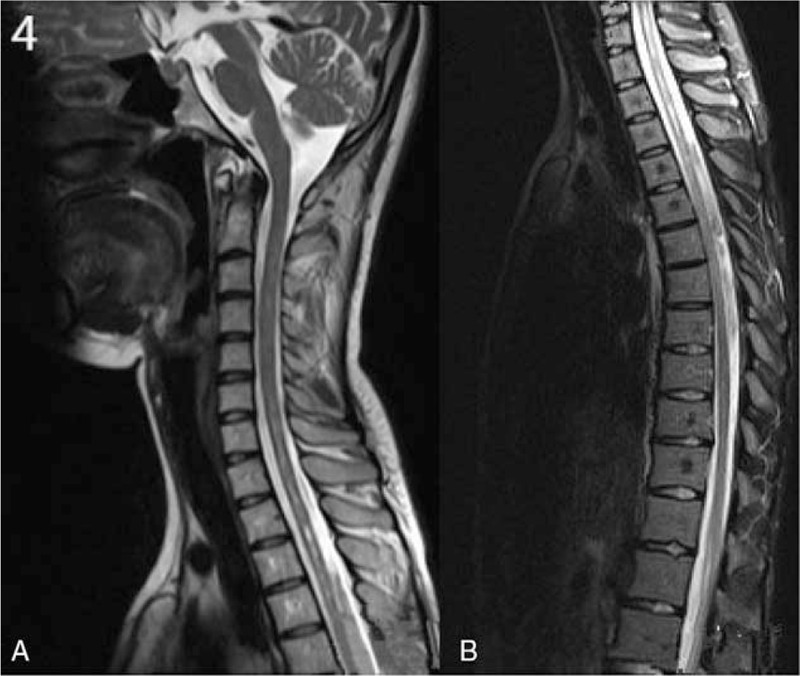
A–B, MRI scan of case 1 12 months after treatment showed hyperintensity signal from C6 to T8 levels on T2-weighed sequence. The lesion was significantly reduced compared with that before treatments, spinal cord slightly atrophy (A–B). MRI = magnetic resonance imaging.
2.2. Case 2
A 21-year-old woman was admitted to our hospital for numbness in bilateral lower limbs and in the chest for 7 days. She had a past medical history of PTB without receiving any treatment for 3 years. On neurological examination, the muscle strength in the upper limbs and lower limbs were 5/5 according to the MRC grade. Hyperreflexia was found in bilateral lower limbs. She was found loss of sensation of pain and touch in the spinal segments below T4. Bilateral Babinski sign were positive. Four days after admission, the muscle strength in bilateral lower limbs were 4/5 according to the MRC grade, with retention of urine. The EDSS of the patient was 4.5 during hospitalization. The MRI scan during hospitalization of cervical and thoracic spinal cord revealed diffuse hyperintense signal extending C3 to T11 on T2W and FLAIR images and hypointense signal on T1W images (Fig. 5A–C). The MRI of the thoracic spinal cord revealed altered medullary signal intensity from C6 to T11 levels, appearing hyperintense on T1W and T2W images (Fig. 5B). The chest CT showed left upper lobe tuberculosis with cavitation (Fig. 5D). CSF routine test showed increased protein levels (0.48 g/L, normal range from 0.15 to 0.45 g/L). The CSF cell numbers were within the normal range (4 × 106/L). The anti-AQP4-Ab was negative both in the serum and in the CSF of the patient. Tuberculosis mycobacterium antibody was negative both in serum and CSF. A combination of corticosteroid and anti-tuberculosis treatment (isonicotinyl hydrazide 0.3 g/d, rifampin 0.45 g/d, pyrazinamide 1 g/d, ethambutol 1 g/d, and dexamethasone 15 mg/d) was given. Neurological dysfunction was ameliorated significantly. The lesion size in the spinal cord and in the lung were significantly reduced 1 month later after discharge from the hospital. The EDSS of the patient dropped to 0 at the follow up time when 3 months after discharge from the hospital.
Figure 5.
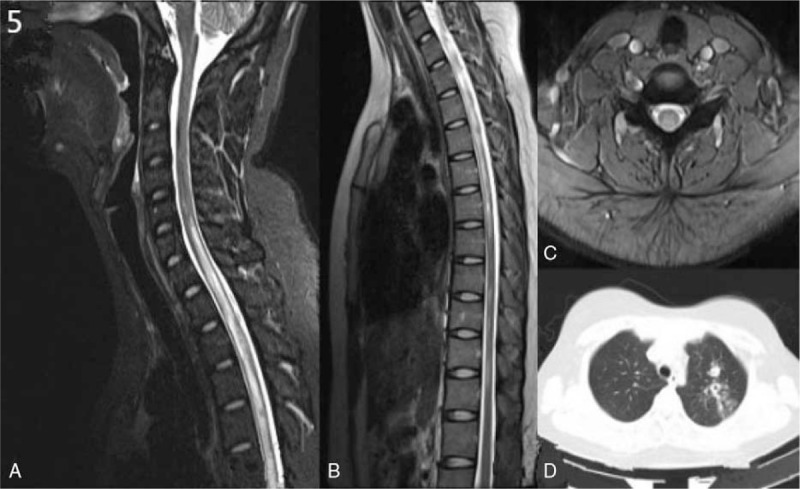
A–D, MRI scan of case 2 showed hyperintensity signal from C3 to T11 on T2-weighed images (A–C). Lung CT scan showed tuberculosis with cavitation in the left upper lobe (D). CT = computed tomography, MRI = magnetic resonance imaging.
3. Discussion
We herein report 2 cases of LETM coincident with PTB. The first case was an anti-AQP4-Ab positive NMOSD patient who had a past history of PTB. The second case was anti-AQP4-Ab negative LETM patient with active PTB. Both patients’ clinical symptoms were improved by the combination of corticosteroid and anti-tuberculosis treatment. Further, the EDSS of both patients decreased to 0 and their lesions size in spinal cord was significantly reduced during the follow-up.
In case 1, the CSF IgG synthesis rate, the blood–brain barrier (BBB) permeability, and the levels of myelin basic protein (MBP) were found increased. In case 2, the ESR, the levels of CSF IgG, anti-myelin oligodendrocyte glycoprotein (MOG) antibody, and anti-MBP antibody in the serum were found increased. Increased IgG synthesis rate or IgG index reflects the presence of humoral immune response. The elevated levels of MBP, anti-MBP antibody, MOG, and anti-MOG antibody reflect the inflammatory demyelinating lesions.[9] The CSF changes of 2 cases showed the presence of demyelination in the central nervous system (CNS). Firstly, direct CNS infection with tuberculosis can cause NMOSD, evidenced by a study conducted by Liu et al,[10] where the authors injected Bacillus Calmette-Guerin (BCG) into the lateral ventricle of mice, and showed the production of anti-AQP4-Ab and demyelination in the brain. Secondly, mycobacterium tuberculosis (MTB) may have common antigens with MBP. Significant inflammatory response and demyelination were found after injecting BCG into sensitized guinea pigs.[11] Moreover, lymphocytes sensitized by MTB are able to recognize and attack the myelin,[12] indicating delayed hyper sensitivity reaction caused by MTB may be one cause of demyelination. Interestingly, in a retrospective study of NMOSD patients with PTB, Silber et al[3] found that after giving anti-tuberculosis treatment, the corresponding impaired neurologic functions of patients were significantly improved.
Both of the patients’ symptoms were improved by anti-tuberculosis and corticosteroid therapy. A case-control study conducted by Feng et al[13] where the cases were treated with anti-tuberculosis therapy, while the control group was treated with corticosteroid, plasmapheresis and immune globulin, and other therapies. The follow-up result showed that the EDSS of patients with anti-tuberculosis treatment decreased gradually, and the recurrence rate was lower than that of the control group. In the early course of disease, the first case had remission despite receiving corticosteroid treatment. Considering her past history of PTB, anti-tuberculosis treatments were given. Her clinical symptoms improved rapidly within 1 month. No recurrence was observed during the follow-up period of 12 months. The MRI lesions in the cervical and thoracic spine were significantly absorbed than before. Our experiences in this case together with Feng's findings indicate that anti-tuberculosis treatment can improve neurological damage and reduce the relapse rate of the disease NMOSD with PTB patients. The second case was anti-AQP4-Ab negative LETM patient with active PTB. The patient received corticosteroid treatment combined with anti-tuberculosis treatment for 1 month, and the symptoms were improved dramatically. The lesions in the lung and in the spinal cord were significant absorbed than before.
In conclusion, a combination of corticosteroid and anti-tuberculosis treatment has better therapeutic effects for patients who had co-occurrence of LETM and MTB. MTB induced activation of the immune system may contribute to the etiology of LETM.
Acknowledgments
The authors are grateful for the support from the grants from the National Science Foundation of China (No. 31600820); The Health and Family Planning Commission of Jilin Province (No. 2016Q036); The Youth Foundation from The First Hospital of Jilin University (No.00400050050); The Programme for JLU Science and Technology Innovative Research Team (JLUSTIRT) (No: 451170301647).
Footnotes
Abbreviations: AQP4-Ab = aquaporin-4 antibody, BBB = blood–brain barrier, BCG = Bacillus Calmette-Guerin, CNS = central nervous system, CSF = cerebral spinal fluid, CT = computed tomography, DWI = diffusion-weighted imaging, EDSS = Expanded Disability Status Scale, ESR = erythrocyte sedimentation rate, FLAIR = fluid-attenuated inversion recovery, Ig = immunoglobulin, LETM = longitudinally extensive transverse myelitis, MBP = myelin basic protein, MOG = myelin oligodendrocyte glycoprotein, MRC = Medical Research Council, MRI = magnetic resonance imaging, NMOSD = neuromyelitis optic spectrum disorder, PTB = pulmonary tuberculosis, T1W = T1-weighted, T2W = T2-weighted, TB = tuberculosis.
YZ and MZ have equally contributed to the work.
Ethical Considerations: Ethical approval was obtained from the Ethics Committee from The First Hospital, of Jilin University, China. Though written informed consent was not obtained, the patient's information was anonymized and de-identified.
The authors declare no conflicts of interest.
References
- [1].Jain RS, Kumar S, Mathur T, et al. Longitudinally extensive transverse myelitis: a retrospective analysis of sixty-four patiens at tertiary care center of North-West India. Clin Neurol Neurosurg 2016;148:5–12. [DOI] [PubMed] [Google Scholar]
- [2].Kitley JL, Leite MI, George JS, et al. The differential diagnosis of longitudinally extensive transverse myelitis. Mult Scler 2012;18:271–85. [DOI] [PubMed] [Google Scholar]
- [3].Silber MH, Willcox PA, Bowen RM, et al. Neuromyelitis optica (Devic's syndrome) and pulmonary tuberculosis. Neurology 1990;40:934–8. [DOI] [PubMed] [Google Scholar]
- [4].Rafai MA, Boulaajaj FZ, Gynerane M, et al. Devic-like syndrome in the course of pulmonary tuberculosis. Acta Neurol Belg 2010;110:196–200. [PubMed] [Google Scholar]
- [5].Siddiqi SA, Hashmi M, Azmat Z, et al. Pulmonary tuberculosis with neuromyelitis optica: an uncommon association of a common disease. J Coll Physicians Surg Pak 2012;22:527–8. [PubMed] [Google Scholar]
- [6].Sellner J, Hemmer B, Mühlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (Devic) syndromes. J Autoimmun 2010;34:37l–9. [DOI] [PubMed] [Google Scholar]
- [7].El Otmani H, Rafai MA, Moutaouakil F, et al. Devic's optic neuromyelitis and pulmonary tuberculosis. Rev Mal Respir 2005;22(1 Pt 1):143–6. [DOI] [PubMed] [Google Scholar]
- [8].Zatjirua V, Butler J, Carr J, et al. Neuromyelitis optica and pulmonary tuberculosis: a case–control study. Int J Tuberc Lung Dis 2011;15:1675–80. [DOI] [PubMed] [Google Scholar]
- [9].Reindl M, Linington C, Brehm U, et al. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 1999;122(Pt 11):2047–56. [DOI] [PubMed] [Google Scholar]
- [10].Liu J-X, Lai R, Guo N, et al. NMO-IgG/AQP4-Ab expression induced by intracerebral mycobacterium bovis bacillus calmette-guerin infection in mice. J SUN Yat-sen Univ (Med Sci) 2013;34:4l–7. [Google Scholar]
- [11].Wisniewski HM, Bloom BR. Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med 1975;141:346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hughes RA, Mair WG. Acute necrotic myelopathy with pulmonary tuberculosis. Brain 1977;100:223–38. [DOI] [PubMed] [Google Scholar]
- [13].Feng YQ, Guo N, Huang F, et al. Anti-tuberculosis treatment for Devic's neuromyelitis optica. J Clin Neurosci 2010;17:1372–7. [DOI] [PubMed] [Google Scholar]


