Abstract
Recently, dynamic risk stratification has been found to be more valuable than static anatomic staging system in nonmedullary thyroid cancer and this strategy has also been accepted in medullary thyroid cancer (MTC). The present study was designed to compare the clinical usefulness of response to initial therapy stratification with a traditional anatomic staging system.
From August 1982 to December 2012, a total of 144 MTC patients underwent thyroidectomy in Yonsei University Hospital. Among them, 117 (82.2%) patients with complete clinical data and sustained follow-up were enrolled in this study. Clinicopathological features and surgical outcomes were analyzed by retrospective medical chart review. Mean follow-up duration was 85.78 ± 62.51 months.
In this study, mean tumor size was 1.94 ± 1.40 cm and 22 (18.9%) patients had hereditary MTC; 95 (81.1%) patients had sporadic MTC. Stage I patients had highest probability of excellent response to initial therapy (92.1%). Stage IV patients had highest probability of biochemical and structural incomplete response to initial therapy (57.5% and 30.3%) and lowest probability of excellent response to initial therapy (12.1%). Both response to initial therapy stratification and TNM staging system offered useful prognostic information in this study. The TNM staging system provided risk stratification pertaining to disease-free survival (DFS), disease-specific survival (DSS), and the probability of having no evidence of disease at final outcome, but did not provide risk stratification pertaining to the probability of having biochemical persistent/recurrence disease at final outcome. However, response to initial therapy stratification provided risk stratification pertaining to not only DFS, DSS, and the probability of having no evidence of disease at final outcome but also the probability of having biochemical persistent/recurrence disease at final outcome.
In this study, we demonstrated that dynamic risk stratification with adjusted response to initial therapy system can offer more useful prognostic information than anatomic staging system in MTC.
Keywords: dynamic risk stratification, medullary thyroid carcinoma, prognosis
1. Introduction
Medullary thyroid carcinoma (MTC) is a rare cancer that accounts for 3% to 10% of all thyroid cancers.[1] MTC has a more aggressive biological behavior than papillary thyroid carcinoma. Metastasis to regional lymph nodes (LNs) in the central compartment is reported in 50% to 81% of patients and to the lateral compartments in 34% to 81%.[2] MTC accounts for 8% to 13% of all thyroid cancer related mortalities because of distant metastasis.[3] For these reasons, recognizing prognostic factors is important. Age, male sex, extrathyroidal invasion, cervical LN metastasis, distant metastasis, sporadic or hereditary disease, delay in diagnosis, and incomplete surgical treatment are known to be prognostic factors for MTC.[3]
The tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) is based on the anatomical and structural extent of thyroid cancer and has a good correlation with disease-related mortality. However, the TNM staging system has static risk estimates that do not change over time as clinical data accumulate. Besides, the TNM staging system does not predict the risk of persistent or recurrent disease because it does not include important prognostic factors such as age and biochemical markers. Dynamic risk stratification (DRS) has been found to be more valuable than the static, anatomical TNM staging system for nonmedullary thyroid cancer (NMTC), and risk-adapted management stratified by response to initial therapy has been recommended.[4] This strategy has also been accepted for MTC. Biochemical markers such as serum calcitonin and carcinoembryonic antigen (CEA) are important for MTC prognosis. The 2015 revised American Thyroid Association (ATA) guidelines recommend that clinicians should consider TNM classification and postoperative serum calcitonin level when predicting outcomes and planning long-term follow-up for patients treated by thyroidectomy for MTC.[5] Several studies found that DRS predicts risk of biochemical and structural persistent or recurrent disease as well as disease-related mortality from MTC.[3,6]
In this study, we evaluated and compared the clinical usefulness of stratification by response to initial therapy with a traditional anatomical TNM staging system for MTC treated at Yonsei University Health System.
2. Materials and methods
2.1. Study population
The medical records of patients diagnosed with MTC from August 1982 to December 2012 were reviewed retrospectively. A total of 144 patients with MTC underwent thyroidectomy in the Yonsei University Heath System. Among these patients, 27 were not followed continuously and had incomplete medical records. Thus, 117 patients were reviewed in this study. All patients had conventional open operations. Mean follow-up duration was 85.78 ± 62.51 months. This study was approved by the local institutional review board.
2.2. Outcome assessment
To evaluate clinicopathological features, clinical and pathological parameters of multi-centricity, bi-laterality, extrathyroidal extension, central LN metastasis, lateral neck LN metastasis, TNM staging, and response to initial therapy were analyzed. We used 7th edition AJCC TNM staging system. In 2016, 8th edition AJCC TNM staging system was published. However, there is no significant change in 8th edition compared with 7th edition.
To evaluate surgical outcomes, all patients underwent regular follow-up ultrasonography (US) in first 6 to 12 months after initial surgery, followed by annual examination after 3 years. Serum calcitonin and CEA were also checked in the first 6 to 12 months after initial surgery, followed by annual examination after 3 years. Patients with evidence of recurrence or distant metastasis were examined using other imaging modalities, such as neck computed tomography (CT) and/or positron emission tomography-CT (PET-CT). Regional neck node and remaining thyroid recurrence were confirmed by US-guided fine needle aspiration biopsy.
We used 3 clinical results for response to initial therapy: excellent, biochemically incomplete, and structurally incomplete response. We used 5 clinical results for final outcomes: no evidence of disease (NED), biochemically persistent, structurally persistent, biochemically recurrent, and structurally recurrent. Response to initial therapy was evaluated during the first 6 to 12 months after initial operation.
Serum calcitonin was measured using immunoradiometric assays (CT-USA-IRMA; Biosource, Nivelles, Belgium) from 1994 to 2009, when these tests were replaced with chemiluminescence DPC immunoassays (Immulite 2000; Siemens, Gwynedd, UK). Normal range was 0 to 10 pg/mL. Serum CEA measurement was by immunoassays (Unicel DxI 800; Beckman Coulter, Pasadena, USA). Normal range was 0 to 5 ng/mL.
Disease-specific survival (DSS) was defined as the time from date of initial surgery to the date of death from thyroid cancer or last follow-up. Disease-free survival (DFS) was defined as the time from date of initial surgery to the date of detection of first recurrence. These factors were analyzed to evaluate prognostic results.
2.3. Definition of surgical outcomes
For response to initial therapy, excellent response was defined as no structural evidence of disease and normalized serum calcitonin and CEA levels after initial operation. Biochemically incomplete response was defined as no structural evidence of disease after initial operation, but serum calcitonin and CEA levels higher than normal range. Structurally incomplete response was defined as structural evidence of disease after initial operation.
We defined NED as undetectable calcitonin and CEA with no structural evidence of disease.[4] Biochemically persistent disease was defined as detectable calcitonin and CEA with no structural evidence of disease. Structurally persistent disease was defined as structural evidence of disease regardless of biochemical markers.[4] Patients with newly developed biochemical or structural evidence of disease after NED were classified as having biochemically or structurally recurrent disease.[4]
2.4. Statistical analysis
We used SPSS software version 20.0 (IBM Inc., Chicago, IL) and R package version 3.1.3 for all statistical analysis. Rates and percentages were calculated for categorical data and medians were calculated for continuous data. DSS and DFS were estimated using Kaplan–Meier survival curves according to the AJCC TNM staging system and DRS and compared by log-rank test with SPSS software. Survival estimates and 95% confidence intervals (CIs) were recorded for 5, 10, 15, 20, and 25 years of follow-up. Relative importance for predicting the likelihood of NED between TNM staging and DRS was confirmed by calculating the proportion of variance explained (PVE) with R package. PVE was calculated using the maximum likelihood ratio (G2) of the Cox model (PVE = 1- exp [-G2/n]). Higher PVE values suggested more accurate prediction of NED.[7,8] We also evaluated the discriminative ability of TNM staging and DRS using integrated areas under the curves (IAUCs) with R package. IAUCs were compared using results of time-dependent receiver operating characteristic (ROC) curves at selected time-points. The statistical test for IAUCs was a Wilcoxon rank sum test for dependent samples. Higher IAUC suggested more accurate risk stratification. P values less than .05 were considered statistically significant.
3. Results
3.1. Clinicopathological characteristics of patients with MTC
Patient clinicopathological characteristics for MTC are summarized in Table 1. Of patients with MTC, a higher proportion was female (68.4% vs 31.6%). Mean tumor size was 1.94 ± 1.40 cm and mean age was 47.98 ± 15.11 years. Hereditary MTC was seen in 22 patients (18.9%). Frequencies were multi-centricity 30.7%, bi-laterality 5.2%, extrathyroidal invasion 35.0%, central LN metastasis 34.1%, and lateral neck LN metastasis 25.6%. Of a total of 117 patients, 115 (98.2%) underwent bilateral total thyroidectomy with 38 (32.4%) undergoing bilateral total thyroidectomy with ipsilateral modified radical neck dissection; 22 with therapeutic purpose and 16 with prophylactic purpose and 16 (13.6%) undergoing bilateral total thyroidectomy with bilateral modified radical neck dissection; 5 with therapeutic purpose and 11 with prophylactic purpose. Although there was no clue of lateral neck LN metastasis in preoperative image, we performed prophylactic lateral neck dissections in patients with MTC in our institution until 2008. Since 2008, lateral neck dissections in patients with MTC have been performed only for therapeutic purpose. In addition, 2 (1.8%) patients underwent less than total thyroidectomy. One patient had undergone thyroid lobectomy for benign nodules previously, followed by completion total thyroidectomy to treat MTC a few years later. The other patient performed thyroid lobectomy for papillary thyroid micro-carcinoma and concurrently pathologically proven occult MTC. We considered completion total thyroidectomy for this patient, but eventually we planned to do no additional surgery with the consent of patient.
Table 1.
Clinicopathological characteristics of patients with medullary thyroid carcinoma (1).
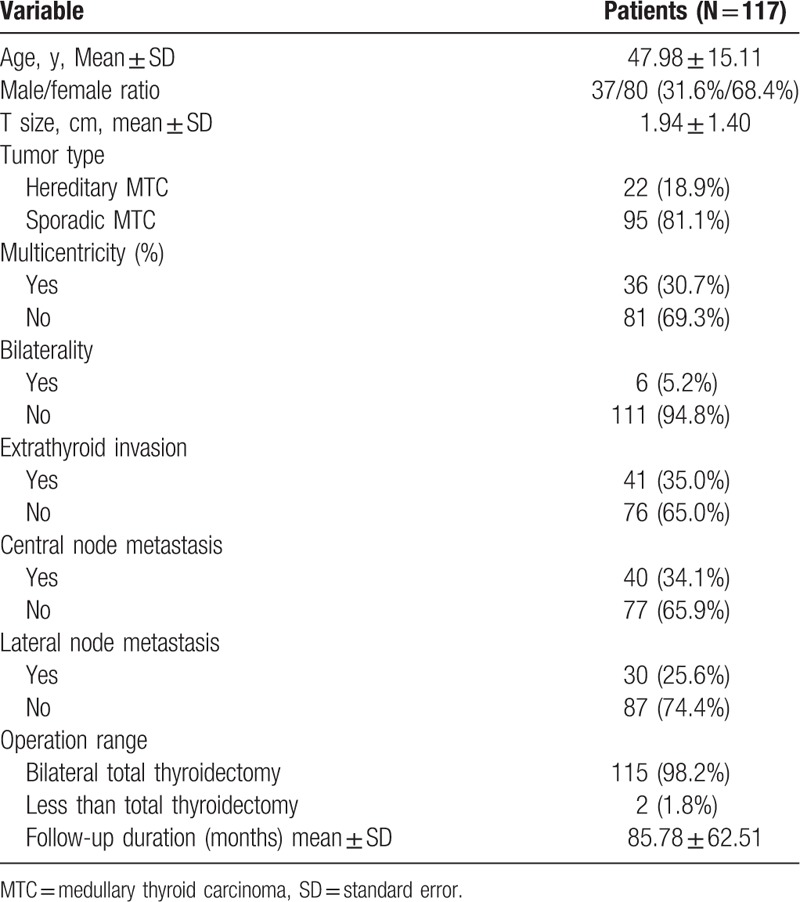
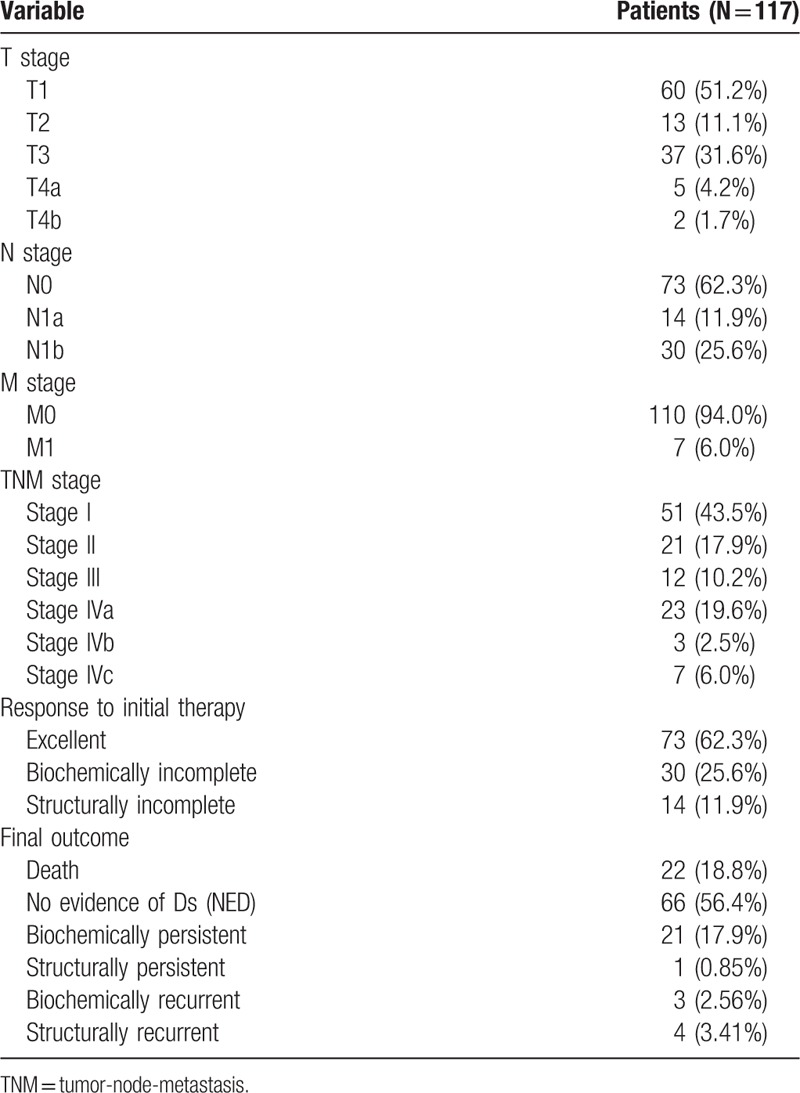
For TNM staging of the MTC patients, 7 (6.0%) had distant metastasis. Among 7 patients with distant metastasis, 2 had lung metastasis and 5 had multiple metastases in lung and bone. All patients died because of disease progression. Staging was stage I 43.5%; stage II, 17.9%; stage III, 10.2%; stage IVa, 19.6%; stage IVb, 2.5%; and stage IVc, 6.0%. Of 117 patients, 73 (62.3%) were classified in the excellent group, 30 (25.6%) were in the biochemically incomplete response group, and 14 (11.9%) were in the structurally incomplete response group. In final outcomes, 66 (56.4%) patients were classified in the NED group, 21 (17.9%) in the biochemically persistent group, and 1 (0.85%) in the structurally persistent group; 3 (2.56%) patients were in the biochemically recurrent group and 4 (3.41%) in the structurally recurrent group, with 22 (18.8%) patients who died, with 15 disease-specific deaths (Table 2).
Table 2.
Clinicopathological characteristics of patients with medullary thyroid carcinoma (2).
3.2. Clinical outcomes according to the AJCC TNM staging system
The AJCC TNM staging system predicted the clinical outcomes of MTC patients. Final outcomes according to the staging system are summarized in Table 3. Of the 15 patients who died from their disease, 12 (80%) had stage IV disease. Classified with NED was 90.1% of stage I patients, 41.6% of stage III patients, and 6.1% of stage IV patients. In addition, 3 (5.8%) patients with stage I and 2 (9.5%) patients with stage II disease were classified in biochemically persistent. All patients classified with structural recurrence were stage IV. Cumulative survivals of MTC patients according to the AJCC TNM staging system showed significant differences in survival. We excluded patients with biochemically/structurally persistent disease in DFS analysis. With higher AJCC TNM staging grades, DFS (P < .001, Fig. 1A) and DSS (P < .001, Fig. 1B) were lower.
Table 3.
Clinical outcomes according to the AJCC TNM Staging System.
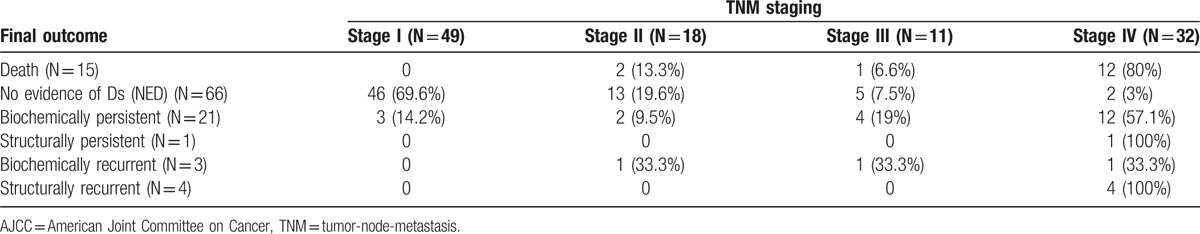
Figure 1.
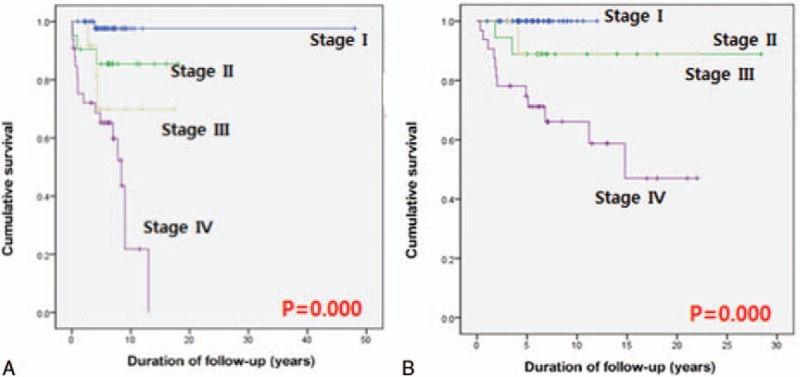
(A) DFS stratified by TNM staging system. The 5-year DFS rates were 100% with stage I, 90% with stage II, 90.5% with stage III, and 63.4% with stage IV disease. The ten-year DFS rates were 98.2% with stage I, 85.7% with stage II, 71.3% with stage III, and 20.5% with stage IV disease. (B) DSS stratified by TNM staging system. The 5-year DSS rates were 100% with stage I, 90.5% with stage II, 90.3% with stage III, and 72.5% with stage IV disease. The 10-year DSS rates were 100% with stage I, 90.5% with stage II, 90.3% with stage III, and 65.5% with stage IV disease.
3.3. Clinical outcomes according to response to initial therapy
A DRS system based on response to initial therapy predicted clinical outcomes of MTC patients. Final outcomes according to the DRS system are summarized in Table 4. MTC patients with an excellent response had the highest likelihood of NED (89%). One patient with a biochemically incomplete response was classified with NED and no patients with structurally incomplete response were categorized with NED. A total of 30 patients had a biochemically incomplete response, 4 (13%) had structurally recurrent disease; 2 patients had lateral neck recurrences and we considered surgical treatments for these patients; 1 patient had 1 lateral neck recurrence and we performed ethanol injection for this patient and there was no progression of disease until now; 1 patient had multiple recurrences in lung and bone and this patient had chemotherapy and 19 (63%) had biochemically persistent disease. In the excellent response group, 3 (4.1%) patients had biochemically recurrent disease and none had structurally recurrent disease at the end of follow-up. No patients in the excellent response group died from MTC. Two (14.2%) patients in the structurally incomplete response group had biochemically persistent disease after reoperation. Analysis of cumulative survival of MTC patients according to the DRS system showed significant differences in survival. We excluded patients with biochemically/structurally persistent disease in DFS analysis. With higher DRS grades, DFS (P < .001, Fig. 2A) and DSS (P < .001, Fig. 2B) were lower.
Table 4.
Clinical outcomes according to response to initial therapy.
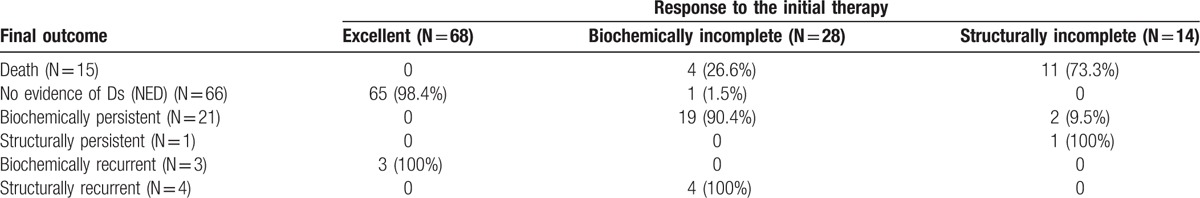
Figure 2.
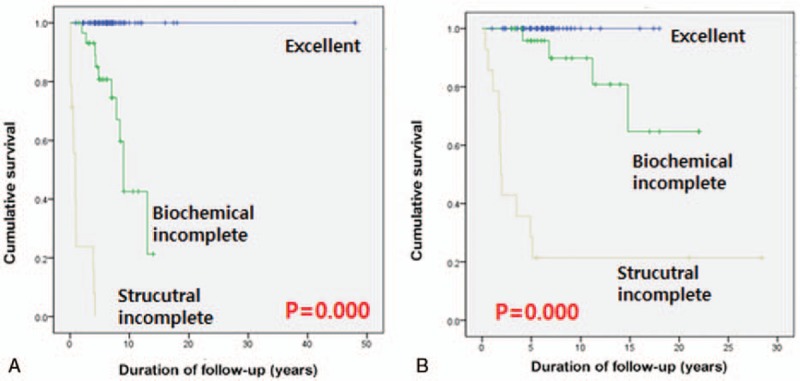
(A) DFS stratified by response to therapy. The 5-year DFS rates were 100% with excellent group, 80.3% with biochemically incomplete group and 0% with structurally incomplete group. The 10-year DFS rates were 100% with excellent group, 42.3% with biochemically incomplete group, and 0% with structurally incomplete group. (B) DSS stratified by response to therapy. The 5-year DSS rates were 100% with excellent group, 95.1% with biochemically incomplete group, and 20% with structurally incomplete group. The 10-year DSS rates were 100% with excellent group, 90.3% with biochemically incomplete group, and 20% with structurally incomplete group.
3.4. Correlation between AJCC TNM staging and DRS
The AJCC TNM staging system demonstrated good correlation with the DRS based on response to the initial therapy (Table 5). Most patients with stage I (92.1%) or stage II (76.1%) disease were also categorized in the excellent response group within 1 year after initial therapy with 50% of patients with stage III and 12% with stage IV MTC categorized in the excellent response group. The biochemically incomplete response group was classified as 5% with stage I, 9% with stage II, 50% with stage III, and 57% with stage I IV disease.
Table 5.
Correlation between AJCC TNM Staging System and DRS.

3.5. Predictive ability with AJCC TNM staging and DRS
To evaluate the relative importance of predicting the likelihood of NED, PVEs were determined for both the TNM staging and the DRS system based on response to initial therapy. PVE was 57.7% for the DRS system, which was higher than the TNM staging system (25.8%). We also calculated IAUCs to evaluate discrimination of the TNM staging and DRS systems based on response to the initial therapy. IAUC was 0.661 (95% CI 0.613–0.701) for the DRS system, which was higher than the TNM staging system (0.615; 95% CI 0.583–0.671) (P = .0012, Fig. 3).
Figure 3.
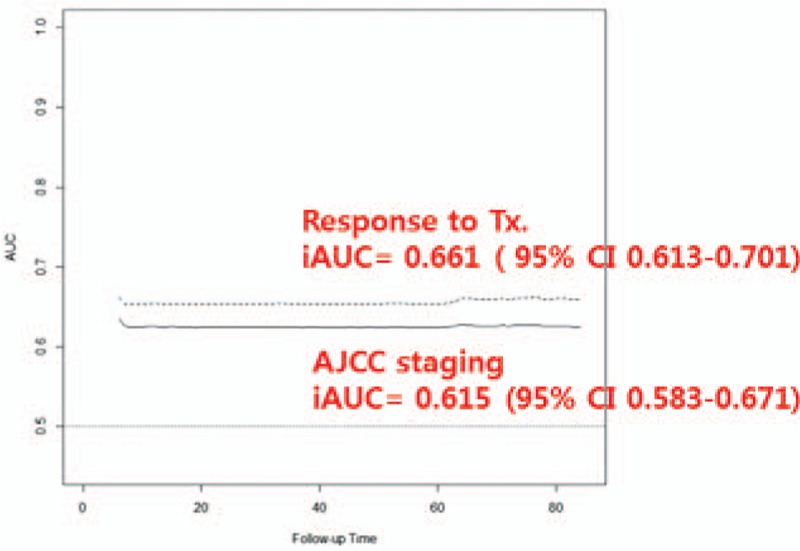
Time-dependent IAUC. IAUC was 0.661 (95% CI 0.613–0.701) for the DRS system based on response to the initial therapy, which was higher than the TNM staging system (0.615; 95% CI 0.583–0.671).
4. Discussion
A DRS system based on response to initial therapy gave more useful clinical prognostic information and was more valuable than the static, anatomical TNM staging system for NMTC. Recently, DRS has been accepted for MTC.[3,4,6,9,10] We evaluated the clinical importance of DRS for MTC patients at our single institution. Both the DRS and TNM staging systems predicted clinical outcomes of MTC patients. The DRS system demonstrated a good correlation with the TNM staging system. However, the DRS system was a more valuable predictor of NED than the TNM system because of its higher PVE values (57.7% vs 25.8%, respectively). The DRS system was also a more accurate predictive system than the TNM system because of its higher IAUC values (0.661; 95% CI 0.613–0.701 vs 0.615; 95% CI 0.583–0.671, respectively).
Although clinical data for MTC patients change after initial therapy, the TNM staging system does not change over time.[3,4,6,10] In our study, 50% of patients with stage III disease and 12% of patients with stage IV MTC were categorized in the excellent response group after initial therapy (Table 5). This finding demonstrated that MTC patients with stage III and IV disease at initial diagnosis could have an excellent response to initial therapy and good clinical outcomes. In the group with excellent response to initial therapy, no patients died from their disease.
The TNM staging system is an independent prognostic factor for DSS.[11,12] According to a previous study, 10-year DSS is 100% for stage I and II disease, 66% for stage III, and 48% for stage IV.[12] In our study, DSS according to TNM staging was significantly different (P < .001, Fig. 1B). Similarly, DSS according to the DRS system was significantly different (P < .001, Fig. 2B). However, mortality rates according to TNM staging were 9.5% for stage II disease, 8.3% for stage III, and 36.3% for stage IV (Table 3). Mortality rates according to the DRS system were 13.3% in the biochemically incomplete response group and 78.5% in the structurally incomplete response group (Table 4). These findings suggested that the DRS system might be more valuable for predicting mortality than TNM staging. Also, we should always consider the possibility of high mortality rates with biochemically incomplete response after initial therapy in MTC patients.
Postoperative serum calcitonin levels are thought to be important prognostic factors in MTC patients.[5,13–15] However, the TNM staging system does not include postoperative serum calcitonin levels. A previous study found that 35% of MTC patients with normal postoperative serum calcitonin showed a 3-year DFS of 94% and 5-year DFS of 90%.[15] In our study, of 30 patients with biochemically incomplete response, 4 (13.3%) patients (stage II, 2; stage III, 1; stage IV, 1) died from their disease and 4 (13.3%) had structurally recurrent disease (Tables 3 and 4). This finding suggested that patients with biochemically incomplete response should receive a careful and strict follow-up for structural evidence of disease.
Previous studies reported that the TNM staging system does not adequately predict risk of recurrent disease in MTC patients.[4,6] In contrast to previous studies, our study showed that DFS according to both the TNM staging and DRS system were significantly different (P < .001, Figs. 1A and 2A). However, with the DRS system, all but 3 patients in the excellent response group reached NED. This finding suggested that the DRS system might be more valuable for predicting recurrent disease than TNM staging. In the excellent response group, 3 patients had biochemically recurrent disease. On the basis of this finding, we might consider the possibility of recurrence for MTC patients with an excellent response after initial therapy rather than for NMTC patients.
In our study, 65 (89%) patients in the excellent response group reached NED and 3 (4.1%) had biochemically recurrent disease. However, 11 (78.5%) patients died from their disease and no patients reached NED in the structurally incomplete response group. One patient in the structurally incomplete response group still had structurally persistent disease at final outcomes (Table 4). Kebebew et al[16] reported that reoperation for MTC patients with grossly residual cancer rarely reach biochemical cure, even if surgery limits progression of disease in some patients. In our data, 2 (14.2%) patients in the structurally incomplete response group had biochemically persistent disease as a result of reoperation, consistent with a previous study. These findings demonstrated that complete surgical resection (R0 resection) at initial therapy was important for survival of MTC patients.
In our study, only 1 patient with biochemically incomplete response reached NED. This patient did not have increased calcitonin levels, but increased CEA levels at the first evaluation after initial treatment. This patient was classified in the biochemically incomplete response group. He eventually reached NED at final outcome with a normalized CEA level. We propose that the increased CEA resulted from other causes such as smoking or infection, in this case.
This study had limitations with the retrospective study and small numbers of patients. Preoperative serum calcitonin and germline RET status were not considered, but we plan to conduct further studies on the associations between prognosis of MTC, gene mutation status, and calcitonin doubling time. Selection bias was the main limitation of this study because our single institution performed both initial therapy and follow-up.
In conclusion, we demonstrated that DRS based on response to the initial therapy offered more useful prognostic information than an anatomical staging system for MTC in this study.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, ATA = American Thyroid Association, CEA = carcinoembryonic antigen, CI = confidence interval, DFS = disease-free survival, DRS = Dynamic Risk Stratification, DSS = disease-specific survival, IAUC = Integrated Areas Under the Curves, LN = lymph node, MTC = medullary thyroid carcinoma, NED = no evidence of disease, NMTC = nonmedullary thyroid carcinoma, PVE = proportion of variance explained, ROC = receiver operating characteristic, TNM = tumor-node-metastasis, US = ultrasonography.
The authors report no conflicts of interest.
References
- [1].Jung KY, Kim SM, Yoo WS, et al. Postoperative biochemical remission of serum calcitonin is the best predictive factor for recurrence-free survival of medullary thyroid cancer: a large-scale retrospective analysis over 30 years. Clin Endocrinol 2016;84:587–97. [DOI] [PubMed] [Google Scholar]
- [2].De Crea C, Raffaelli M, Milano V, et al. Intraoperative high-dose calcium stimulation test in patients with sporadic medullary thyroid carcinoma is highly accurate in predicting lateral neck metastases. Surgery 2016;159:70–6. [DOI] [PubMed] [Google Scholar]
- [3].Kwon H, Kim WG, Jeon MJ, et al. Dynamic risk stratification for medullary thyroid cancer according to the response to initial therapy. Endocrine 2016;53:174–81. [DOI] [PubMed] [Google Scholar]
- [4].Lindsey SC, Ganly I, Palmer F, et al. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid 2015;25:242–9. [DOI] [PubMed] [Google Scholar]
- [5].Wells SA, Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tuttle RM, Ganly I. Risk stratification in medullary thyroid cancer: moving beyond static anatomic staging. Oral Oncol 2013;49:695–701. [DOI] [PubMed] [Google Scholar]
- [7].Jeon MJ, Kim WG, Park WR, et al. Modified dynamic risk stratification for predicting recurrence using the response to initial therapy in patients with differentiated thyroid carcinoma. Eur J Endocrinol 2014;170:23–30. [DOI] [PubMed] [Google Scholar]
- [8].Brierley JD, Panzarella T, Tsang RW, et al. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer 1997;79:2414–23. [PubMed] [Google Scholar]
- [9].Yang JH, Lindsey SC, Camacho CP, et al. Integration of a postoperative calcitonin measurement into an anatomical staging system improves initial risk stratification in medullary thyroid cancer. Clin Endocrinol 2015;83:938–42. [DOI] [PubMed] [Google Scholar]
- [10].Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2655–63. [DOI] [PubMed] [Google Scholar]
- [11].Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139–48. [DOI] [PubMed] [Google Scholar]
- [12].Pelizzo MR, Boschin IM, Bernante P, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol 2007;33:493–7. [DOI] [PubMed] [Google Scholar]
- [13].Elisei R, Pinchera A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol 2012;8:466–75. [DOI] [PubMed] [Google Scholar]
- [14].Barbot N, Calmettes C, Schuffenecker I, et al. Pentagastrin stimulation test and early diagnosis of medullary thyroid carcinoma using an immunoradiometric assay of calcitonin: comparison with genetic screening in hereditary medullary thyroid carcinoma. J Clin Endocrinol Metab 1994;78:114–20. [DOI] [PubMed] [Google Scholar]
- [15].Pellegriti G, Leboulleux S, Baudin E, et al. Long-term outcome of medullary thyroid carcinoma in patients with normal postoperative medical imaging. Br J Cancer 2003;88:1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kebebew E, Kikuchi S, Duh QY, et al. Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg (Chicago, Ill: 1960) 2000;135:895–901. [DOI] [PubMed] [Google Scholar]


