Abstract
Lymph node metastasis plays a crucial role in predicting prognosis in advanced gastric cancer (AGC). In the present study, we formulated a fibrosis ratio (FR), defined as the number of metastatic lymph nodes with fibrosis divided by the total number of lymph nodes, and sought to determine whether it can be used to predict the prognosis of patients with AGC and improve on existing node staging. We retrospectively analyzed 161 patients who underwent curative resection for node-positive AGC between 2001 and 2010, evaluating the association between FR, lymph node ratio (LNR), and micrometastasis, and the relationship between FR and clinicopathologic findings, overall survival (OS) and disease-free survival (DFS). A high FR was significantly related to T stage (P < .001), N stage (P < .001), tumor stage (P < .001), lymphatic invasion (P < .001), and venous invasion (P = .007). FR was significantly correlated with an increased number of metastatic lymph nodes (P = .001, R = 0.869) and LNR (P = .001, R = 0.943), but not with total harvested lymph nodes. Patients with micrometastases had a lower FR, compared with those without micrometastases (P < .001). A survival analysis showed poor OS for patients in the entire cohort (P < .001); N1 (P = .002), N2 (P = .004), N3a (P = .010), and N3b (P = .003) stages; and groups with high LNR (P = .013) and low LNR (P = .001). DFS was also poor for the entire cohort (P < .001) and the N2 (P = .013), N3b (P = .002), high-LNR (P = .036), and low-LNR (P = .001) groups, but not the N1 or N3a group. Univariate and multivariate analyses revealed that high FR was an independent prognostic factor for OS (hazard ratio [HR], 2.780; CI, 1.655–4.670; P < .001) and DFS (HR, 2.051; CI, 1.199–3.508; P = .009) in AGC. Collectively, our findings indicate that high FR is associated with adverse clinicopathologic parameters in AGC, clearly establishing nodal fibrosis as a pathological finding with value in predicting poor prognosis of patients with AGC. Thus, combining current N stage and LNR diagnostics with FR could improve prognostic prediction in AGC.
Keywords: fibrosis, gastric cancer, metastatic lymph node, tumor stroma
1. Introduction
Gastric cancer (GC) is one of the most common cancers and the 3rd most common cause of cancer-related mortality.[1] Despite advancements in surgical and oncologic therapies, the prognosis of patients with advanced GC (AGC) is poor, with overall 5-year survival remaining at 14% to 25%.[2] Currently, prognosis prediction and determination of therapeutic plans for patients with GC are dependent on a staging system. The American Joint Committee on Cancer (AJCC) TNM system, which stratifies patients based on the depth of invasion of the primary tumor, number of regional lymph nodes with metastasis, and distant metastasis,[3–5] is widely used in clinical practice. Of these components, nodal metastasis has been demonstrated to be powerful in predicting prognosis, especially in patients with resected GC.[6–8] However, debates persist about whether simply determining the number of positive lymph nodes conveys sufficient information about metastatic lymph nodes.[9,10] The lymph node ratio (LNR), defined as the number of positive lymph nodes divided by the number of lymph nodes examined, is an improved metric designed to supplant the present N status that current method for determining N status. Compared that has been shown to be more effective in predicting the prognosis of patients.[11–13]
The effectiveness of N status evaluation is dependent on the thoroughness of the pathological examination used to assess metastatic lymph nodes. Considering the various histopathologic features manifested by metastatic lymph nodes, simply evaluating the absence or presence of tumor cells in a metastatic lymph node may be insufficient for proper stratification of patients with GC. However, these various histopathologic features, which could reflect the tumor microenvironment of metastatic lymph nodes, are not included in current assessments of N status.
The tumor microenvironment consists of tumor cells, immune cells, fibroblasts, and vessels.[14,15] Among these, fibroblasts adjacent to tumor cells – so-called “cancer-associated fibroblasts” – produce fibrosis, which is known to be a predictor of poor prognosis in various malignant tumors.[16–18] Metastatic lymph nodes also create a tumor microenvironment that contributes to tumor progression.[15] Among the factors apart from tumor cells that make up the microenvironment of a metastatic lymph node, tumor-associated fibrosis is easily detectable by routine microscopic examination.
In this study, we formulated a metastatic lymph node fibrosis ratio (FR), defined as the number of positive lymph nodes with fibrosis divided by the total number of lymph nodes, and sought to determine whether it can be used to predict the prognosis of patients with GC and improve existing node staging.
2. Materials and methods
2.1. Patients
We consecutively collected a total of 161 patients with node-positive advanced gastric carcinoma from 2006 to 2010 at Soonchunhyang University Cheonan Hospital and analyzed them retrospectively. All patients underwent R0 resection and D2 lymph node dissection, and cases with at least 15 harvested lymph nodes were selected. None of the patients had received neoadjuvant chemo- or radiotherapy. Clinical data, including age, sex, and follow-up dates, were obtained from electronic medical records. Tumor stage was reevaluated according to the 2010 TMN classification system (AJCC staging manual, 7th edition). Briefly, T stage was classified as T1, mucosal invasion; T2, proper muscle invasion; T3, subserosal invasion; or T4, serosal or other organ invasion; and N stage was classified as N0, no metastasis in regional lymph nodes; N1, metastasis in 1 to 2 regional lymph nodes; N2, metastasis in 3 to 6 regional lymph nodes; N3a, metastasis in 7 to 15 regional lymph nodes; or N3b, metastasis in 16 or more regional lymph nodes. The institutional review board of Soonchunhyang Cheonan Hospital approved this study.
2.2. Pathology
The histopathologic features of all cases were reviewed by 2 gastrointestinal pathologists (SAH and JC). All paraffin blocks containing lymph nodes were sectioned as 2 serial and 2 deeper sections, and then stained with hematoxylin and eosin. Immunohistochemical staining for cytokeratin (AE1/AE3) was performed to detect low-volume tumors, as necessary. The diagnosis, pathologic TNM stage, Lauren classification, other histologic findings, and absence or presence of micrometastases, defined as tumor cell clusters measuring 0.2 to 2.0 mm, were reevaluated. LNR was calculated as the number of positive lymph nodes divided by the number of lymph nodes examined. A cut-off value of 0.25 was established for LNRs. The absence or presence of fibrosis in metastatic lymph nodes was evaluated based on the FR, calculated by dividing the number of metastatic lymph nodes with fibrosis by the total number of lymph nodes collected.
2.3. Statistical analysis
A maximally selected rank statistics was used to determine the optimal cut-off value for FR in the entire cohort, and in N1, N2, N3a, N3b, low-LNR, and high-LNR groups using the Maxstat package in R 3.3.1 (R Development Core Team, Vienna, Austria, http://www.R-project.org).[19] Groups were dichotomized into low- and high-FR groups based on the cut-off values for FR determined in each group.
Associations between the FR and clinicopathologic parameters were analyzed using Fisher exact test or the chi-square test, as appropriate. Differences in FR according to nodal stage were analyzed by Kruskal–Wallis and Mann–Whitney U tests, and differences in FR according to the absence or presence of micrometastases were evaluated by Student t test. Correlations between the FR, the number of retrieved lymph nodes, the number of metastatic lymph nodes, and the LNR of metastatic lymph nodes were evaluated using a Pearson correlation test. Overall survival (OS) was defined as the time from the date of surgery to the date of death from any cause. Disease-free survival (DFS) was defined as the time from the date of surgery to the date of first recurrence or disease-free last follow-up date. Survival was calculated using the Kaplan–Meier method, and statistically significant differences were identified using the log-rank test. Univariate and multivariate Cox proportional hazards analyses were conducted to identify independent prognostic factors for survival. Differences were considered to be significant at a P-value < .05.
3. Results
3.1. Clinicopathologic features associated with a high FR in metastatic lymph nodes
Fibrosis associated with metastatic tumor cells was frequently accompanied by a destructive nodal architecture, containing a nodal capsule, sinus, cortex, and medulla (Fig. 1A and B), whereas metastatic tumors without fibrosis appeared to be restricted to the lymphatic channel around the capsule of the lymph node (Fig. 1C and D). The cut-off value for high FR was determined to be 0.08 for the entire cohort, based on patient survival. A high FR was associated with high T stage (P < .001), N stage (P < .001), AJCC stage (P < .001), lymphatic invasion (P < .001), and venous invasion (P = .007) (Table 1). The mean values (mean ± standard deviation) of FRs according to N stage were 0.018 ± 0.041 for N1, 0.087 ± 0.069 for N2, and 0.314 ± 0.208 for N3. As the N stage increased, the mean value of the FR also significantly increased (N1 vs N2, P < .001; N1 vs N3, P < .001; N2 vs N3, P < .001) (Fig. 2). FR was significantly correlated with the number of metastatic lymph nodes (R = 0.869; P = .001) and metastatic LNR (R = 0.943; P < .001) (Fig. 3A and C). However, the total number of lymph nodes was not correlated with FR (Fig. 3B). FR was significantly increased in patients without micrometastases compared with those with micrometastases (P < .001; Fig. 4).
Figure 1.
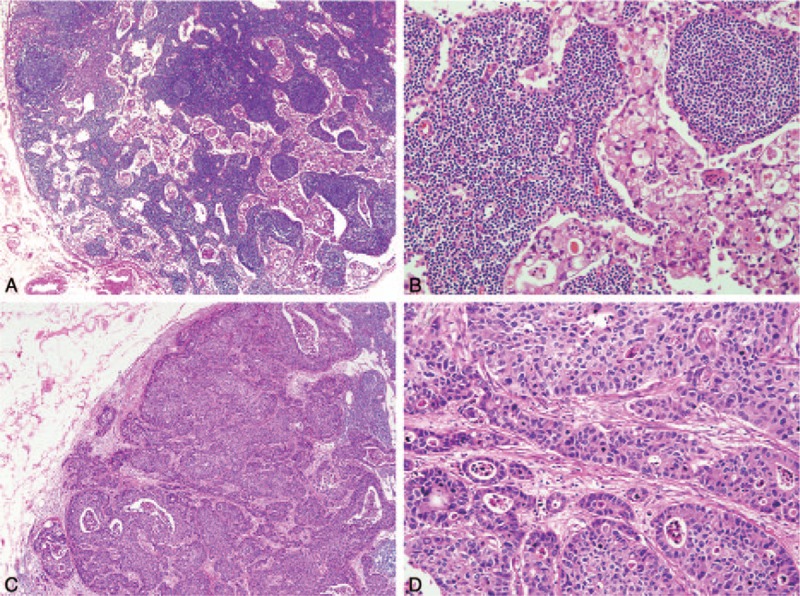
Representative histologic findings in metastatic lymph nodes. (A) Metastatic tumor cells were distributed throughout nodal sinuses. (B) Metastatic tumor cells were not accompanied by fibrosis. (C) Metastatic tumor cells with extensive disruption of nodal structure were observed. (D) Dense fibrosis was found in the tumor stroma.
Table 1.
Correlation between fibrosis ratio and clinicopathologic features in AGC patients.
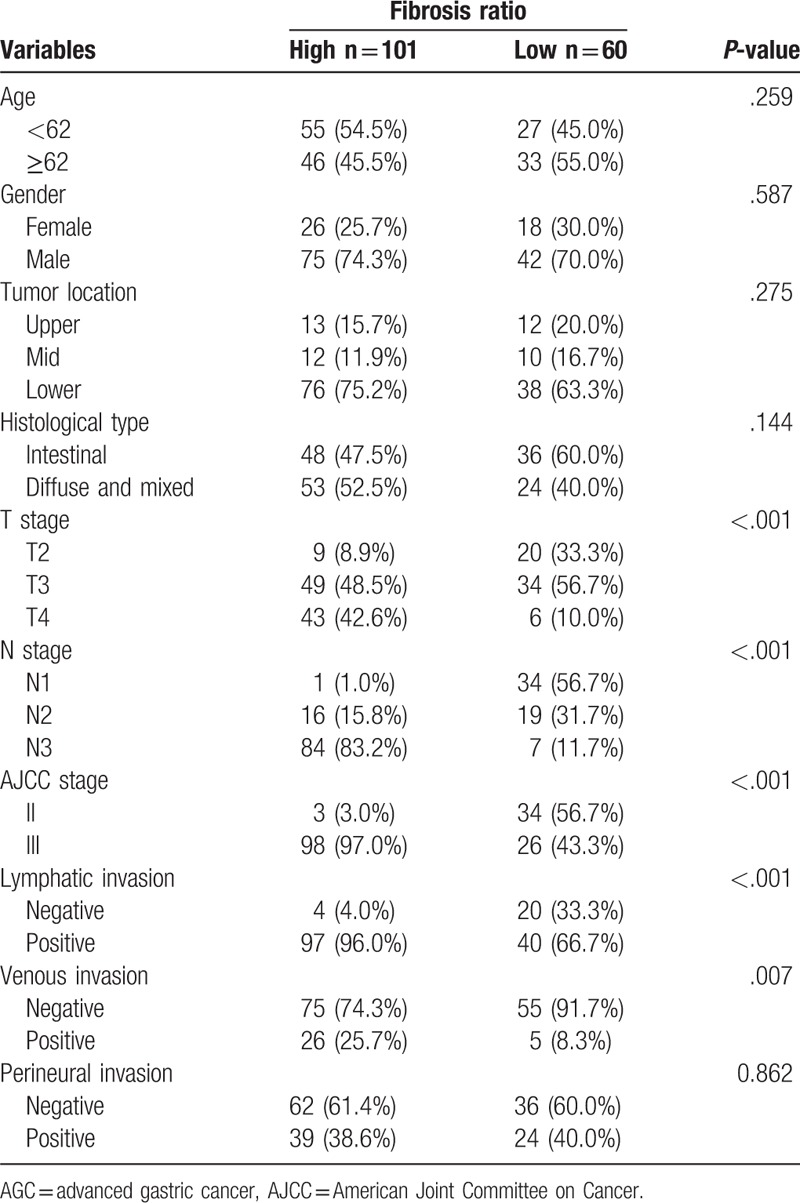
Figure 2.
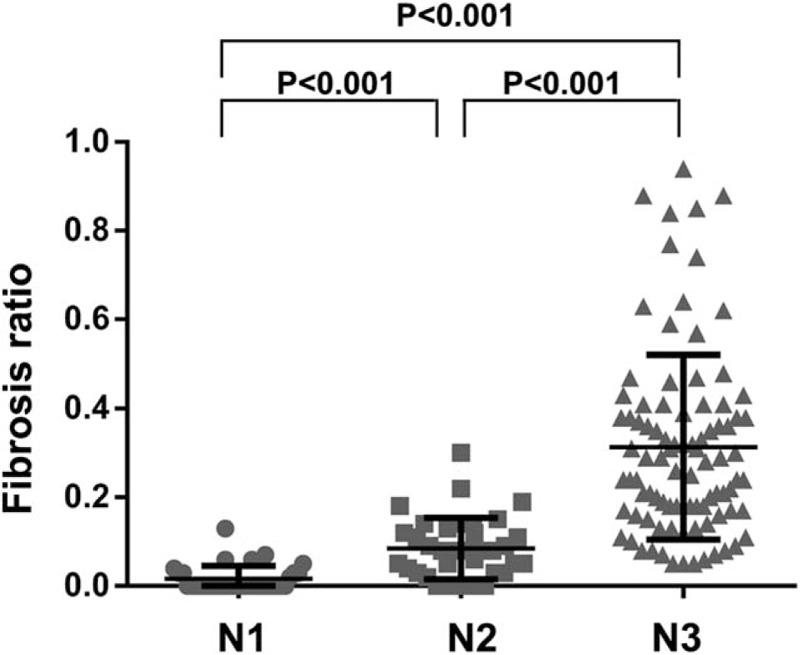
Fibrosis ratio (FR) differed significantly according to N stage.
Figure 3.
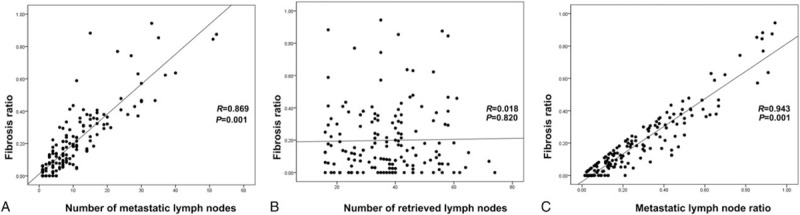
Correlation of FR with the (A) number of metastatic lymph nodes, (B) number of retrieved lymph nodes, and (C) LNR. R, correlation coefficient. FR = fibrosis ratio, LNR = lymph node ratio.
Figure 4.
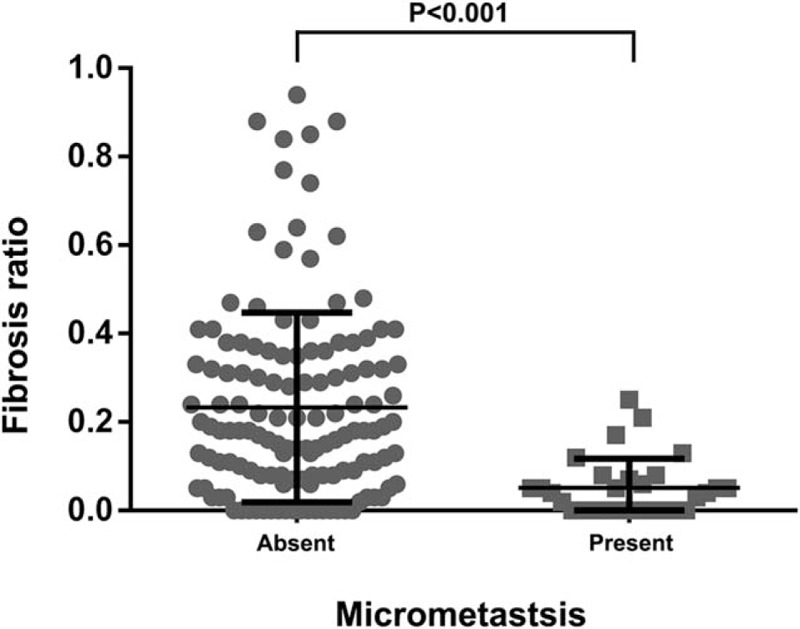
Fibrosis ratio (FR) differed significantly according to micrometastasis status.
3.2. Impact of FR on nodal stage and LNR
Survival curve analyses revealed a significantly shorter OS (P < .001) and DFS (P < .001) in the high-FR group compared with the low-FR group among all patients with AGC (Fig. 4A and B).
To determine whether a high FR could better stratify patients with the same N stage and LNR, we performed survival curve analyses of the respective N groups, and high-LNR (>0.16) and low-LNR (≤0.16) groups. Cut-off values for high FR that showed the best ability to predict patient prognosis were as follows: entire cohort, 0.07; N1, 0.00; N2, 0.05; N3a, 0.08; and N3b, 0.41; low LNR, 0.07; and high LNR, 0.63. These analyses showed that the high-FR group for each N stage had significantly shorter OS (N1, P = .002; N2, P = .004; N3a, P = .010; and N3b, P = .003) (Fig. 5C, E, G, and I). DFS was significantly decreased in high-FR N2 (P = .013) and N3b (P = .002) stage patients, but not in N1 (P = .140) or N3a (P = .069) stage patients (Fig. 5D, F, H, and J).
Figure 5.
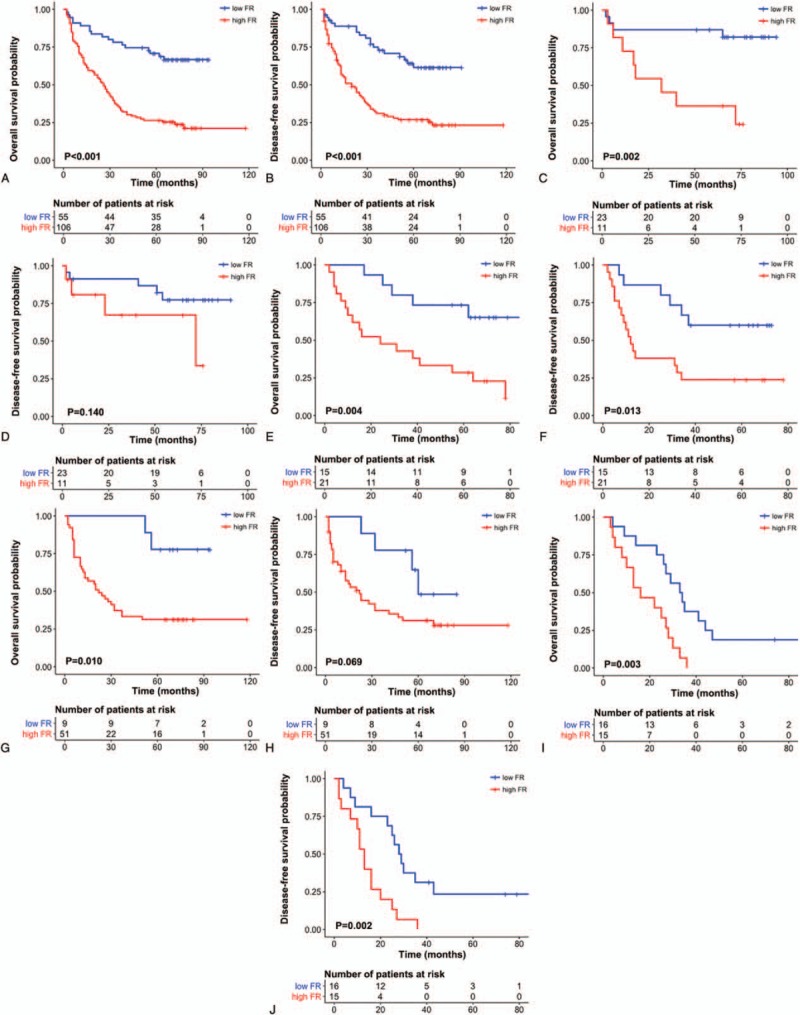
Kaplan–Meier plots illustrating overall survival and disease-free survival probability according to fibrosis ratio (FR) in all patients (A and B) and N1 (C and D), N2 (E and F), N3a (G and H), and N3b (I and J) stage patients.
In the low- and high-LNR groups, patients with high FR showed a significantly shorter OS (low LNR, P = .001; high LNR, P = .013) and DFS (low LNR, P = .001; high LNR, P = .036) compared with those with low FR (Fig. 6A–D).
Figure 6.
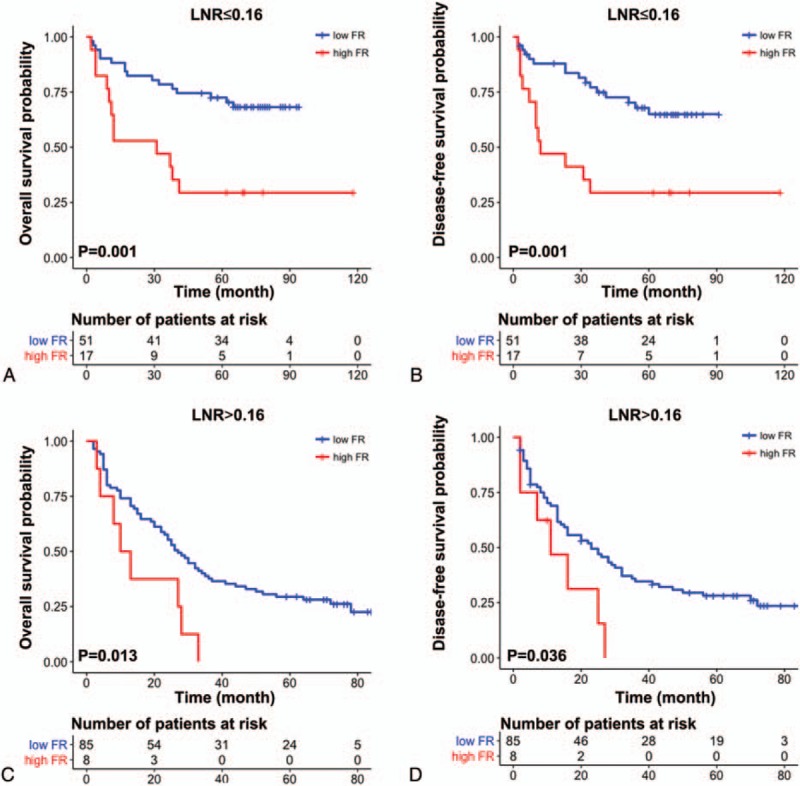
Overall survival and disease-free survival probability according to FR in low LNR (A and B) and high LNR (C and D) patients. FR = fibrosis ratio, LNR = lymph node ratio.
3.3. Univariate and multivariate analyses of factors affecting survival in patients with AGC
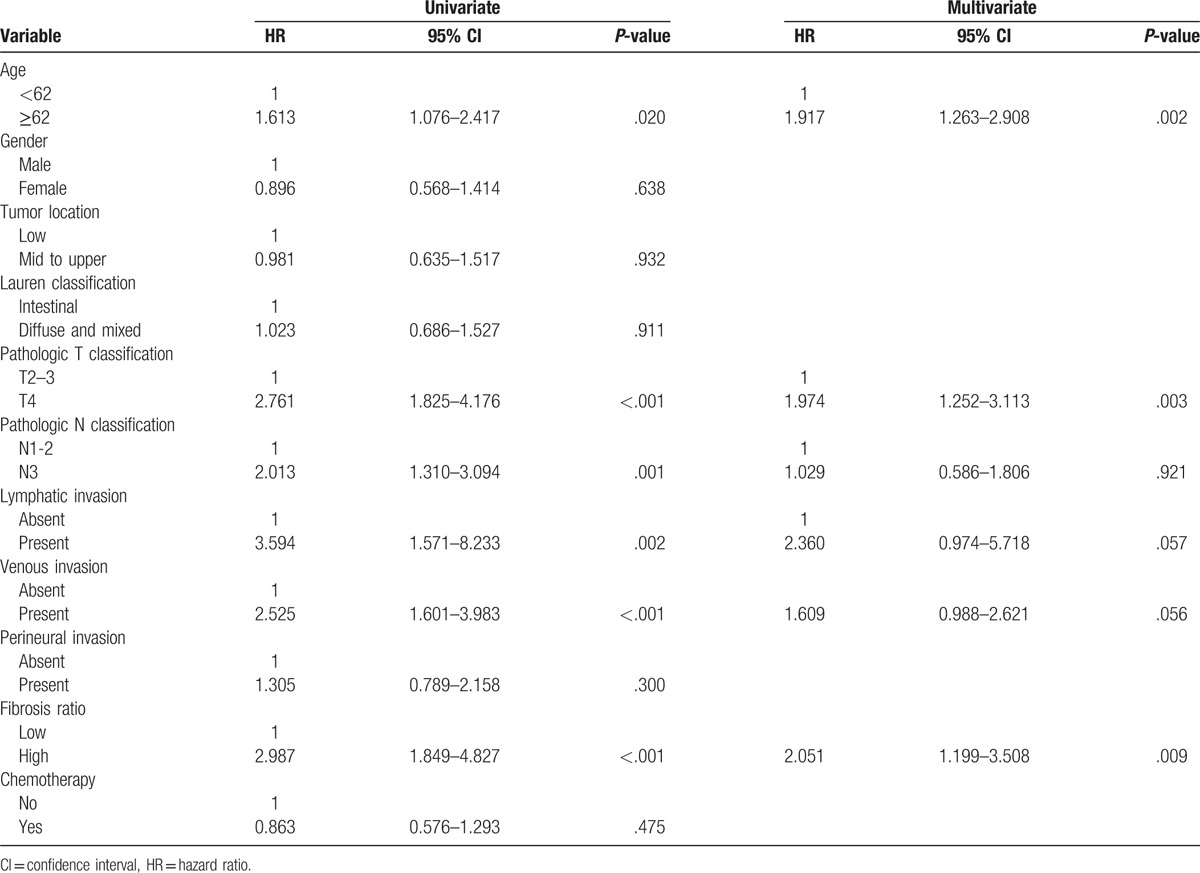
Finally, we conducted univariate and multivariate analyses using a Cox proportional-hazards regression analysis. Univariate analyses showed that adverse prognostic factors for both OS and DFS included age, T stage, N stage, lymphatic invasion, venous invasion, and high FR. In multivariate analyses, high FR was found to be an independent prognostic indicator for both OS (hazard ratio [HR], 2.780; CI, 1.655–4.670, P < .001) (Table 2) and DFS (HR, 2.051; CI, 1.199–3.508, P = .009) (Table 3).
Table 2.
Univariate and multivariate Cox multiple regression analysis for overall survival in patients with advanced gastric carcinoma.
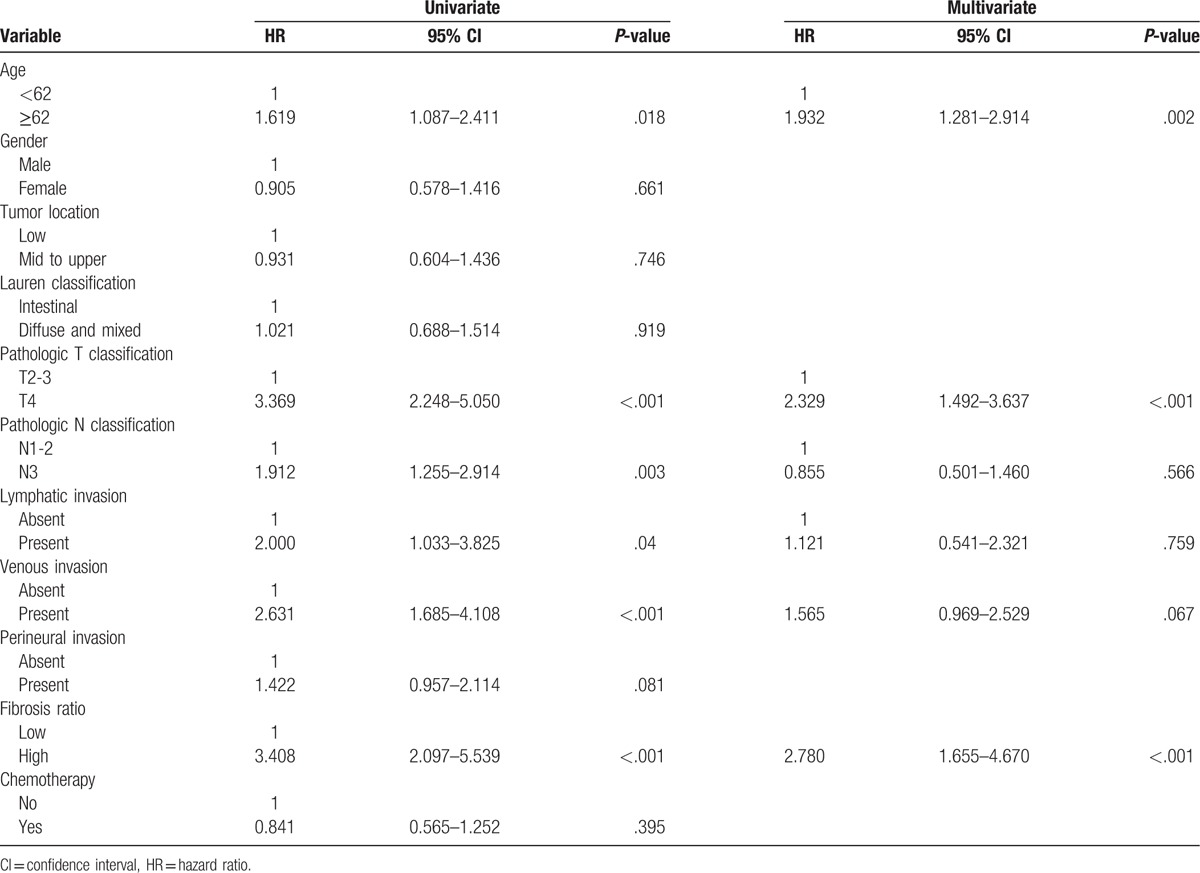
Table 3.
Univariate and multivariate Cox multiple regression analysis for disease-free survival in patients with advanced gastric carcinoma.
4. Discussion
In this study, we first investigated the prognostic relevance of the FR in metastatic lymph nodes. Our results demonstrating an association of high FR with adverse clinicopathologic parameters suggest that a high FR in metastatic lymph nodes of AGC patients is an indicator of tumor progression that has value for predicting survival of AGC patients with node metastasis.
Nodal status in AGC is the most important factor for predicting prognosis and determining a therapeutic plan, especially in surgically resected GC without distant metastasis.[20,21] However, to our knowledge, there is only a single study[22] that emphasized the adverse prognostic impacts of histopathologic characteristics of metastatic lymph nodes, including fibrotic foci and extracapsular invasion, on GC patients with metastasis to a single lymph node. However, histopathologic features of metastatic lymph nodes were not analyzed according to nodal stage in this study.
We found significant differences in FR between N1, N2, and N3 stages (P < .001), showing that, as N stage increased, FR also increased. These findings could indicate that FR is associated with advanced nodal stage. Although it is conceivable that FR may depend on N stage, our data showed that the FR successfully stratified patients at all N stages for OS and at N2 and N3b for DFS. In N3a stage, there were relatively fewer patients with low FR (n = 9, 15.0%), compared with patients in N1, N2, or N3b stage. This could indicate that the benefits of classifying patients according to FR were limited in the case of N3a stage patients. However, despite the small number of cases, we found that patients in the low-FR group showed longer OS than those in the high-FR group (P = .010). These results suggest that dichotomizing patients based on FR could be a novel strategy for refining current N stage according to prognosis in AGC patients.
Compared with that of other N stages, the prognostic effect of N3b is “saturated” because there are no higher stages. Notably, however, we found that high FR predicted shorter survival in N3b stage patients. Moreover, in a multivariate analysis, the prognostic value of N stage was not retained, whereas high FR remained an independent prognostic factor. On the basis of these results, we suggest that FR could replace or be used in conjunction with the present N stage system.
In the N1 group, the best cut-off value for FR of 0.00 indicated that simply the presence or absence of fibrosis in the metastatic lymph node was a powerful parameter. From a practical standpoint, fibrosis could be a conveniently and easily applied pathologic finding that does not require calculation, especially in the N1 group. Therefore, we recommend that the presence or absence of fibrosis be included in pathology reports.
In the present study, FR showed a high correlation with the number of metastatic lymph nodes (P = .001, R = 0.869) and LNR (P = .001, R = 0.943). Previous studies have reported that LNR has a role in the prognosis of GC and in preventing stage migration.[23–25] Although the cut-off values for LNR varied in previous studies, LNR has shown superiority compared with the present N classification. In addition, LNR can be flexibly applied to retrieved lymph nodes, whether less than 15 or more than 16.[12,26,27] In our study, the cut-off value of LNR that showed the best correlation with patient prognosis was 0.16. Our data demonstrated that high FR was associated with shorter survival in both the low-LNR (OS, P < .001; DFS, P < .001) and high-LNR (OS, P = .013; DFS, P = .036) groups. However, using an LNR cut-off value of 0.25, as typically reported in previous studies,[28,29] all patients in the high-LNR group in our study would be categorized as high FR. Thus, the prognostic value of high FR might be limited in high-LNR compared with low-LNR groups. One possible explanation for these findings is that high LNR might be a sufficient survival-related factor on its own, and thus no additional pathologic parameter is needed. On the other hand, we hypothesize that low LNR requires additional pathologic findings, such as FR, which provide additional power in predicting survival in AGC patients.
Previous studies have demonstrated that the number of harvested lymph nodes is related to prognosis in GC.[30,31] However, the utility of this relationship could be limited by the fact that the number of harvested lymph nodes may depend on surgical technique, extent of lymphadenectomy, the relative eagerness of the pathologist to retrieve lymph nodes, the patient's fat volume, and the number of innate lymph nodes.[32] In the present study, the number of harvested lymph nodes was not correlated with FR, indicating that the FR could be independent of the number of harvested lymph nodes if at least 16 lymph nodes are obtained. However, the present staging system and previous studies emphasize that a minimum of 16 retrieved lymph nodes is suitable for staging and preventing stage migration. Thus, determining whether applying FR prevents stage migration related to retrieved lymph nodes in cases of D1 dissection or insufficient number of harvested lymph nodes will require additional studies.
The impact of micrometastases, defined as metastatic tumors with a size between 0.2 and 2 mm, on prognosis in GC has been a matter of controversy.[33] According to Kim et al[34] and Cao et al,[35] micrometastases are associated with a shorter 5-year survival rate and are an independent prognostic factor in multivariate analyses. In contrast, Morgagni et al[36] reported that the presence or absence of micrometastases had no significant impact on 10-year survival rate in pT1N0 stage GC. Similarly, a study by Fukagawa et al[37] concluded that micrometastases have no significant prognostic value in predicting 5- and 10-year OS rates in T2N0 and T3N0 stage GC. However, most studies on micrometastasis in GC have been limited by their focus on the prognostic impact of newly detected micrometastases in N0 and/or early GC cases. In our study, FR was inversely correlated with the presence of micrometastases, indicating that the FR could be an indication of the high burden of the metastatic tumor as well as the number of metastatic lymph nodes and LNR. Based on the association between FR and micrometastases and clinical impact of FR on AGC, we cautiously suggest that micrometastases could have clinical value in AGC distinct from that of macrometastases.
The mechanism underlying the poor prognosis associated with fibrotic metastatic lymph nodes presumably involves the tumor microenvironment.[38] Tumor cells secrete transforming growth factor-9β, platelet-derived growth factor, and fibroblast growth factor, which create a unique tumor stroma.[17] The tumor stroma, variously referred to as a “desmoplastic stroma” or the product of a “desmoplastic reaction”,[39] is characterized by dense fibrosis, which is a hallmark of the proliferation of so-called cancer-associated fibroblasts (CAFs).[40] CAFs are not simply participants in tumor infiltration, they also actively cross-talk with their tumor cell counterparts to promote cancer progression by inducing proliferation of tumor cells, inflammation, blood vessel growth, intratumoral hypoxia, and metastasis in various tumors, including GC.[38,41] Previous studies have reported that CAFs play a role in metastatic lymph nodes analogous to that in the primary tumor in terms of tumor progression and immunophenotype.[42,43] Therefore, the adverse prognostic impact of high FR reported here likely reflects the association of CAFs with tumor progression.
One possible limitation of this study is its nonrandomized, retrospective design. This resulted in small and unevenly distributed sample sizes in the various nodal stages, and meant that a power calculation for the appropriate number of patients could not be performed. As a result, the cut-off values for high FR used in this study may not be absolute. Further large-scale prospective validation studies are required to obtain optimized cut-off values.
In summary, a high FR was associated with adverse clinicopathologic parameters in AGC, indicating that nodal fibrosis is clearly a valuable pathologic finding for predicting poor prognosis in patients with AGC. Thus, combining current N stage and LNR diagnostics with FR could improve prognostic prediction in AGC.
Acknowledgments
The authors thank the Soonchunhyang University Research Fund for the support.
Footnotes
Abbreviations: AGC = advanced gastric cancer, AJCC = American Joint Committee on Cancer, DFS = disease-free survival, FR = fibrosis ratio, GC = gastric cancer, LNR = lymph node ratio, OS = overall survival.
Funding/support: This research was supported by the Soonchunhyang University Research Fund.
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Huang Q, Fang C, Shi J, et al. Differences in clinicopathology of early gastric carcinoma between proximal and distal location in 438 Chinese patients. Sci Rep 2015;5:13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marrelli D, Morgagni P, de Manzoni G, et al. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg 2012;255:486–91. [DOI] [PubMed] [Google Scholar]
- [4].Rausei S, Dionigi G, Boni L. Evaluation of the Seventh American Joint Committee on Cancer/International Union against cancer classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2011;117:2823–4. [DOI] [PubMed] [Google Scholar]
- [5].Washington K. 7th Edition of the AJCC Cancer Staging Manual: stomach. Ann Surg Oncol 2010;17:3077–9. [DOI] [PubMed] [Google Scholar]
- [6].Manfe A, Segalina P, Maffei FA. Prognostic factors in gastric cancer. Our experience and review of the literature. Minerva Chir 2000;55:299–305. [PubMed] [Google Scholar]
- [7].Coburn NG, Swallow CJ, Kiss A, et al. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer 2006;107:2143–51. [DOI] [PubMed] [Google Scholar]
- [8].Deng J-Y, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol 2014;20:3967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Warneke VS, Behrens H-M, Hartmann JT, et al. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol 2011;29:2364–71. [DOI] [PubMed] [Google Scholar]
- [10].Yoon HM, Ryu KW, Nam BH, et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg 2012;214:88–96. [DOI] [PubMed] [Google Scholar]
- [11].Inoue K, Nakane Y, Iiyama H, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol 2002;9:27–34. [DOI] [PubMed] [Google Scholar]
- [12].Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg 2012;255:478–85. [DOI] [PubMed] [Google Scholar]
- [13].Spolverato G, Ejaz A, Kim Y, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg 2015;262:991–8. [DOI] [PubMed] [Google Scholar]
- [14].Whiteside T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008;130:1091–103. [DOI] [PubMed] [Google Scholar]
- [16].Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 2004;432:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401. [DOI] [PubMed] [Google Scholar]
- [18].Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001;411:375. [DOI] [PubMed] [Google Scholar]
- [19].Chan JC, Chan DL, Diakos CI, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg 2017;265:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kwon S, Kim G. Prognostic significance of lymph node metastasis in advanced carcinoma of the stomach. Br J Surg 1996;83:1600–3. [DOI] [PubMed] [Google Scholar]
- [21].Kodera Y, Yamamura Y, Shimizu Y, et al. The number of metastatic lymph nodes: a promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg 1998;187:597–603. [DOI] [PubMed] [Google Scholar]
- [22].Okamoto T, Tsuburaya A, Kameda Y, et al. Prognostic value of extracapsular invasion and fibrotic focus in single lymph node metastasis of gastric cancer. Gastric Cancer 2008;11:160–7. [DOI] [PubMed] [Google Scholar]
- [23].Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007;245:543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol 2003;10:1077–85. [DOI] [PubMed] [Google Scholar]
- [25].Bilici A, Seker M, Ustaalioglu BBO, et al. Determining of metastatic lymph node ratio in patients who underwent D2 dissection for gastric cancer. Med Oncol 2010;27:975–84. [DOI] [PubMed] [Google Scholar]
- [26].Kutlu OC, Watchell M, Dissanaike S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg Oncol 2015;24:84–8. [DOI] [PubMed] [Google Scholar]
- [27].Sun Z, Zhu GL, Lu C, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol 2009;20:897–905. [DOI] [PubMed] [Google Scholar]
- [28].Smith DD, Nelson RA, Schwarz RE. A comparison of five competing lymph node staging schemes in a cohort of resectable gastric cancer patients. Ann Surg Oncol 2014;21:875–82. [DOI] [PubMed] [Google Scholar]
- [29].Kim Y, Squires MH, Poultsides GA, et al. Impact of lymph node ratio in selecting patients with resected gastric cancer for adjuvant therapy. Surgery 2017;162:285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:7114–24. [DOI] [PubMed] [Google Scholar]
- [31].Jiao X-G, Deng J-Y, Zhang R-P, et al. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J Gastroenterol 2014;20:3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Komatsu S, Ichikawa D, Nishimura M, et al. Evaluation of prognostic value and stage migration effect using positive lymph node ratio in gastric cancer. Eur J Surg Oncol 2017;43:203–9. [DOI] [PubMed] [Google Scholar]
- [33].Zeng Y-J, Zhang C-D, Dai D-Q. Impact of lymph node micrometastasis on gastric carcinoma prognosis: a meta-analysis. World J Gastroenterol 2015;21:1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim JH, Park JM, Jung CW, et al. The significances of lymph node micrometastasis and its correlation with E-cadherin expression in pT1-T3N0 gastric adenocarcinoma. J Surg Oncol 2008;97:125–30. [DOI] [PubMed] [Google Scholar]
- [35].Cao L, Hu X, Zhang Y, et al. Adverse prognosis of clustered-cell versus single-cell micrometastases in pN0 early gastric cancer. J Surg Oncol 2011;103:53–6. [DOI] [PubMed] [Google Scholar]
- [36].Morgagni P, Saragoni L, Scarpi E, et al. Lymph node micrometastases in early gastric cancer and their impact on prognosis. World J Surg 2003;27:558–61. [DOI] [PubMed] [Google Scholar]
- [37].Fukagawa T, Sasako M, Mann GB, et al. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer 2001;92:753–60. [DOI] [PubMed] [Google Scholar]
- [38].Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hewitt RE, Powe DG, Ian Carter G, et al. Desmoplasia and its relevance to colorectal tumour invasion. Int J Cancer 1993;53:62–9. [DOI] [PubMed] [Google Scholar]
- [40].Östman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev 2009;19:67–73. [DOI] [PubMed] [Google Scholar]
- [41].Lee D, Ham IH, Son SY, et al. Intratumor stromal proportion predicts aggressive phenotype of gastric signet ring cell carcinomas. Gastric Cancer 2007;20:591–601. [DOI] [PubMed] [Google Scholar]
- [42].LeBedis C, Chen K, Fallavollita L, et al. Peripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-I and EGF. Int J Cancer 2002;100:2–8. [DOI] [PubMed] [Google Scholar]
- [43].Mundim FG, Pasini FS, Nonogaki S, et al. Breast carcinoma–associated fibroblasts share similar biomarker profiles in matched lymph node metastasis. Appl Immunohistochem Mol Morphol 2016;24:712–20. [DOI] [PubMed] [Google Scholar]


