Abstract
Please cite this paper as: bin‐Reza et al. (2012) The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza and Other Respiratory Viruses 6(4), 257–267.
There are limited data on the use of masks and respirators to reduce transmission of influenza. A systematic review was undertaken to help inform pandemic influenza guidance in the United Kingdom. The initial review was performed in November 2009 and updated in June 2010 and January 2011. Inclusion criteria included randomised controlled trials and quasi‐experimental and observational studies of humans published in English with an outcome of laboratory‐confirmed or clinically‐diagnosed influenza and other viral respiratory infections. There were 17 eligible studies. Six of eight randomised controlled trials found no significant differences between control and intervention groups (masks with or without hand hygiene; N95/P2 respirators). One household trial found that mask wearing coupled with hand sanitiser use reduced secondary transmission of upper respiratory infection/influenza‐like illness/laboratory‐confirmed influenza compared with education; hand sanitiser alone resulted in no reduction. One hospital‐based trial found a lower rate of clinical respiratory illness associated with non‐fit‐tested N95 respirator use compared with medical masks. Eight of nine retrospective observational studies found that mask and/or respirator use was independently associated with a reduced risk of severe acute respiratory syndrome (SARS). Findings, however, may not be applicable to influenza and many studies were suboptimal. None of the studies established a conclusive relationship between mask/respirator use and protection against influenza infection. Some evidence suggests that mask use is best undertaken as part of a package of personal protection especially hand hygiene. The effectiveness of masks and respirators is likely linked to early, consistent and correct usage.
Keywords: Influenza, mask, pandemic, respirator
Introduction
Personal protective equipment to help reduce transmission of influenza is generally advised according to the risk of exposure to the influenza virus and the degree of infectivity and human pathogenicity of the virus. The paucity of scientific evidence upon which to base guidance for the use of masks and respirators in healthcare and community settings has been a particularly vexing issue for policymakers.
The Health Protection Agency (HPA) undertook a scientific evidence‐based review of the use of masks and respirators in an influenza pandemic to inform relevant guidance following the emergence of pandemic A (H1N1) 2009 influenza. The Department of Health commissioned the HPA to update the review in support of the revision of the United Kingdom (UK) influenza pandemic preparedness strategy. 1 The review was published on‐line at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_125425.pdf. A further update of the evidence base subsequently was performed in January 2011 and described herein.
Methods
Search strategy
We generally followed the approach detailed in the University of York’s Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. 2
The original search of the PubMed database was conducted on 7 November 2009; subsequent updates of the PubMed database search were undertaken on 23 June 2010 and 12 January 2011. 1 The November 2009 search also included the following scientific databases: Bandolier, the Cochrane Library Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the Health Technology Assessment database, the National Health Service (NHS) Economic Evaluation database, the UK Database of Uncertainties about the Effects of Treatments, the NHS Centre for Reviews and Dissemination and the Cumulative Index to Nursing and Allied Health Literature. 2 No additional publications resulted from these databases. The initial search in November 2009 had no time period restrictions.
A limited effort was made to identify additional studies: reference lists of review articles were examined; the European Centre for Disease Prevention and Control’s (ECDC) Antimicrobial Resistance and Health Care Associated Infection Programme was consulted; and MEC’s and AN’s hardcopy literature files were hand‐searched.
Study selection
We included the following types of studies listed in the hierarchical order of study design quality: randomised controlled trials (i.e. randomised cross‐over trial and cluster randomised trial); quasi‐experimental studies (i.e. non‐randomised controlled study, before‐and‐after study and interrupted time series); and observational studies (cohort study and case–control study). Only human studies published in English which had an abstract were included (Table 1).
Table 1.
Summary of criteria for the review
| Inclusion criteria |
| Type of study: Randomised controlled trial, quasi‐experimental and observational studies |
| Participants: Humans |
| Setting: Healthcare or community |
| Language: English only |
| Abstract: Available |
| Outcome: Laboratory‐confirmed or clinically‐diagnosed influenza and other viral respiratory infections |
| Exclusion criteria |
| Type of study: Case series, case report, mathematical modelling and human/non‐human experimental laboratory studies, reviews |
| Participants: Animals |
| Setting: Laboratory |
| Language: non‐English |
| Abstract: not available |
Infection with pandemic strains, seasonal influenza A or B viruses and zoonotic viruses such as swine or avian influenza were included because mask/respirator guidance is needed for all types of influenza. Studies that evaluated the effect of masks/respirators on transmission of other respiratory viruses were included as a proxy for influenza.
Study selection and validity assessment
A two‐stage selection process was used to identify studies that appeared to meet the inclusion criteria. Firstly, Fb‐R or VLC scanned and excluded papers on the basis of the ‘title’ for relevance; in the second and third searches, some relevant titles were excluded because they had been selected for review during a prior search. Secondly, to enhance the reliability of the selection process, Fb‐R, VLC, MEC and AN independently reviewed the abstracts for the remaining papers.
Fb‐R or VLC used a pre‐designed form to perform an initial data extraction of the full article and make an initial determination regarding its eligibility. MEC or AN subsequently reviewed all of the papers, supplemented Fb‐R’s and VLC’s initial abstraction as necessary and re‐assessed each paper for inclusion in the review. Any differences were resolved by mutual agreement. MEC and AN assessed the quality of the eligible studies using the Critical Appraisal Skills Programme tools 3 for randomised controlled trials, case–control studies and cohort studies.
Results
The three separate database searches yielded a total of 6015 titles; five articles were identified by scanning the reference lists of review articles and three articles were from MEC’s hard copy collection (Figure 1). Full papers were obtained for 76 articles; of these, 17 studies were eligible for inclusion. Descriptions, findings and comments for these studies are detailed in 2, 3, 4.
Figure 1.
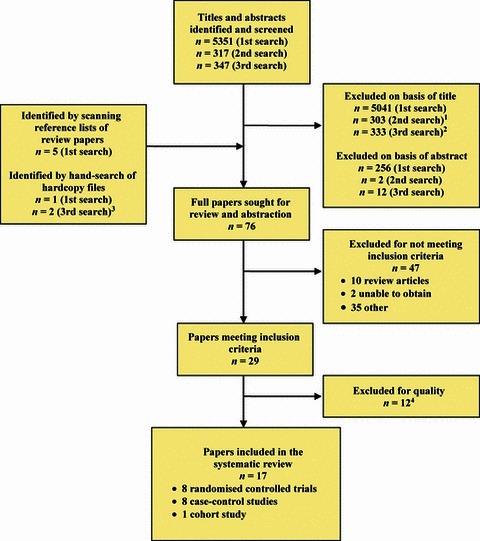
Diagram of search strategy results and article selection for three searches. 1Includes 3 papers that were sought for review and abstraction in the first search. 2Includes 6 papers that were sought for review and abstraction in the second search. 3One of these papers (reference no. 6) became available on‐line on 27 January 2011. 4Reasons for exclusion included an inability to distinguish the effect of mask use from other personal protective equipment or lack of quantitative data.
Table 2.
Synopsis of randomised controlled trials evaluating mask and respirator use for influenza
| Author/country/year of exposure/(reference) | Study design and participants | Reported results | Limitations |
|---|---|---|---|
| Jacobs/Japan 2008 (4) | Block randomisation to 2 arms and analysed as mask group (17 HCWs wore surgical mask on duty) and no mask group (15 HCWs only wore mask if job‐required e.g. surgical nurse). Outcome measure: Self‐reported cold symptoms scaled to severity. | No difference between two groups; HCWs living with children reported higher severity scores. 84·3% of participants reported full compliance with mask use and non‐use. | Underpowered study; no exposure data; compliance self‐reported; no confirmatory laboratory testing. |
| Loeb/Canada 2008/09 (5) | Non‐inferiority randomisation of 446 nurses in emergency departments and medical and paediatric units in 8 hospitals to 2 arms and analysed as surgical mask group (212 nurses) and fit‐tested N95 respirator group (210 nurses); mask/respirator worn when caring for patients with febrile respiratory illness during influenza season; assigned respiratory device worn for aerosol‐generating procedures. Outcome measure: Laboratory confirmed influenza by PCR; serology only if no receipt of 2008/09 vaccine. | No difference in influenza infection: 50 (23·6%) of 212 in mask group versus 48 (22·9%) of 210 in N95 group (absolute risk difference, −0·73%; 95% CI −8·8% to 7·3%; P = 0·86). Limited audit found high compliance. | Hard to generalise findings given lack of control arm. Incomplete assessment of compliance and lack of detailed descriptions of exposures. |
| Cowling/China ‐ Hong Kong 2007 (7) | Cluster randomisation of 198 HHs (index case and HH contacts) to 3 arms and analysed as control (71 HHs and 205 contacts), surgical masks (21 HHs and 61 contacts) or hand hygiene (30 HHs and 84 HH contacts); index cases and contacts asked to wear masks as often as possible at home during the 7‐day follow‐up period (including when with index patient outside of the household). Outcome measure: Culture‐confirmed influenza; self‐reported influenza symptoms. | No difference in laboratory‐confirmed secondary attack ratios in controls 0·06 (95% CI 0·03–0·10), mask 0·07(95% CI 0·02–0·16) and hand hygiene groups 0·06 (95% CI 0·02–0·13), P = 0·99. | Underpowered pilot study; some index cases wore masks in control and hand hygiene arms; difficulty in starting the intervention quickly may have underestimated its true effect. Compliance low: 45% (21%) of index cases (HH contacts) wore mask often/always. |
| Cowling/China ‐ Hong Kong 2008 (8) | Cluster randomisation of 407 HHs (index case and HH contacts) to 3 arms and analysed as control (91 HHs and 279 contacts), surgical masks and hand hygiene by both index case and contacts (83 HHs and 258 contacts) or hand hygiene (85 HHs and 257 contacts); index cases and contacts asked to wear masks as often as possible at home during the 7‐day follow‐up period (including when with index patient outside of the household). Outcome measure: RT‐PCR positive confirmed influenza; self‐reported influenza symptoms. | No difference in laboratory‐confirmed secondary attack ratios in controls 10% (95% CI 6–14), hand hygiene 5% (95% CI 3–9) and mask plus hand hygiene groups 7% (95% CI 4–11); P = 0·22. Significant reduction in secondary attack ratio if either intervention applied within 36 hours of index case’s onset. | Control and hand hygiene arms ‘contaminated’ as some index cases wore masks; delay in starting intervention quickly may have underestimated its true effect Adherence low: 49% (26%) of index cases (HH contacts) wore mask often/always. Cannot distinguish relative contributions of hand hygiene and mask as they were combined. |
| MacIntyre/Australia 2006/07 (9) | Cluster randomisation of 145 HHs (index case and HH contacts >16 years) to 3 arms and analysed as control (50 HHs and 100 contacts) or surgical mask (47 HHs and 94 contacts) or P2 respirator (46 HHs and 92 contacts); mask/respirator to be worn at all times when in room with index case. Outcome measure: ILI or laboratory‐ confirmed respiratory virus infection. | No significant differences between ILI rates in controls 16 (16·0%) of 100, in surgical mask group 21 (22·3%) of 94 (RR 1·29, 95%.CI 0·69–2·31, P = 0·46) and in P2 respirator group 14 (15·2%) of 92 (RR 0·95, 95%.CI = 0·49–1·84, P = 1); no difference in respiratory virus isolation rates in controls 3 (3·0%) of 100; in surgical mask group 6 (6·4%) of 94 (RR 2·13, 95% CI 0·55–8·26, P = 0·32); and in P2 respirator group 8 (8·7%) of 92 (RR 2·90, 95% CI 0·79–10·6, P = 0·12). Reduced risk for ILI with adherent mask or respirator use (hazard ratio 0·26, CI 0·09–0·77, P = 0·015). | Underpowered to detect differences between 2 interventions; low level of self‐reported adherence (21% of contacts in the surgical mask and respirator arms wore mask often/always). Interval between index case’s symptom onset and start of intervention not stated; if delayed may have underestimated true effect of intervention. |
| Aiello/USA, 2006/07 (10) | Cluster parallel randomisation of 1437 students living in university residence halls to 3 arms and analysed as control group (552 students); mask plus hand sanitiser group (367 students); and mask‐only group (378 students); instructed to wear mask as much as possible in residence hall during 6 week intervention period; encouraged to wear outside residence hall also. Outcome measure: self‐reported ILI. | Adjusted analyses found ILI significantly reduced in mask plus hand sanitiser hygiene group compared with controls (during weeks 4–6), ranging from 35% (95% CI 9–53%) to 51% (95% CI 13–73%); reductions in the mask group not significant at P < 0·025. | Hard to generalise given limited age group and specialised setting. Study underpowered to detect small reductions in ILI across arms and the relative contributions of hand hygiene and masks. |
| Larson/USA 2006/08 (11) | Block randomisation of 617 urban HHs allocated into education (control) group (174 HHs); hand sanitiser group (169 HHs); and hand sanitiser and mask group (166 HHs); household caretaker to wear mask when within 3 feet of person with ILI for 7 days or until symptoms disappeared and to change mask between interactions; ill person encouraged to wear mask when within 3 feet of other HH members. Outcome measure: Self‐reported ILI/URI symptoms and viral culture. | Hand sanitiser group more likely to report no symptomatic HH members (545/946 [57·6%] compared with education (447/904 [49·4%] and hand sanitiser/mask (363/938 [38·7%] groups, P < 0·01; no significant differences in rates of URI, ILI or influenza infection by intervention group in multivariate analyses. Hand sanitiser/mask group had significant reduction in secondary attack rates for URI/ILI/influenza infection (OR 0·82, 95% CI 0·70–0·97) compared with education. No reduction with hand sanitiser alone (OR 1·01, 95% CI 0·85–1·21). | Poor self‐reported compliance with mask use: 22 (50%) of 44 HHs reporting ILI used masks within 48 hours of episode onset; average of 2 (range 0–9) masks/day/ILI episode used. Limited power to detect differences amongst 3 groups; some use of hand sanitiser in control group in response to media reports about methicillin‐resistant Staphylococcus aureus. |
| MacIntyre/China –Beijing/2008/09 (6) | Cluster, stratified (by size of hospital and level of infection control) randomisation of 1441 HCWs in 15 Beijing hospitals into mask group (492 HCWs/5 hospitals); N95 fit‐tested group (461 HCWs/5 hospitals; and N95 non‐fit‐tested group (488 HCWs/5 hospitals); supplemented with convenience sample of non‐mask‐wearing HCWs from 9 hospitals; participants wore the mask/respirator on every shift for 4 consecutive weeks after being shown when/how to wear it. Outcome measure: Self‐reported CRI, ILI and laboratory‐confirmed viral infection by PCR. | For all outcomes N95 respirators had lower, but not significant, rates compared with masks. Intention‐to‐treat analysis adjusted for clustering of hospitals found only non‐fit‐tested N95s protective against CRI (16/488 [3·3%], OR 0·48, 95% CI 0·24–0·98, P = 0·045) compared with mask group (33/492 [6·7%]) as ref. Multivariate analysis found wearing N95s and hospital level each reduced odds of CRI and laboratory‐confirmed infection. | Monitored and self‐reported compliance good (68–76%) in the 3 arms; however, monitoring by HCWs’ supervisors not optimal method. Limited power to detect differences amongst 3 groups as observed attack rates low. Authors note 46% probability of incorrectly finding one significant difference. Despite stratified randomisation, mask group comprised of only level 3 (most sophisticated) hospitals. Hard to generalise beyond unique study population. Detailed data on potential exposures and information on community levels of influenza not provided. |
HCW, healthcare worker; PPE, personal protective equipment; RT‐PCR, reverse transcription‐polymerase chain reaction; ILI, influenza‐like illness; HH, household; URI, upper respiratory infection; CRI, clinical respiratory illness; ref, reference group.
Table 3.
Synopsis of observational case–control studies evaluating mask and respirator use for SARS
| Author/country (reference) | Study design and participants | Reported results | Comments |
|---|---|---|---|
| Chen/China (12) | 91 SARS IgG positive HCWs compared with 657 SARS IgG negative HCWs who cared for SARS patients in two hospitals. | Double‐layer cotton mask (versus a single‐layer cotton mask) protective against SARS infection in univariate analysis (OR 2·53, 95% CI 1·57–4·07); not significant in multivariate analysis. | Possible recall bias as questionnaire survey conducted 4 months after outbreak; limited data on frequency and type of exposures to SARS patients. |
| Lau/China‐Hong Kong (13) | 72 HCWS with SARS from 5 hospitals compared with 144 matched controls; PPE use examined during (i) direct contact with SARS patient; (ii) general contact with SARS and non‐SARS patients; and (iii) no patient contact. | Almost all HCWs wore N95 respirator or surgical mask in all patient settings. Unadjusted univariate analysis found inconsistent use of masks or respirators not associated with higher risk of SARS in any of the 3 contact settings; multivariate analysis found inconsistent use of >1 type of PPE during direct contact independent risk for SARS. | No serological testing of controls; reporting bias possible. |
| Nishiura/Viet Nam (14) | Period 1: Time from admission of index case to occurrence of secondary cases in one hospital: 25 laboratory‐confirmed SARS cases compared with 90 controls (HCWs and relatives of patients). Period 2: During a nosocomial outbreak in the hospital with strict isolation procedures, quarantine of HCWs and increased use of PPE: 4 laboratory‐confirmed SARS cases compared with 26 controls with only physicians and nurses in both groups. | Period 1: univariate analysis found masks (OR 0·3, 95%CI 0·1–0·7) and gowns (OR 0·2, 95%CI 0·0–0·8) protective; in logistic regression analyses, only masks protective (OR = 0·29, 95% CI 0·11–0·73) Period 2: use of masks (OR < 0·1, 95% CI 0·0–0·3) and gowns (P = 0·010, OR and CI not calculable) associated with non‐infection for doctors and nurses. | Possible recall bias; exposures imprecisely quantified; no serological testing of controls. |
| Nishiyama/Viet Nam (15) | Risk factors for serologically‐ confirmed SARS infection assessed for 85 case and control HCWs who had direct contact with SARS patients. | Multivariate logistic regression analysis found significant risk for SARS amongst HCWs who never wore mask compared with those who always wore a mask (OR 12·6, 95% CI 2·0–80·0, P < 0·01) | Possible reporting bias as interview conducted 7 months after outbreak; nature of exposures to SARS not specified; community exposures not assessed. |
| Seto/China ‐ Hong Kong (16) | 13 SARS‐infected HCWs with no community exposures compared with 241 HCWs without clinical SARS; all reported direct contact with 11 SARS patients in 5 hospitals. | Univariate analysis found HCWs who used surgical masks or N95 respirators, gowns or hand washing less likely to develop SARS; logistic regression analysis found use of any mask significant (OR 13, 95% CI 3–60). | No serological testing of controls; reporting bias possible as interviews conducted a month after cases identified; community exposures not assessed. |
| Teleman/Singapore (17) | Evaluated risk factors for serologically‐confirmed SARS amongst 36 ill case‐HCWs exposed to 3 highly infectious source patients and 50 well control‐HCWs that came within 1 m of serologically‐confirmed SARS patients. | Adjusted logistic regression analyses found that wearing N95 respirator during each patient contact (adj OR 0·1, 95% CI 0·02–0·86, P = 0·04) and hand washing after patient contact (adj OR 0·07, 95% CI 0·008–0·66, P = 0·02) protective. | Small sample size; no serological testing of the controls; limited recall of precise exposure data; no assessment of community/household exposures. |
| Lau/China ‐ Hong Kong (19) | 330 probable SARS cases with ‘undefined’ source of infection compared with 660 controls recruited by random telephone survey matched for age, sex and reference time for behaviours in question. | Matched multivariate analyses found using mask frequently in public places 27·9% of 330 cases versus 58·7% of 660 controls (OR = 0·36, 95% CI 0·25–0·52); washing one’s hands >10 times a day (OR = 0·58, 95% CI 0·38–0·87) and disinfecting living quarters (OR = 0·41, 95% CI 0·29–0·58) protective. | Likely misclassification because no laboratory testing for most cases and no testing of controls; non‐specific questions about exposures and potential protective measures. |
| Wu/China (20) | 94 unlinked, probable clinical SARS cases without reported contact with other SARS cases and 281 community‐based age‐ and sex‐matched controls in Beijing recruited by sequential digit dialling. | Multivariate analysis found ‘sometimes’ and ‘always’ wearing mask when outside home protective (matched OR 0·4, 95% CI 0·2–0·9, P = 0·03 and OR 0·3, 95% CI 0·1–0·6, P = 0·002, respectively). | Likely misclassification because no laboratory testing for most cases and no testing of controls; lack of information about community exposures; recall and self‐selection bias possible. |
SARS, severe acute respiratory syndrome; HCW, healthcare worker.
Table 4.
Synopsis of an observational cohort study evaluating mask and respirator use for SARS
| Author/country (reference) | Study design and participants | Reported results | Comments |
|---|---|---|---|
| Loeb/Canada (18) | Retrospective cohort of 43 nurses who worked in ICU or CCU when laboratory‐confirmed SARS patient in unit; analysis limited to 32 nurses who entered patient’s room at least once. | 3 (13%) of 23 nurses who consistently wore mask (either surgical or N95 respirator) developed SARS compared with 5 (56%) of 9 nurses who did not consistently wear either (RR 0·23, P = 0·02). 2 (13%) of 16 nurses who consistently wore N95 respirator developed SARS compared with 1 (25%) of 4 nurses who consistently wore a surgical mask (RR = 0·50, P = 0·51). | Underpowered study; recall bias possible; community exposure not explored; no serological testing of controls. |
SARS, severe acute respiratory syndrome; PPE, personal protective equipment; ILI, influenza‐like illness; ICU, intensive care unit; CCU, coronary care unit.
Randomised controlled trials
Three of the randomised trials were hospital‐based studies, 4 , 5 , 6 and five were conducted in community settings. 7 , 8 , 9 , 10 , 11 Two of these studies compared N95 respirators (designed to seal tightly to the wearer’s face and filter out very small particles or aerosols that may contain viruses) and surgical masks (used to block large droplets from coming into contact with the wearer’s mouth or nose) amongst healthcare workers; one trial found a lower rate of clinical respiratory illness associated with the use of non‐fit‐tested N95 respirators compared with medical masks, 6 whilst a non‐inferiority trial found that masks and respirators offered similar protection to nurses against laboratory‐confirmed influenza infection. 5 A trial conducted amongst crowded, urban households found that, despite poor compliance, mask wearing coupled with hand sanitiser use, reduced secondary transmission of upper respiratory infection/influenza‐like illness/laboratory‐confirmed influenza compared with education; hand sanitiser alone resulted in no reduction in this aggregated outcome. 11
Although the remaining five trials found no significant differences between control and intervention groups, there were some notable findings. Household contacts who wore a P2 respirator (considered to have an equivalent rating to an N95 respirator) ‘all’ or ‘most’ of the time for the first 5 days were less likely to develop an influenza‐like illness compared with less frequent users in one study. 9 Another study found a significant reduction in laboratory‐confirmed influenza amongst household contacts that began hand hygiene or hand hygiene plus a mask within 36 hours of the index case’s illness. 8 A trial conducted amongst resident university students detected significant reductions in influenza‐like illness during weeks 4–6 in the mask and hand hygiene group after adjusting for vaccine receipt and other potential confounders. 10
The requirements for mask/respirator wearing and subsequent compliance varied by study (Table 2); for example, in MacIntyre’s study of healthcare workers in China in December 2008 through January 2009 6 ‘participants wore the mask or respirator on every shift for 4 consecutive weeks after being shown when to wear it’, whilst nurses in Canada wore a mask or respirator during the 2008/09 influenza season when caring for patients with febrile respiratory illness and during aerosol‐generating procedures. 5
Observational studies
All of the observational studies evaluated mask and respirator use following the outbreaks of severe acute respiratory syndrome (SARS) in 2003; 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 seven studies were conducted amongst healthcare workers and two were community‐based. All but two 12 , 13 of the case–control studies in healthcare workers reported that wearing masks and/or respirators appeared to protect workers from acquiring SARS. 14 , 15 , 16 , 17 A retrospective cohort study of nurses who worked in two Toronto hospital intensive care units found that the relative risk of SARS for nurses who consistently wore a N95 respirator was half that for nurses who consistently wore a surgical mask; however, the difference was not significant because of a small sample size. 18
Discussion
None of the studies we reviewed established a conclusive relationship between mask/respirator use and protection against influenza infection. Some useful clues, however, could be gleaned. Subanalyses performed for one of the larger randomised controlled studies in a household setting found evidence of reduced rates of influenza‐like illness if household contacts consistently wore the mask or respirator. 9 The authors of a randomised trial of mask plus alcohol‐based sanitiser and mask‐only group amongst U.S. university students living in residence halls noted that their study may have been better positioned to identify a protective effect because participants initiated the interventions at the beginning of the influenza season. 10 Cowling’s 8 finding that there was a significant reduction in the secondary attack ratio if the hand hygiene and mask plus hand hygiene interventions were begun within 36 hours of the index case lends support to this hypothesis.
Anticipating the paucity of studies that focused solely on influenza, we included the effect of masks/respirators on respiratory viruses other than influenza. Such studies have often been used to support infection control guidance for influenza. However, the difficulties in interpreting the observational studies of SARS suggest that they are of limited use for guiding policy on influenza. Firstly, SARS is an unusual acute viral respiratory infection with a very different epidemiology to almost all other respiratory viral infections. It is fundamentally different from human influenza: it rarely infects children, has a long incubation period, transmits little early on, mostly transmits in healthcare settings, is not prone to extensive global spread and has only appeared once. Secondly, the studies were poorly designed, had many weaknesses and so were very difficult to interpret. Issues of concern include the use of a non‐specific definition for exposure to a SARS patient (e.g. coming within one metre of a patient), inconsistency in providing information about the comparability of cases and controls and collection of data after a lengthy period following the outbreak. Several lacked microbiological confirmation of cases or controls and it would seem likely that a number of the SARS cases were not cases at all. Because all the cases knew they were cases, recall bias was highly likely. The single case–control study that tried to address some of these limitations did not find that inconsistent use of masks or respirators was associated with SARS infection. 13
It is important to note three considerations when assessing the practical implications of the review’s findings. Firstly, development of evidence‐based guidance about mask/respirator use is inextricably linked to what is known about how influenza is spread and specific risk factors that can affect transmissibility (e.g. host factors, pathogen factors, environmental factors and particle size). However, this is an area equally fraught with uncertainty; there are limited and conflicting evidence regarding the relative importance and frequency of direct contact, indirect contact, droplet and aerosol modes of transmission. 21 , 22 Historically, transmission has been thought to occur principally through respiratory droplets and masks have been used as a barrier against droplets emitted by coughing and sneezing. In the last decade, there has been increasing interest in a possible role for aerosol transmission of influenza and the advisability of filtering respirators to block such transmission. For example, studies have found that infected patients can produce aerosol particles containing influenza virus 23 and that hospital airflow patterns can influence influenza transmission via aerosols. 24
Secondly, although the focus of this review has been on masks and respirators, limiting transmission of influenza in both healthcare and community settings requires a multifaceted approach, of which masks and respirators are but one component. In the healthcare setting, this ‘hierarchy of controls’ includes administrative controls help to reduce the introduction and spread of infection (e.g. policies to restrict entrance of ill visitors and workers, vaccination of healthcare workers); environmental/ engineering controls (e.g. adequate ventilation); and lastly, use of personal protective equipment and hand hygiene. 25 In the community setting, a similarly structured approach is advised. However, during both the planning for an eventual pandemic and the subsequent public health response to the H1N1 pandemic, concern over policy and guidance related to mask/respirator use has at times seemed to overshadow other important controls. 26 It is somewhat paradoxical that whilst continued effort and resources are needed to assess the independent effect of masks and respirators on influenza transmission, their use would always be recommended in combination with other control measures.
Thirdly the practical implications of policy, guidance and recommendations on mask/respirator use and other infection control measures must be considered. The only two studies that compared mask and respirators to protect healthcare workers from influenza infection essentially reached different conclusions 5 , 6 illustrating the difficulties facing policymakers. 27 Further, a simulation study found that strict adherence to guidance about personal protective equipment (which included masks and respirators) compromised normal ward functioning in a UK hospital setting. 28
This review had a prescribed narrow focus that permitted us to examine a relatively small number of studies. We considered employing quantitative techniques, but on analysis found the studies comprised a range of study designs, pathogens, participants, interventions and opportunities for bias and confounding would render any meta‐analysis findings open to criticism. A review that included interventions other than mask/respirator use, experimental laboratory and/animal–human studies on mask/respirator efficacy, cost‐effectiveness studies and the occurrence of adverse events would present a more comprehensive picture.
Several systematic reviews of interventions to limit the transmission of respiratory viral infections and/or specifically influenza have been undertaken. Most have considered a range of interventions; 29 , 30 , 31 , 32 , 33 one focused specifically on respiratory protection. 34 Within the boundaries established by our inclusion criteria, our search strategy captured essentially the same studies on masks and respirators that others have identified. Jefferson et al derived pooled estimates of the effectiveness of wearing an N95 respirator (91%) and wearing a mask (68%) for any respiratory viral infection; 29 however, these estimates were derived from the analyses of six SARS studies whose methodology was problematic. We carefully noted how well exposures in various studies were detailed and if cases and controls were laboratory‐confirmed to avoid misclassification bias. We did not feel that such a heterogeneous group of studies could be combined even for SARS.
In conclusion, there is a limited evidence base to support the use of masks and/or respirators in healthcare or community settings. Mask use is best undertaken as part of a package of personal protection, especially including hand hygiene in both home and healthcare settings. Early initiation and correct and consistent wearing of masks/respirators may improve their effectiveness. However, this remains a major challenge – both in the context of a formal study and in everyday practice.
Continued research on the effectiveness masks/respirators use and other closely associated considerations remains an urgent priority with emphasis being on carefully designed observational studies and trials best conducted outside a crisis situation. 35 However, examination of the literature has highlighted that well‐designed studies in this field are challenging. 27 Studies need to be adequately powered to assess potentially small differences between interventions and the independent effect of mask/respirator wearing when a second intervention (e.g. hand hygiene) is employed; an appropriate control group must be identified (e.g. no use of masks/respirators). Most of the studies we examined were too small to reliably detect what would be anticipated to be moderate effects. Perhaps, one solution is to fund large multi‐centre trials with similar protocols in different sites for multiple years to achieve sufficient power. Protocols should include the collection of detailed exposure data, objective monitoring of compliance and assessment of potential confounders. It may be difficult to design studies employing a control group that does not use any protective equipment (including masks/respirators), particularly in healthcare settings, as such precautions are routinely recommended. Finally, there is a striking paucity of published studies with microbiologically proven influenza infection as an outcome; inclusion of laboratory outcomes is essential in any future study of masks/respirators on transmission of influenza.
Funding
Supported by funding from the Health Protection Agency and the European Centre for Disease Prevention and Control.
Declarations of interest
Mary E Chamberland provided assistance to the Health Protection Agency, U.S. Centers for Disease Control and Prevention and the World Health Organization in the development of infection control recommendations for pandemic influenza. Angus Nicoll helped develop the ECDC infection control guidance for pandemic, seasonal and avian influenza.
Authors’ contributions
Fb‐R, VLC, AN and MEC analysed the data. Fb‐R and MEC were the principal writers of the manuscript with contributions from AN and VLC.
Acknowledgements
We gratefully acknowledge the librarians at the Health Protection Agency (Sheila O’Malley) and ECDC (Ana‐Belen Escriva and Indu Kadlac) for assistance with the literature reviews; and Anthony Kessel, Jeremy Hawker, Anna Cichowska, John Watson and Nick Phin of the HPA, Marc Struelens (ECDC) and others for helpful suggestions.
Faisal bin‐Reza, Angus Nicoll and Mary E Chamberland undertook this work whilst at the Health Protection Agency but no longer work at the HPA.
An earlier version of this review was published on‐line by the Department of Health at: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_125425.pdf. This version has been updated and revised.
Footnotes
Search terms for PubMed database search: [1] Respiratory viruses: influenza OR influenza[tw] OR flu OR flu[tw] OR common cold OR common cold[tw] OR rhinovirus OR rhinovirus*[tw] OR adenoviridae OR adenovirus*[tw] OR coronavirus OR coronavirus infections OR coronavirus*[tw] OR respiratory syncytial viruses OR respiratory syncytial virus infections OR respiratory syncytial virus*[tw] OR respiratory syncitial virus[tw] OR parainfluenza virus 1 OR parainfluenza virus 2 OR parainfluenza virus 3 OR parainfluenza virus 4 OR parainfluenza[tw] OR parainfluenza[tw] OR parainfluenza[tw] OR severe acute respiratory syndrome OR severe acute respiratory syndrome[tw] OR SARS[tw] OR acute respiratory infection*[tw] OR acute respiratory tract infection*[tw] OR influenza‐like illness OR influenza‐like illness[tw] OR ILI OR Severe acute respiratory infection OR Severe acute respiratory infection[tw] OR pandemic influenza OR pandemic flu [2] Interventions and population groups: masks OR mask*[tw] OR patient isolators OR personal protective equipment OR face protection OR N95 OR FFP2 OR FFP3 OR respirator OR home OR household* OR community OR nursing home OR nosocomial OR HCAI OR healthcare associated infection OR healthcare associated infections OR airborne precautions OR droplet precautions OR non‐pharmaceutical intervention OR nonpharmaceutical intervention OR aerosol‐generating procedures OR healthcare workers OR healthcare workers OR HCW OR healthcare personnel OR healthcare personnel.
Search terms for the additional databases were respiratory viruses, mask, respirator, N95, FFP, FFP2, FFP3, influenza.
References
- 1. Department of Health . UK influenza pandemic preparedness strategy 2011: strategy for consultation. 22 March 2011. Available at http://www.dh.gov.uk/en/Consultations/Liveconsultations/DH_125316 (Accessed 25 March 2011).
- 2. Centre for Reviews and Dissemination, University of York . CRD’s guidance for undertaking reviews in health care. 2009. Available at http://www.york.ac.uk/inst/crd/systematic_reviews_book.htm (Accessed 22 December 2009).
- 3. National Health Service Critical Appraisal Skills Programme . Available at http://www.phru.nhs.uk/casp/CASP.htm (Accessed 16 November 2009).
- 4. Jacobs JL, Ohde S, Takahashi O, Tokuda Y, Omata F, Fukui T. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am J Infect Control 2009; 37:417–419. [DOI] [PubMed] [Google Scholar]
- 5. Loeb M, Dafoe N, Mahony J et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA 2009; 302:1865–1871. [DOI] [PubMed] [Google Scholar]
- 6. MacIntyre CR, Wang Q, Cauchemez S et al. A cluster randomized clinical trial comparing fit‐tested and non‐fit‐tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respi Viruses 2011; 5:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowling BJ, Fung ROP, Cheng CKY et al. Preliminary findings of a randomized trial of non‐pharmaceutical interventions to prevent influenza transmission in households. PLoS ONE 2008; 3:e2101. doi:10.1371/journal.pone.0002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowling BJ, Chan K‐H, Fang VJ et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med 2009; 151:437–446. [DOI] [PubMed] [Google Scholar]
- 9. MacIntyre CR, Cauchemez S, Dwyer DE et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis 2009; 15:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aiello AE, Murray GF, Perez V et al. Mask use, hand hygiene, and seasonal influenza‐like illness among young adults: a randomized intervention trial. J Infect Dis 2010; 201:491–498. [DOI] [PubMed] [Google Scholar]
- 11. Larson EL, Ferng Y‐H, Wong‐McLoughlin J, Wang S, Haber M, Morse SS. Impact of non‐pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep 2010; 125:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen W‐Q, Ling W‐H, Lu C‐Y et al. Which preventive measures might protect health care workers from SARS? BMC Public Health 2009; 9:81. doi:10.1186/1471‐2458‐9‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau JTF, Fung KS, Wong TW et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 2004; 10:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishiura H, Kuratsuji T, Quy T et al. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. Am J Trop Med Hyg 2005; 73:17–25. [PubMed] [Google Scholar]
- 15. Nishiyama A, Wakasugi N, Kirikae T et al. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis 2008; 61:388–390. [PubMed] [Google Scholar]
- 16. Seto WH, Tsang D, Yung RWH et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003; 361:1519–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health‐care workers in Singapore. Epidemiol Infect 2004; 132:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loeb M, McGeer A, Henry B et al. SARS among critical care nurses, Toronto. Emerg Infect Dis 2004; 10:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lau JTF, Tsui H, Lau M, Yang X. SARS transmission, risk factors, and prevention in Hong Kong. Emerg Infect Dis 2004; 10:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Xu F, Zhou W et al. Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis 2004; 10:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis 2007; 7:257–265. [DOI] [PubMed] [Google Scholar]
- 22. Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface 2009; 6(Suppl 6):S783–S790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lindsley WG, Blachere FM, Thewlis RE et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS ONE 2010; 5:e15100. doi:10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong BCK, Lee N, Li Y et al. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin Infect Dis 2010; 51:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Department of Health and Health Protection Agency . Pandemic (H1N1) 2009 influenza – a summary of guidance for infection control in healthcare settings. 8 Jan 2010. Available at http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_110902 (Accessed 12 February 2010).
- 26. Srinivasan A, Perl TM. Respiratory protection against influenza (editorial). JAMA 2009; 302:1903–1904. [DOI] [PubMed] [Google Scholar]
- 27. Killingley B. Respirators versus medical masks: evidence accumulates but the jury remains out (editorial). Influenza Other Respi Viruses 2011; 5:143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phin NF, Rylands A, Allan J, Edwards C, Enstone JE, Nguyen‐Van‐Tam J. Personal protective equipment in an influenza pandemic: a UK simulation exercise. J Hosp Infect 2009; 71:15–21. [DOI] [PubMed] [Google Scholar]
- 29. Jefferson T, Del Mar C, Dooley L et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review [published online 21September 2009]. BMJ 2009; 339:b3675. doi:10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jefferson T, Foxlee R, Del Mar C et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review [published online 27 November 2007]. BMJ 2008; 336:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jefferson T, Del Mar C, Dooley L et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev 2010: CD006207. doi: 10.1002/14651858.CD006207.pub3. [DOI] [PubMed] [Google Scholar]
- 32. Aledort JE, Lurie N, Wasserman J, Bozzette SA. Non‐pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base. BMC Public Health 2007; 7:208. doi: 10.1186/1471‐2458‐7‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gamage B, Moore D, Copes R et al. Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. Am J Infect Control 2005; 33:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cowling BJ, Zhou Y, Ip DKM, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review [published online January 22, 2010]. Epidemiol Infect 2010; 138:449–456. [DOI] [PubMed] [Google Scholar]
- 35. Aiello AE, Coulborn RM, Aragon TJ et al. Research findings from nonpharmaceutical intervention studies for pandemic influenza and current gaps in the research. Am J Infect Control 2010; 38:251–258. [DOI] [PubMed] [Google Scholar]


