Abstract
Please cite this paper as: Higa et al. (2012) Role of neuraminidase inhibitor chemoprophylaxis in controlling nosocomial influenza: an observational study. Influenza and Other Respiratory Viruses 6(4), 299–303.
Background An influenza outbreak might result in disruption of services at acute care setting hospitals.
Objectives In this study, we retrospectively evaluated the use of neuraminidase inhibitor chemoprophylaxis for prevention of nosocomial spread of influenza in a university hospital.
Patients/Methods During the 3‐year study period, 202 index cases of influenza [30 hospitalized patients and 172 healthcare workers (HCW)] and 762 individuals who had had close contact with the index cases (248 hospitalized patients and 514 HCW) were identified. Of these contacts, 416 received neuraminidase inhibitor chemoprophylaxis.
Results When both the index cases and the close contacts were hospitalized patients, the incidence of influenza was lower among the close contacts who received chemoprophylaxis than among those who did not (odds ratio, 0·07; confidence interval, 0·01–0·49; P = 0·012). In contrast, when the index cases were HCW, the incidence of influenza was not different between close contacts who did or did not receive chemoprophylaxis.
Conclusions This study suggests that chemoprophylaxis might be useful to prevent nosocomial spread of infection between hospitalized patients.
Keywords: Chemoprophylaxis, infection control, influenza, neuraminidase inhibitor
Introduction
Outbreaks of influenza might significantly increase the workload and negatively impact services in both acute hospital settings and healthcare facilities for the care of chronic diseases. Infection control measures for preventing influenza outbreaks include vaccination, standard precautions, and personal protective equipment (PPE). 1 , 2 When an index case is identified, it is important to take prompt measures to prevent droplet transmission of the influenza virus. However, accidental exposure to the influenza virus in hospitals is inevitable. Vaccination of healthcare workers (HCW) is associated with substantially decreased mortality among their patients. 3 However, hospitalized patients are not necessarily vaccinated and may also have impaired immune systems that prevent them from responding to vaccination. Therefore, chemoprophylaxis for those who have had close contact with index cases may supplement vaccination and infection control measures to limit the spread of infection. 4
Neuraminidase inhibitors (NIs) such as oseltamivir and zanamivir are useful for both treatment and prophylaxis of influenza. 5 , 6 Early administration of NIs reduces the duration and severity of symptoms as well as the overall risk of complications. 7 , 8 , 9 Several observational studies have reported that post‐exposure NI prophylaxis is effective in controlling outbreaks. 10 , 11 , 12 A double‐blind randomized control trial found that long‐term use of oseltamivir for influenza prophylaxis in a vaccinated frail population led to a 92% reduction in the incidence of influenza. 13 On the other hand, it has been pointed out that extensive use of chemoprophylaxis may be impractical and costly. 14
The efficacy of NI chemoprophylaxis in the acute hospital setting is unknown. In our hospital, chemoprophylaxis has often been used for individuals who have had unprotected (i.e., not wearing PPE) close contact with index cases. This retrospective study was performed to evaluate the use of chemoprophylaxis in close contacts in an acute hospital setting.
Methods
Study population
Hospitalized patients and HCW present at the affiliated hospital of University of the Ryukyus between April 2007 and March 2010 were included in this study.
Only patients who did not have influenza symptoms at the time of admission were included in this study. Patients who were hospitalized for treatment of influenza were excluded from this study, as these patients were isolated and droplet precautions were taken during their care from the time of their admission to the hospital.
Almost all HCW (93·6–94·8%) received annual conventional influenza vaccination during the study period. The influenza virus A/H1N1pdm strain was prevalent between August 2009 and February 2010. Most of the HCW (>86%) were vaccinated for A/H1N1pdm between October and December 2009. The vaccination statuses of the hospitalized patients could not be ascertained. During regional epidemics of influenza, HCW used surgical masks while on duty.
Identification of index cases and close contacts
Influenza‐like illness was identified by self‐reported symptoms. An immunochromatographic test (ICT) for influenza virus A and B antigens (Tauns Laboratories Inc., Shizuoka, Japan) was used to diagnose index cases. The ICT test was repeated if necessary. Examples of close contact with the index cases included the following: (i) physical care, (ii) verbal communication without PPE, and (iii) sharing a room. Individuals who were considered to have had close contact with the index cases were actively monitored for symptoms for 10 days after identification. In close contacts, influenza was diagnosed by either positive ICT or a characteristic clinical presentation consisting of influenza‐like illness, known close contact with a definitive influenza case, and absence of other febrile diseases.
Influenza chemoprophylaxis
Chemoprophylaxis was recommended for hospitalized patients who were considered to have had close contact with index cases. When multiple cases were identified in a single ward, the recommendation for chemoprophylaxis was extended to the HCW in the ward.
Neuraminidase inhibitors (either oseltamivir or zanamivir) were used for influenza chemoprophylaxis. Written informed consent was obtained for the administration of these drugs. Adults with normal renal function received 75 mg/day oseltamivir for 5–7 days. Patients on hemodialysis received a single daily dose of 75 mg of oseltamivir. Pediatric patients received oseltamivir at 2 mg/kg/day. For certain patients with normal renal function, 10 mg of zanamivir was administered once daily for 10 days.
Statistical analysis
Statistical analysis was performed using commercial software (SPSS version 15.0J; SPSS Japan Inc., Tokyo, Japan). A P‐value <0·05 was considered statistically significant.
Results
During the 3‐year investigation, 202 index cases were identified among the hospitalized patients and HCW. The incidence of influenza clearly increased with the regional epidemic in late 2009 and early 2010 (Figure 1). We identified 172 index cases among the HCW and 30 among the hospitalized patients (Table 1). Most of the hospitalized patients developed flu‐like symptoms within 2 days of admission, suggesting that they had been infected with influenza virus prior to admission. Five hospitalized patients contracted influenza via nosocomial transmission from another patient with influenza sharing the same ward. Among the HCW, 12 influenza cases were thought to be hospital‐acquired, while the remainder were community‐acquired.
Figure 1.
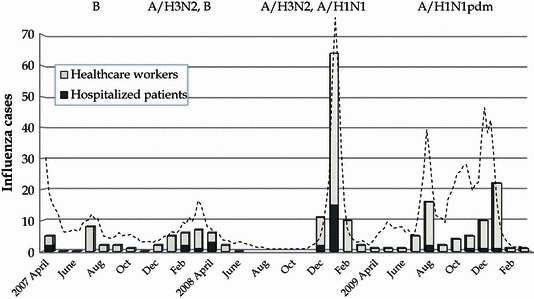
Incidence of influenza in hospitalized patients and healthcare workers during the fiscal years 2007–2009. Bars indicate the incidence of influenza in each month. Dotted line represents regional epidemic curve in Okinawa, where the hospital was located.
Table 1.
Incidence of influenza in hospitalized patients and healthcare workers by fiscal year
| Fiscal year* | 2007 | 2008 | 2009 | Total |
|---|---|---|---|---|
| Hospitalized patients | 5 | 20 | 5 | 30 |
| Healthcare workers | 33 | 75 | 64 | 172 |
| Total | 38 | 95 | 69 | 202 |
*The Japanese fiscal year starts on April 1 and ends on March 31.
After diagnosis of each index case, an infection control team performed a survey to identify the individuals who might have had close contact with the index case; 762 close contacts (248 hospitalized patients and 514 HCW) were identified during the study period. The mean numbers of contacts for hospital index patients and for HCW index patients were 8·3 and 4·9, respectively. Among the close contacts, influenza chemoprophylaxis was recommended for the hospitalized patients, and the recommendation was extended to the HCW when multiple cases were identified in a single ward. Healthcare workers who might have had close contact also received chemoprophylaxis upon request. Overall, 416 persons received chemoprophylaxis (Table 2). Prophylactic treatment of contacts usually commenced within several hours of diagnosis of the index case and began within 24 hours in all cases.
Table 2.
Chemoprophylaxis after accidental exposure to influenza cases in a hospital
| Index cases (n) | Close contacts (n) | CP* | Disease/Contacts | Odds ratio (CI) | P † |
|---|---|---|---|---|---|
| All (202) | All (762) | Yes | 5/416 | 0·34 (0·12–0·97) | 0·047 |
| No | 12/346 | ||||
| Hospitalized patients (30) | All (248) | Yes | 3/153 | 0·22 (0·06–0·84) | 0·024 |
| No | 8/95 | ||||
| Hospitalized patients (116) | Yes | 2/102 | 0·07 (0·01–0·49) | 0·012 | |
| No | 3/14 | ||||
| Healthcare workers (132) | Yes | 1/51 | 0·30 (0·03–2·68) | 0·405 | |
| No | 5/81 | ||||
| Healthcare workers (172) | All (514) | Yes | 2/263 | 0·47 (0·09–2·61) | 0·440 |
| No | 4/251 | ||||
| Hospitalized patients (156) | Yes | 0/146 | – | 1 | |
| No | 0/10 | ||||
| Healthcare workers (358) | Yes | 2/123 | 0·95 (0·17–5·29) | 1 | |
| No | 4/235 |
*Chemoprophylaxis.
†Fisher’s exact probability test.
Five of the 416 contacts (1·2%) with chemoprophylaxis and 12 of the 346 contacts (3·5%) without it became infected with influenza (Table 2). Diagnosis was based on clinical symptoms and ICT. Fourteen cases were confirmed by positive antigen test, although two of these required repeated ICTs to detect the antigen. Three cases were ICT‐negative for influenza but satisfied the characteristic clinical presentation criteria for influenza diagnosis. When the index cases were hospitalized patients, the incidence of influenza was lower among the close contacts who had received chemoprophylaxis than among those who had not (odds ratio, 0·22; confidence interval, 0·06–0·84; P = 0·024). In contrast, when the index cases were HCW, chemoprophylaxis did not change the incidence of influenza among close contacts.
Between January and February 2009, the hospital experienced a regional epidemic of influenza A/H1N1 with reduced oseltamivir susceptibility. During that time, zanamivir was preferentially used for chemoprophylaxis. Between August 2009 and February 2010, there was an influenza A/H1N1pdm pandemic. During that time, a single failure of zanamivir chemoprophylaxis occurred in a hospitalized patient who had had close contact with an index case. Zanamivir was used in 23 close contacts, while oseltamivir was used in 393 close contacts. These numbers were too small to identify any difference in the prophylactic efficacies of oseltamivir and zanamivir.
Discussion
Several studies have assessed the usefulness of chemoprophylaxis for the control of influenza outbreaks. 15 , 16 The present study showed that the efficacy of chemoprophylaxis depended on whether the index cases were hospitalized patients or HCW. Chemoprophylaxis did not influence the incidence of influenza when the index cases were HCW. During regional epidemics of influenza, most HCWs wore surgical masks while on duty, while the hospitalized patients who were unaware of their illness did not wear masks and may have unintentionally transmitted influenza virus to fellow patients. The use of surgical masks may have played a role in reducing transmission of influenza virus from HCW to others. Standard and droplet precautions are recommended for reducing nosocomial transmission of influenza, 2 and this study suggested that in certain situations, chemoprophylaxis may be a useful adjunct for reducing nosocomial influenza. Healthcare workers received chemoprophylaxis only when multiple cases were identified in the ward. This strategy might have influenced the efficacy of chemoprophylaxis among HCWs.
This study did not identify which NI was most effective for chemoprophylaxis, but genotypic characterization of the prevalent influenza virus might influence the choice of NI. During the study period, an epidemic of A/H1N1 with reduced susceptibility to oseltamivir (December 2008–February 2009) occurred in the region. 17 , 18 During the epidemic, chemoprophylaxis with oseltamivir failed in 1 case; consequently, zanamivir was used for prophylaxis in subsequent cases. No prophylaxis failure with zanamivir was noted during the A/H1N1 2008–2009 epidemic, while one zanamivir failure was noted during the A/H1N1pdm pandemic.
The incidence of influenza among close contacts did not increase during the A/H1N1pdm pandemic. Vaccination was not available until late November 2009, but the incidence among and nosocomial transmission of influenza to the HCWs did not change after the vaccine became available (data not shown). It is conceivable that other infection control measures including droplet precautions and chemoprophylaxis for close contacts were effective during the pandemic even in the absence of vaccination. However, the emergence of oseltamivir‐resistant strains during chemoprophylaxis was reported, 19 and therefore, judicious application of chemoprophylaxis may be prudent.
The retrospective approach used may limit the conclusions of this study. Identification of the index cases was based on self‐reporting, and several cases, especially mild or subclinical infections, may not have been detected, which may have introduced a selection bias. By contrast, close contacts were under active surveillance, and the incidences of secondary infection are therefore reliable. The diagnostic sensitivity of the rapid test kits is not high, and we carefully surveyed the close contacts and sometimes repeated antigen tests or diagnosed cases based on clinical presentation. Overall, we believe that the data presented here are useful for evaluation of the incidence of secondary influenza infections in a hospital setting.
This study suggested that chemoprophylaxis might be a worthwhile supplement to standard and droplet precautions for reducing nosocomial transmission of influenza. Post‐exposure chemoprophylaxis is especially warranted when both index cases and close contacts are hospitalized patients.
Conflicts of interest
None to declare.
References
- 1. CDC . Prevention strategies for seasonal influenza in healthcare settings. Available at http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm (Accessed 11 July 2011).
- 2. Siegel JD, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee . 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings, 2007. Available at http://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html (Accessed 11 July 2011) [DOI] [PMC free article] [PubMed]
- 3. Carman WF, Elder AG, Wallace LA et al. Effects of influenza vaccination of health‐care workers on mortality of elderly people in long‐term care: a randomised controlled trial. Lancet 2000; 355:93–97. [DOI] [PubMed] [Google Scholar]
- 4. Harper SA, Bradley JS, Englund JA et al. Seasonal influenza in adults and children – diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong AK, Hayden FG, John F. Enders lecture 2006: antivirals for influenza. J Infect Dis 2007; 196:181–190. [DOI] [PubMed] [Google Scholar]
- 6. Khazeni N, Bravata DM, Holty JE, Uyeki TM, Stave CD, Gould MK. Systematic review: safety and efficacy of extended‐duration antiviral chemoprophylaxis against pandemic and seasonal influenza. Ann Intern Med 2009; 151:464–473. [DOI] [PubMed] [Google Scholar]
- 7. Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835. [DOI] [PubMed] [Google Scholar]
- 8. Hayden FG, Atmar RL, Schilling M et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 1999; 341:1336–1343. [DOI] [PubMed] [Google Scholar]
- 9. Hayden FG, Treanor JJ, Fritz RS et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 1999; 282:1240–1246. [DOI] [PubMed] [Google Scholar]
- 10. Bush KA, McAnulty J, McPhie K et al. Antiviral prophylaxis in the management of an influenza outbreak in an aged care facility. Commun Dis Intell 2004; 28:396–400. [DOI] [PubMed] [Google Scholar]
- 11. Seale H, Weston KM, Dwyer DE et al. The use of oseltamivir during an influenza B outbreak in a chronic care hospital. Influenza Other Respi Viruses 2009; 3:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shijubo N, Yamada G, Takahashi M, Tokunoh T, Suzuki T, Abe S. Experience with oseltamivir in the control of nursing home influenza A outbreak. Intern Med 2002; 41:366–370. [DOI] [PubMed] [Google Scholar]
- 13. Peters PH Jr, Gravenstein S, Norwood P et al. Long‐term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc 2001; 49:1025–1031. [DOI] [PubMed] [Google Scholar]
- 14. Harling R, Hayward A, Watson JM. Implications of the incidence of influenza‐like illness in nursing homes for influenza chemoprophylaxis: descriptive study. BMJ 2004; 329:663–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee VJ, Yap J, Cook AR et al. Oseltamivir ring prophylaxis for containment of 2009 H1N1 influenza outbreaks. N Engl J Med 2010; 362:2166–2174. [DOI] [PubMed] [Google Scholar]
- 16. Hsu EB, Millin MG. A hospital‐based strategy for setting priorities for antiviral prophylaxis during an influenza pandemic. Biosecur Bioterror 2008; 6:171–178. [DOI] [PubMed] [Google Scholar]
- 17. Garrison M, Welden L, Brantley P et al. Oseltamivir‐resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis – North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972. [PubMed] [Google Scholar]
- 18. Gooskens J, Jonges M, Claas EC, Meijer A, van den Broek PJ, Kroes AM. Morbidity and mortality associated with nosocomial transmission of oseltamivir‐resistant influenza A(H1N1) virus. JAMA 2009; 301:1042–1046. [DOI] [PubMed] [Google Scholar]
- 19. Baz M, Abed Y, Papenburg J, Bouhy X, Hamelin ME, Boivin G. Emergence of oseltamivir‐resistant pandemic H1N1 virus during prophylaxis. N Engl J Med 2009; 361:2296–2297. [DOI] [PubMed] [Google Scholar]


