Abstract
Please cite this paper as: Kammerer et al. (2012). Influenza‐like illness surveillance on the California‐Mexico border, 2004–2009. Influenza and Other Respiratory Viruses 6(5), 358–366.
Background Since 2004, the Naval Health Research Center, with San Diego and Imperial counties, has collaborated with the US Centers for Disease Control and Prevention to conduct respiratory disease surveillance in the US‐Mexico border region. In 2007, the Secretariat of Health, Mexico and the Institute of Public Health of Baja California joined the collaboration.
Objectives The identification of circulating respiratory pathogens in respiratory specimens from patients with influenza‐like illness (ILI).
Methods Demographic, symptom information and respiratory swabs were collected from enrollees who met the case definition for ILI. Specimens underwent PCR testing and culture in virology and bacteriology.
Results From 2004 through 2009, 1855 persons were sampled. Overall, 36% of the participants had a pathogen identified. The most frequent pathogen was influenza (25%), with those aged 6–15 years the most frequently affected. In April 2009, a young female participant from Imperial County, California, was among the first documented cases of 2009 H1N1. Additional pathogens included influenza B, adenovirus, parainfluenza virus, respiratory syncytial virus, enterovirus, herpes simplex virus, Streptococcus pneumoniae, and Streptococcus pyogenes.
Conclusions The US‐Mexico border is one of the busiest in the world, with a large number of daily crossings. Due to its traffic, this area is an ideal location for surveillance sites. We identified a pathogen in 36% of the specimens tested, with influenza A the most common pathogen. A number of other viral and bacterial respiratory pathogens were identified. An understanding of the incidence of respiratory pathogens in border populations is useful for development of regional vaccination and disease prevention responses.
Keywords: Bacterial infections, human, influenza, respiratory tract infections, sentinel surveillance
Introduction
Acute respiratory infections (ARIs) are the most common illnesses among persons of all ages. 1 Approximately 2 million deaths occur globally each year from ARI, mostly among the elderly and young children. 2 The burden of disease is greatest in low‐income countries where ARIs are the cause of up to 25% of all pediatric deaths. 3 In Mexico, ARIs are the leading cause of disease (http://www.dged.salud.gob.mx/contenidos/evaluacion_programas/descargas/CUARTO_INFORME.pdf). In the United States, annual influenza epidemics result in projected lost earnings due to illness and loss of life of $16·3 billion annually and a total economic burden (using projected statistical life values) of $87·1 billion. 4 Respiratory illnesses cause more disease and death than any other infection in the United States. 5 The annual Northern and Southern hemisphere trivalent inactivated or live‐attenuated influenza vaccines are the best way to prevent the spread of influenza and reduce disease related morbidity and mortality in the communities. Nevertheless, limited availability and use of these vaccines in under‐resourced settings put a large proportion of the world’s population at risk. 6
The migration of persons and products across national borders contributes to the spread of infectious diseases 7 . The US‐Mexico border region has been defined as the area of land 100 km (62·5 miles) north and south of the actual international border. This land area has an estimated population of approximately 12 million inhabitants (http://www.borderhealth.org/border_region.php). With 300 million two‐way crossings estimated in 2001, the US‐Mexico border is one of the busiest in the world. Incidence rates for infectious diseases, such as diphtheria, hepatitis A, measles, mumps, rabies, rubella, and salmonellosis, have been found to be significantly higher in the United States along the Mexican border than in non‐border regions. 8 This surveillance program was initiated to identify the respiratory pathogens responsible for illness near the border region and to detect emerging respiratory pathogens in this area, allowing a more timely public health response.
The Centers for Disease Control and Prevention (CDC) Border Infectious Disease Surveillance (BIDS) program 9 and the CDC/California Department of Public Health Early Warning Infectious Disease Surveillance (EWIDS) program, in collaboration with the Naval Health Research Center (NHRC), County of San Diego Health and Human Services Agency and the Imperial County Public Health Department have conducted influenza‐like illness (ILI) surveillance since 2004. In 2007, the Mexico Secretariat of Health and the Institute of Public Health of Baja California joined the collaboration. Here, we describe the etiologies associated with ILI in participants who had a specimen collected from 2004 through 2009.
Materials and methods
Site selection and enrollment
Surveillance investigators selected sites along the US‐Mexico border following discussion with local health officials. Sites selected were local health clinics, chosen due to their proximity to the border region. (Figure 1). Eligible patients, of all ages, with ILI were voluntarily enrolled. Surveillance was conducted August through June in 2004–2006 and year‐round beginning August, 2006 through September, 2009; The NHRC institutional review board reviewed this study protocol and deemed it public health surveillance.
Figure 1.
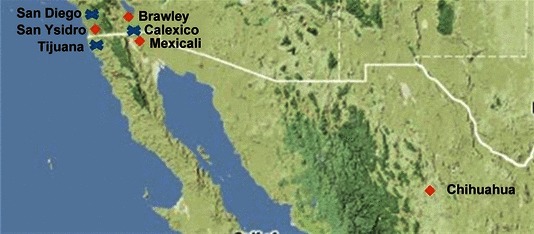
Map of surveillance sites. Diamonds indicate active sites at the start of 2008–2009. Crosses indicate former sites.
Specimen collection
Surveillance personnel obtained two nasal swabs and one throat swab from all patients with ILI (oral temperature ≥100°F (37·8°C) and presence of either cough or a sore throat in the absence of a known cause other than influenza). This ILI case definition was used at all sites. Specimens that did not meet the case definition (n = 6) were removed from the data. For throat swabs, both tonsils and the posterior pharynx were swabbed, and specimens were stored in tryptic soy broth with 15% glycerol (TSB; Remel, Lenexa, KS, USA). For nasal swabs, the nares with observed discharge were swabbed. If no discharge was observed, one side was chosen. The nares were swabbed twice, with one swab placed in viral transport media (VTM) (M4RT; Remel) and the other tested on site by using a rapid influenza diagnostic test (Quidel Corporation, San Diego, CA, USA). Specimens in TSB and VTM were frozen at −70°C within 4 hours of sample collection and stored in an ultra‐low freezer or on dry ice. Frozen specimens were either shipped on dry ice to NHRC or collected by NHRC personnel, who maintained the cold chain.
Extraction
Extraction of RNA was performed using the QIAamp 96 DNA Blood and Body Fluid Kit (Qiagen, Valencia, CA, USA) for conventional polymerase chain reaction (PCR) or the QIAamp Viral RNA Mini Kit (Qiagen) for real‐time PCR reaction, according to manufacturer’s instructions.
PCR
PCR was used to detect influenza A and adenovirus (AdV) only. Samples were tested for influenza A as previously described 10 using universal primers made against the M gene. Adenovirus PCR was conducted using AdV universal β primers 11 and the BCE multiplex as previously described. 12 Reactions were performed using an iCycler PCR machine (Bio‐Rad Laboratories, Inc., Hercules, CA, USA) or a DNA Engine machine (Bio‐Rad).
Influenza subtyping
Samples determined to be positive by comparison to influenza A universal primers were subtyped for H1 and H3 on the LightCycler 2.0 (Roche, Basel, Switzerland) using primers that target the hemagglutinin gene. Primers and probe used for H1 subtyping were H1F (5′‐GYAGTCTTCCTTTCCAGAATGT‐3′), H1R (5′ AGTCCTGTAACCATCCTTAATTT TG‐3′), and H1P (5′‐6FAM‐TAGGAGAGTGTCCAAAGTATGTCAGGA‐TAMRA‐3′). Primers and probe used for H3 subtyping were H3F (5′‐ TGTCTCCAGCAGAATAAGC ATCT‐3′), H3R (5′‐CCCACTTCGTATTTTGAAGTAACC‐3′), and H3P (5′‐6FAM‐TGGACAATAGTAAAACCGGGAGACATACTTTTG‐TAMRA‐3′) (primers developed in house by Luke Daum). Real‐time reactions were performed using the RNA UltraSense One‐Step Quantitative RT‐PCR System (Invitrogen Corporation, Carlsbad, CA, USA), according to manufacturer’s instructions with the modification of a final concentration of probe to 0·3 μm per 25 μl reaction.
pH1N1 testing
Samples were assayed for pH1N1 by using the Emergency Use Authorized CDC Swine Influenza Virus Real‐Time RT‐PCR Detection Panel as previously published (http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/)
PCR/Mass spectrometry assay
PCR amplicons representing internal gene segments were generated with tandem mass spectra analysis conducted on 15 μl aliquots of purified products by using protocols described previously. 13 Nucleotide base compositions were derived from the exact mass measurements of the complementary single‐stranded oligonucleotides. Relationships were determined by comparing profiles with published sequences (Abbott Laboratories, Abbott Park, IL, USA).
Virus isolation and identification
We isolated respiratory viruses following co‐culture in rhesus monkey kidney (RMK) and A549 cells. RMK cells were used to isolate influenzas A and B, and parainfluenza viruses (PIVs) 1, 2, and 3. A549 cells were used to isolate AdV, respiratory syncytial virus (RSV), and enterovirus. Cultures exhibiting cytopathic effect were identified and tested using an immunofluorescence assay with type‐specific monoclonal antibodies for viral identification. Hemagglutination inhibition was used to serotype influenza isolates using the World Health Organization Influenza Reagent Kit for Identification of Influenza Isolates for that year.
Bacteriologic testing
Throat swabs collected in TSB were plated onto Blood Agar and Chocolate plates (BAP, CHOC; Hardy Diagnostics, Santa Maria, CA, USA), and Regan‐Lowe plates [BBL; Becton, Dickinson and Company (BD)]. Blood Agar and Chocolate plates were observed daily for growth of group A streptococcus, S. pneumoniae, Neisseria meningitidis, Haemophilus influenza, and Staphylococcus aureus. Regan‐Lowe plates were incubated for 7 days and observed daily for growth of Bordetella pertussis. Suspicious colonies were Gram stained, catalase tested, and placed into the BD Phoenix (Becton, Dickinson and Company) for identification. The BD BBL Crystal was used to identify Neisseria and Haemophilus species.
Statistical analysis
All data were double‐entered into a Microsoft Access database. We used Pearson Chi‐square analysis to obtain P values for comparison of laboratory results by age group, season, and country. To identify which pathogens contributed to any observed differences among the study populations, 95% confidence intervals for pathogen proportions among subgroups were calculated using the Wilson 14 procedure. P values were calculated to compare proportions between two independent groups. 15
Results
The number and location of sites varied from 2004 through 2009 (Table 1). At study onset, one site was selected near the border in San Ysidro, California. Calexico was an influenza surveillance site in 2004–2005 for the Imperial County Public Health Department, as part of California’s sentinel site program. Calexico joined our collaboration as a second site in 2005–2006. In 2007, at the request of the Mexico Secretariat of Health, two sites in Mexico were added, one in Mexicali and one in Tijuana. At the start of the 2008–2009 influenza season, enrolling sites included two in California (San Ysidro and Brawley) and two in Mexico (Mexicali and Chihuahua) (Figure 1). As more collaborating surveillance sites were trained, the number of specimens collected rose (Table 1). Overall, the San Ysidro Health Center had the highest number of specimens submitted (725), followed by the Mexicali clinic. (543). Although the number of specimens collected per year varied among all sites (average of 373 per year), the largest increase in specimen collection was seen when sites in Mexico were added and with the onset of the 2009 pandemic, 16 when the number of sites was temporarily expanded to 14 (Table 1).
Table 1.
Number of specimens per site
| Year | No. sites USA | No. sites Mexico | Enrollees USA | Enrollees Mexico |
|---|---|---|---|---|
| 2004–2005 | 1 | 0 | 122 | 0 |
| 2005–2006 | 2 | 0 | 224 | 0 |
| 2006–2007 | 3 | 0 | 207 | 0 |
| 2007–2008 | 4 | 2 | 243 | 143 |
| 2008–2009 | 12 | 2 | 506 | 410 |
| Total | 1302 | 553 |
From 2004 through September 2009, a total of 1855 participants (1302 from the US, 553 from Mexico) were enrolled with laboratory samples (Table 2). Among 1677 participants with known gender, 960 (57%) were female. The majority (70%) of participants reported living in the United States (1222), with 99% of US participants reporting US residency and 100% of Mexico clinic participants reporting Mexico residency. Of those reporting age (n = 1826), the most frequent age range represented was 16–54 years (38%), followed by 6–15 years (24%), and 1–5 years (22%). There was a higher percentage of the 16–54 age group in Mexico (P < 0·001) and the >54 (P < 0·001) and 1–5 (P < 0·05) age groups in the US. No significant difference was seen in the two other age groups. The majority of participants were Hispanic (1677; 94%). White non‐Hispanic participants represented 2%, and Asian, African American, and Native American study subjects accounted for the remaining 3%. Influenza vaccination status was self reported for 98% of ILI cases and the proportion vaccinated was 28% at US clinics and 19% at Mexico clinics (P < 0·05; data not shown). In the US, 19% of the vaccinated and 32% of the unvaccinated were positive for influenza A or B. In Mexico, 15% of the vaccinated and 17% of the unvaccinated were positive for influenza A or B. The vaccination rate for 0–5 years old was higher in the US (34%) than that for 6 years and older in the US (26%) (P < 0·05; data not shown). The 0–5 year old vaccination rate was higher in Mexico (24%) than that for 6 years and older in Mexico (19%), but the difference was not significant (P = 0·20; data not shown). The difference in the 0–5 year old vaccination rate between the two countries was significant (P < 0·05; data not shown). Of the 1855 participants, 663 (36%) had a pathogen identified. The most frequent pathogen identified was influenza A, with 363 (20%) cases positive by either PCR or viral culture (Table 3). Additional pathogens identified were influenza B (5%), AdV (4%), PIVs 1–3 (1%), RSV (0·6%); other viral pathogens included enterovirus and herpes simplex virus (0·4%). Bacterial pathogens identified included S. pneumoniae, S. pyogenes, H. influenzae, β‐hemolytic streptococcus (not group A), group C streptococcus, and Moraxella catarrhalis, which made up the remaining 7% of diagnoses. No pathogen was identified in 1192 (64%) of participants. A significantly higher proportion of US clinic cases than Mexico clinic cases were positive for influenza A (22% versus 13%), whereas a significantly higher proportion of Mexico clinic cases than US clinic cases were positive for bacterial pathogens (10% versus 6%, P < 0·05). Thirty‐six coinfections and one triple infection were noted. Of these, 29 were viral/bacterial, seven were viral, and one was bacterial.
Table 2.
Demographics of participants
| Characteristics | USA n = 1302 (70%) n (%) | Mexico n = 553 (30%) n (%) |
|---|---|---|
| No. (%) male | 532 (43) | 185 (41) |
| No. (%) female | 696 (57) | 264 (59) |
| <1 year | 49 (4) | 22 (4) |
| 1–5 year | 300 (23) | 101 (19) |
| 6–15 year | 322 (25) | 120 (22) |
| 16–54 year | 438 (34) | 254 (47) |
| >54 year | 181 (14) | 39 (7) |
| % Hispanic | 1167 (92) | 510 (99) |
| % White/non‐Hispanic | 40 (3) | 3 (0·6) |
| Other | 64 (5) | 2 (0·4) |
Table 3.
Disease etiologies by location*
| Inf A n (%) (95% CI) | Inf B n (%) (95% CI) | AdV n (%) (95% CI) | RSV n (%) (95% CI) | Other n (%) (95% CI) | Neg n (%) (95% CI) | Total | |
|---|---|---|---|---|---|---|---|
| US | 288 (22) (20·0–24·5) | 72 (5) (4·4–6·9) | 56 (4) (3·3–5·5) | 9 (0·7) (0·4–1·3) | 106 (8) (6·6–9·6) | 799 (60) (58·7–64·0) | 1330 (28 co‐inf) |
| Mexico | 75 (13) (11·0–16·7) | 15 (3) (1·7–4·4) | 16 (3) (1·8–4·6) | 2 (0·3) (0·1–1·3) | 62 (11) (8·7–13·9) | 393 (70) (67·2–74·7) | 563 (eight co‐inf, 1 tri‐inf) |
| Total | 363 (19) | 87 (5) | 72 (4) | 11 (0·6) | 168 (9) | 1192 (63) | 1893 |
AdV, adenovirus; CI, confidence interval; Inf, influenza; Neg, negative; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
*Other pathogens – enterovirus, herpes simplex 1, PIV 1–3, Streptococcus pneumoniae, S. pyogenes, Haemophilus influenzae, β‐hemolytic streptococcus (not group A), group C streptococcus, Moraxella catarrhalis.
Study participants aged 6–15 years had a higher proportion of influenza A than did other age groups (32% versus 15%, P < 0·05; Table 4). With specimens collected during the pandemic separated from pre‐pandemic specimens, the higher proportion of influenza A in the 6–15 year age group was still significant (P < 0·05; data not shown) when compared with the 1–5, 16–54, and >54 age groups. The rate of influenza A in the 6–15 year age group was not significantly different from that of the <1 year age group. These relationships were seen in both the pre‐pandemic and pandemic specimens. RSV was only isolated from the 1–5 and >54 years age groups, whereas PIV was identified in all but those younger than 1 year. The percentage of samples in which no pathogen was identified was higher among those aged under 1 year and 16 years and older compared with those between 1 through 15 years old (73% versus 52%, P < 0·05).
Table 4.
Disease etiologies by age*
| Age (year) | Inf A n (%) (95% CI) | Inf B n (%) (95% CI) | AdV n (%) (95% CI) | RSV n (%) (95% CI) | Other n (%) (95% CI) | Neg n (%) (95% CI) | Total |
|---|---|---|---|---|---|---|---|
| <1 | 9 (12) (6·8–22·4) | 1 (1) (0·3–7·6) | 4 (6) (2·2–12·6) | 0 (0) (0·0–5·1) | 6 (8) (3·9–17·0) | 52 (72) (62·0–82·2) | 72 (one co‐infection) |
| 1–5 | 74 (18) (15·0–22·5) | 21 (5) (3·5–7·9) | 25 (6) (4·3–9·0) | 9 (2) (1·2–4·2) | 54 (13) (10·2–16·8) | 228 (55) (52·0–61·6) | 411 (10 co‐infections) |
| 6–15 | 144 (32) (28·4–37·1%) | 30 (7) (4·8–9·5) | 18 (4) (2·6–6·3) | 0 (0) (0·0–0·9) | 42 (9) (6·9–12·3) | 220 (48) (45·1–54·4) | 454 (10 co‐infections; one triple co‐infection) |
| 16–54 | 107 (15) (13·0–18·3) | 23 (3) (2·2–4·9) | 16 (2) (1·4–3·7) | 0 (0) (0·0–0·6) | 51 (7) (5·6–9·4) | 505 (72) (69·6–76·2) | 702 (12 co‐infections) |
| >54 | 24 (11) (7·4–15·7) | 10 (4) (2·5–8·2) | 8 (4) (1·9–7·0) | 2 (0·9) (0·3–3·3) | 12 (5) (3·1–9·2) | 166 (75) (69·4–80·7) | 222 (two co‐infections) |
AdV, adenovirus; CI, confidence interval; Inf, influenza; Neg, negative; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
*Other pathogens – enterovirus, herpes simplex 1, PIV 1–3, Streptococcus pneumoniae, S. pyogenes, Haemophilus influenzae, β‐hemolytic streptococcus (not group A), group C streptococcus, Moraxella catarrhalis.
The proportion of influenza‐positive cases varied by year, ranging from 13·9% in 2008–2009 (prior to the onset of the pandemic) to 44% in 2005–2006 (Table 5). Type and subtype also varied by year, with influenza A predominant in every year except 2004–2005, when influenza B was more common. Of the influenza A subtypes, A/H3N2 was predominant during the 2004–2005, 2005–2006, and 2007–2008 seasons, whereas influenza A/H1N1 was predominant during 2006–2007, and pandemic influenza A/H1N1 was predominant during 2008–2009 (Table 6). There was no significant difference (P = 0·056) in influenza A subtypes isolated on either side of the border (data not shown).
Table 5.
Specimen test results by year*,**
| Influenza A n (%) (95% CI) | Influenza B n (%) (95% CI) | Other n (%) (95% CI) | Negative n (%) (95% CI) | Total | |
|---|---|---|---|---|---|
| 2004–2005 | 7 (6) (2·9–11·7) | 26 (22) (16·2–31·2) | 15 (12) (7·2–18·9) | 72 (60) (50·3–67·8) | 120 |
| 2005–2006 | 91 (40) (35·1–47·9) | 9 (4) (2·1–7·4) | 29 (13) (8·8–17·4) | 98 (43) (36·0–48·8) | 227 |
| 2006–2007 | 33 (16) (11·6–21·6) | 11 (5) (3·0–9·3) | 52 (24) (18·9–30·6) | 116 (55) (47·6–61·0) | 212 |
| 2007–2008 | 56 (14) (11·6–18·7) | 38 (10) (7·5–13·6) | 60 (15) (11·9–19·0) | 237 (61) (54·9–64·7) | 391 |
| 2008–2009 (through 20 April) | 44 (13) (10·3–17·8) | 3 (0·9) (0·3–2·7) | 61 (18) (14·5–22·9) | 223 (67) (63·7–73·8) | 331 |
| 2009 (21 April through 16 September) | 132 (22) (19·3–26·0) | 0 (0) (0·0–0·6) | 34 (6) (3·9–7·6) | 446 (73) (69·7–76·7) | 612 |
| Total | 363 (19) | 87 (5) | 251 (13) | 1192 (63) | 1893 |
CI, confidence interval.
*Starting in 2005–2006, bacteriological testing was added.
**Other pathogens – adenovirus, enterovirus, herpes simplex 1, parainfluenza virus 1–3, respiratory syncytial virus,Streptococcus pneumoniae, S. pyogenes, Haemophilus influenzae, β‐hemolytic streptococcus (not group A), group C streptococcus, Moraxella catarrhalis.
Table 6.
Influenza A subtype by year*
| H1N1 (seasonal) n (%) (95% CI) | H3N2 n (%) (95% CI) | pH1N1 n (%) (95% CI) | |
|---|---|---|---|
| 2004–2005 | 0 (0) (0·0–39·0) | 6 (100) (61·0–100) | 0 (0) (0·0–39·0) |
| 2005–2006 | 0 (0) (0·0–5·8) | 63 (100) (94·3–100) | 0 (0) (0·0–5·8) |
| 2006–2007 | 25 (86) (69·4–95·0) | 4 (14) (5·5–30·6) | 0 (0) (0·0–11·7) |
| 2007–2008 | 19 (37) (24·8–50·1) | 33 (63) (49·9–75·2) | 0 (0) (0·0–6·9) |
| 2008–2009 | 29 (16) (11·5–22·3) | 20 (11) (7·4–16·6) | 130 (73) (65·7–78·6) |
*CI, confidence interval.
On March 30, 2009, a specimen was collected from a 9‐year‐old female participant from Brawley, California, meeting the ILI case definition. When standard reverse transcriptase (RT‐PCR) assays found infection with an untyped influenza A virus, these samples were analyzed by RT‐PCR and electrospray ionization mass spectrometry assay on an Ibis T5000 platform (Ibis Biosciences, Inc., Carlsbad, CA, USA). 17 This analysis showed a novel reassortant influenza virus, with swine, human, and avian components as the highest probability match. Subsequent characterization at CDC determined that the isolated virus from the participant was identical to the pandemic influenza A/H1N1 virus collected 2 days later from a 10‐year‐old boy from San Diego. These were the first two laboratory identified cases captured and recorded during the initial outbreak (16).
Discussion
Acute respiratory infections contribute significantly to morbidity and mortality, especially in persons younger than 5 years of age. 1 , 18 Mexico, Central, and South America have some of the highest ARI rates. 19 , 20 , 21 By elucidating the causes of ILI, public health responses can be implemented to minimize the effect of disease. In this study, we describe the etiologies associated with ILI in participants sampled at clinic sites on the US‐Mexico border from 2004 through 2009. The most common etiology diagnosed was influenza (25%). We did not find differences in the types of pathogens isolated from clinics on each respective side of the US‐Mexico border, but we found significant differences in the frequency at which influenza A and bacterial pathogens were isolated across the border. The finding of a higher proportion of bacterial pathogens from the Mexico clinics is unexpected, and we do not have a ready explanation for this difference. Continuing surveillance in these populations will allow tracking of this difference, if it persists. The higher proportion of influenza A seen at US clinics compared with Mexico clinics was somewhat surprising with influenza vaccination reported less in Mexico enrollees. When analyses were limited to only seasons having meaningful participation of both US and Mexican clinics (2007–2009), the proportion positive for influenza A was 20% for US clinics and 14% for Mexico clinics, still a significant difference (P < 0·05; data not shown). It is possible that storage or transport conditions may have contributed to the lower yield among Mexico samples; however, all study samples were ostensibly collected, stored, and transported using the same procedures. The higher proportion of influenza A at US clinics could reflect a focus in Mexico starting during the 2006–2007 winter season to extend influenza vaccination to include all children aged 6–35 months which has resulted in a higher vaccination rate in this age group than was seen in our self‐reported vaccination data. 22 However, the 2008–2009 seasonal vaccine would not have had much, if any, protective effect against the pandemic influenza virus. This is seen in the negligible difference between the proportion of vaccinated and unvaccinated influenza positive cases in Mexico. This probably reflects the fact that the pandemic occurred in the second year of Mexico’s participation, for which that year’s influenza vaccine was not well matched.
Most studies of ARI in Mexico have focused on children, but very few have conducted testing to determine their precise etiologies. Those studies that have performed viral testing have shown varying results, as expected. Furthermore, most of the studies have investigated RSV, PIV, and Mycoplasma pneumoniae, especially in children during the first 2 years of life. 23 , 24 , 25 Cabello et al. 26 studied Mexican children under the age of 5 years and found RSV was the most prevalent cause of ARI. Another group studied Mexican children with rhinopharyngitis and a history of asthma and wheezing. They found RSV was the predominant cause in preschool‐aged children, and influenza A was the main cause among school‐aged children and adolescents. 27 A study in a cohort of 100 Mexican children who were aged 6–12 years, in the same school, with ARI, determined significant PCR evidence that AdV C was the leading cause of ARI, with 23% of cases being AdV positive. 28 ARI has a different case definition than ILI (ARI – presence of two or more of the following symptoms: fever, cough, sore throat, sneezing, congestion, aphonia, or rhinorrhea) and this may result in different rates for pathogens identified compared with this surveillance, which used the ILI case definition.
In our surveillance, we found influenza was the most common cause of ILI in all age groups, but especially so in the 6–15 years age group. This trend was seen in both pre‐pandemic and pandemic specimens. We found lower prevalence of RSV (0·6%) and AdV (4%) than earlier studies. RSV tends to be seasonal, causing localized outbreaks mainly affecting young children, older adults, and immunocompromised patients. Sample collection technique is critical in RSV testing. The best and most frequently used sample is a nasal aspirate or wash. Our study used nasal swab as the specimen type, which may partially explain the low levels of RSV we found. However, the RSV‐positive cases we did find were in the expected 1–5 and >54 years age groups.
Adenovirus infections occur worldwide in humans and are common in all age groups, causing both hospital‐ and community‐acquired epidemics. AdV probably accounts for 3% of the infections in the civilian population (http://www.who.int/healthinfo/global_burden_disease/en/index.html). In children younger than age 5 years, AdV causes approximately 5% of upper tract respiratory infections 29 and are probably responsible for approximately 10% of the pneumonias in childhood. By age 6, 95% of children are seropositive for AdV. 30 The prevalence of AdV infection in our study (4%) was similar to those in these studies.
Limitations of this surveillance may include collection from a limited number of sites and the clinic population possibly not being representative of the border population as a whole. In addition, at the clinics, not all patients with ILI may be sampled. Self reported data collected on the case report form may introduce a recall bias. The clinics may vary on the quality of the data collected on the case report form and collection, handling, storage, and shipping of the specimens. During the pandemic, many new sites started to collect specimens, often with limited or no training, which could have affected the quality of the specimens and/or data. The data from the sites in Mexico was collected over 2 years with the majority of specimens (410 of 553) collected in a single surveillance year. Differences between the US and Mexico may reflect this shorter period of collection in Mexico and not an overall trend.
Ongoing knowledge of the circulating pathogens in this region contributes to public health preparedness. In <2 weeks after being received at NHRC, an ILI specimen was found to be unsubtypeable, was analyzed on the IBIS T‐5000 and sent to the CDC. The identification of a second influenza case with a nearly, if not identical, previously unseen influenza virus was a harbinger of the coming 2009 pandemic. The discovery of one of the first identified cases of pandemic H1N1 influenza in this population illustrated the importance of surveillance in border regions. Timely transport, testing, and communication allowed health officials to act quickly to diagnose and treat patients, and initiate epidemiologic studies to define groups at risk for severe disease. Across the border region, the number of surveillance sites were increased and severe acute respiratory illness surveillance has been initiated in local hospitals. In summary, this surveillance system met its primary objectives – to identify the respiratory pathogens responsible for ILI and to detect emerging respiratory pathogens in the border region.
This collaborative research has fostered cooperation, joint training, and communication between the participating entities. Mexico has responded to the challenge of pandemic H1N1 and made great strides in strengthening its viral respiratory laboratory diagnostic capacity in state health departments. These binational collaborative relationships will continue to be important in the event of other public health emergencies.
Additional authors’ addendum
A. W. Hawksworth (Naval Health Research Center, San Diego, CA, USA): conceived program, data analysis, write manuscript; D. J. Faix (Naval Health Research Center, San Diego, CA, USA): conceived program, write manuscript; M. L. Nava (ISESALUD, Baja California, Mexico): conceived program; L. Wong Lopez (ISESALUD, Baja California, Mexico): conceived program; E. Palacios (Department of Population Studies, El Colegio de la Frontera Norte, Tijuana, B. C., Mexico): conceived program; R. Flores (National Institute of Epidemiological Reference, Secretariat of Health, Mexico): conceived program, write manuscript; M. Fonseca‐Ford (Centers for Disease Control and Prevention, Atlanta, GA, USA): conceived program, data analysis; A. Phippard (Centers for Disease Control and Prevention, Atlanta, GA, USA): conceived program, write manuscript; K. Lopez (Imperial County Public Health Department, CA, USA):conceived program; J. Johnson (County of San Diego Health and Human Services Agency, CA, USA): write manuscript; J. G. Bustamante Moreno (ISESALUD, Baja California, Mexico): conceived program; K. L. Russell (Armed Forces Health Surveillance Center, Silver Spring, MD): conceived program; S.H. Waterman (Centers for Disease Control and Prevention, Atlanta, GA, USA): conceived program, write manuscript.
Biographical sketch
Dr. Kammerer works at the Naval Health Research Center in San Diego on a number of respiratory illness surveillance projects. He received his Doctor of Medicine degree from Hahnemann University in Philadelphia and his Master of Public Health degree from San Diego State University.
Acknowledgements
We thank Melinda Balansay, Daisy Cabrera, Rob Coon, Christian Hansen, Karen Ferran, Akiko Kimura, Angelica Pon, and the staffs of participating county and jurisdictional health departments and clinics. This represents report 11‐15, supported by the Department of Defense, under work unit no. 60501. This study was also funded by grants from the Centers for Disease Control and Prevention, the Armed Forces Health Surveillance Center Global Emerging Infections Surveillance and Response System, and the US Department of State Biosecurity Engagement Program. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, the US Government, or the CDC. Approved for public release; distribution is unlimited. This research has been conducted in compliance with all applicable federal regulations governing protection of human subjects in research.
In this surveillance project, influenza virus was the most commonly identified pathogen in influenza‐like illness specimens from both sides of the border from 2004 through 2009.
References
- 1. Monto AS, Lehmann D. Acute respiratory infections (ARI) in children: prospects for prevention. Vaccine 1998; 16:1582–1588. [DOI] [PubMed] [Google Scholar]
- 2. Mulholland K. Global burden of acute respiratory infections in children: implications for interventions. Pediatr Pulmonol 2003; 36:469–474. [DOI] [PubMed] [Google Scholar]
- 3. Weissenbacher M, Carballal G, Avila M et al. Etiologic and clinical evaluation of acute lower respiratory tract infections in young Argentinian children: an overview. Rev Infect Dis 1990; 12(Suppl 8):S889–S898. [DOI] [PubMed] [Google Scholar]
- 4. Molinari NA, Ortega‐Sanchez IR, Messonnier ML et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–5096. [DOI] [PubMed] [Google Scholar]
- 5. Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008; 358:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poland GA, Tosh P, Jacobson RM. Requiring influenza vaccination for health care workers: seven truths we must accept. Vaccine 2005; 23:2251–2255. [DOI] [PubMed] [Google Scholar]
- 7. MacPherson DW, Gushulak BD, Baine WB et al. Population mobility, globalization, and antimicrobial drug resistance. Emerg Infect Dis 2009; 15:1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doyle TJ, Bryan RT. Infectious disease morbidity in the US region bordering Mexico, 1990–1998. J Infect Dis 2000; 182:1503–1510. [DOI] [PubMed] [Google Scholar]
- 9. Weinberg M, Waterman S, Lucas CA et al. The U.S.‐Mexico Border Infectious Disease Surveillance project: establishing bi‐national border surveillance. Emerg Infect Dis 2003; 9:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freed NE, Myers CA, Russell KL et al. Diagnostic discrimination of live attenuated influenza vaccine strains and community‐acquired pathogenic strains in clinical samples. Mol Cell Probes 2007; 21:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metzgar D, Osuna M, Kajon AE, Hawksworth AW, Irvine M, Russell KL. Abrupt emergence of diverse species B adenoviruses in US military recruit training centers. J Infect Dis 2007; 196:1465–1473. [DOI] [PubMed] [Google Scholar]
- 12. Metzgar D, Skochko G, Gibbins C, Hudson N, Lott L, Jones MS. Evaluation and validation of a real‐time PCR assay for detection and quantitation of human adenovirus 14 from clinical samples. PLoS One 2009; 4:e7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall TA, Budowle B, Jiang Y et al. Base composition analysis of human mitochondrial DNA using electrospray ionization mass spectrometry: a novel tool for the identification and differentiation of humans. Anal Biochem 2005; 344:53–69. [DOI] [PubMed] [Google Scholar]
- 14. Wilson EB. What is statistics? Science 1927; 65:581–587. [DOI] [PubMed] [Google Scholar]
- 15. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927; 22:209–212. [Google Scholar]
- 16. Ginsberg M, Hopkins J, Maroufi A et al. Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep 2009; 58:400–402. [PubMed] [Google Scholar]
- 17. Sampath R, Hall TA, Massire C et al. Rapid identification of emerging infectious agents using PCR and electrospray ionization mass spectrometry. Ann N Y Acad Sci 2007; 1102:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garbino J, Gerbase MW, Wunderli W et al. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med 2004; 170:1197–1203. [DOI] [PubMed] [Google Scholar]
- 19. Noyola DE, Rodriguez‐Moreno G, Sanchez‐Alvarado J, Martinez‐Wagner R, Ochoa‐Zavala JR. Viral etiology of lower respiratory tract infections in hospitalized children in Mexico. Pediatr Infect Dis J 2004; 23:118–123. [DOI] [PubMed] [Google Scholar]
- 20. Reyes M, Hedlund KO, Lorenzana I, Ehrnst A. Respiratory infection and iatrogenic diarrhea in Honduras and El Salvador during the 1991–1992 season. Am J Trop Med Hyg 1996; 54:260–264. [DOI] [PubMed] [Google Scholar]
- 21. Bellei N, Carraro E, Perosa A, Watanabe A, Arruda E, Granato C. Acute respiratory infection and influenza‐like illness viral etiologies in Brazilian adults. J Med Virol 2008; 80:1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vacunacion Universal Y Semanas Nacionales De Salud Lineamientos Generales, 2008. Available at http://www.censia.salud.gob.mx/descargas/vacunacion/vac_univ08_lin_pre.pdf (Accessed 27 October 2010).
- 23. Golubjatnikov R, Allen VD, Olmos‐Blancarte MP, Inhorn SL. Serologic profile of children in a Mexican highland community: prevalence of complement‐fixing antibodies to Mycoplasma pneumoniae, respiratory syncytial virus and parainfluenza viruses. Am J Epidemiol 1975; 101:458–464. [DOI] [PubMed] [Google Scholar]
- 24. Tirado R, Sarmiento RE, Bustos J, Thompson O, Gomez B. Occurrence of respiratory syncytial virus subtypes in Mexican infants with acute lower respiratory tract disease. Arch Med Res 1995; 26:121–126. [PubMed] [Google Scholar]
- 25. Bustamante‐Calvillo ME, Velazquez FR, Cabrera‐Munoz L et al. Molecular detection of respiratory syncytial virus in postmortem lung tissue samples from Mexican children deceased with pneumonia. Pediatr Infect Dis J 2001; 20:495–501. [DOI] [PubMed] [Google Scholar]
- 26. Cabello C, Manjarrez ME, Olvera R, Villalba J, Valle L, Paramo I. Frequency of viruses associated with acute respiratory infections in children younger than five years of age at a locality of Mexico City. Mem Inst Oswaldo Cruz 2006; 101:21–24. [DOI] [PubMed] [Google Scholar]
- 27. Lopez Perez G, Morfin Maciel BM, Navarrete N, Aguirre A. Identification of influenza, parainfluenza, adenovirus and respiratory syncytial virus during rhinopharyngitis in a group of Mexican children with asthma and wheezing. Rev Alerg Mex 2009; 56:86–91. [PubMed] [Google Scholar]
- 28. Rosete DP, Manjarrez ME, Barron BL. Adenoviruses C in non‐hospitalized Mexican children older than five years of age with acute respiratory infection. Mem Inst Oswaldo Cruz 2008; 103:195–200. [DOI] [PubMed] [Google Scholar]
- 29. Lazzaro T, Hogg G, Barnett P. Respiratory syncytial virus infection and recurrent wheeze/asthma in children under five years: an epidemiological survey. J Paediatr Child Health 2007; 43:29–33. [DOI] [PubMed] [Google Scholar]
- 30. Bourgeois FT, Valim C, McAdam AJ, Mandl KD. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics 2009; 124:e1072–e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]


