Abstract
Please cite this paper as: Hernandez et al. (2012) Impact of the 2009/2010 influenza A (H1N1) pandemic on trends in influenza hospitalization, diagnostic testing, and treatment. Influenza and Other Respiratory Viruses 6(5), 305–308.
Analysis of a US hospitalization database demonstrated that more influenza patients were hospitalized and the age distribution of hospitalizations was younger during the 2009 (H1N1) influenza A pandemic compared with the three previous influenza seasons. The duration of hospital stay remained stable in all four seasons. A higher proportion of patients was treated with antivirals (P < 0·0001), comprised almost entirely of neuraminidase inhibitors, and the proportion was highest in those with influenza confirmed by diagnostic testing (P < 0·0001). Approximately one‐third remained untreated. Young children had the lowest rate of neuraminidase‐inhibitor treatment during the 2009 pandemic (P < 0·05).
Keywords: Antivirals, hospitalization, influenza
Introduction
The 2009 (H1N1) influenza A pandemic created a public health emergency in the USA. 1 Rates of hospitalization, admission to ICUs, and invasive life support seemed higher than observed for seasonal influenza. 2 , 3 By mid‐December, an estimated 55 million were infected and 246 000 were hospitalized in the USA; 4 a modeling approach used in another study provided even higher estimates. 5 The Centers for Disease Control and Prevention and the World Health Organization recommended early treatment with antivirals in patients hospitalized with 2009 (H1N1) influenza A. 6 , 7 In uncontrolled cohort studies in hospitalized influenza patients, early use of antivirals, particularly neuraminidase inhibitors (NAIs), was associated with improved survival and shorter hospital stays. 8 , 9 We used a large electronic database to evaluate the impact of the 2009 (H1N1) pandemic on the diagnosis of, hospitalization of, and treatment for influenza in the USA compared with previous seasons.
Study design
The SDI database used for this study contained standardized, patient‐level, anonymized healthcare encounter records for all patients admitted to a large sample (approximately 20%) of non‐government‐funded hospitals in the USA. In 2009, the database included patients from hospitals in all 50 states that were urban (85%) and medium‐ to large‐sized (100–500 beds) (72%); 36% were teaching hospitals. Records from October 2006 through March 2010 were included in the study; they were representative of the population with regard to age, gender, and regional distribution and included all payers (i.e., Medicare/Medicaid, third‐party payer claims, and cash). De‐identified HIPAA‐compliant patient information was made available in the database within 2–4 weeks of discharge.
We selected records containing a discharge diagnosis of influenza (ICD‐9 codes 487–488) for patients discharged during October 2006–March 2010. We compared the influenza seasons of 2006–2007, 2007–2008, and 2008–2009 (October–April) to two waves of the 2009 (H1N1) pandemic (May–July 2009 and August 2009–March 2010) for the number of hospitalized influenza cases, age of patients, use of diagnostic tests for influenza [current procedural terminology (CPT) codes for rapid antigen test, polymerase chain reaction test, immunofluorescence, culture, or antibodies], antiviral treatments, and duration of hospital stay.
In the population of patients who were hospitalized, we compared the proportions by age group, use of diagnostic testing, and use of antiviral treatment between seasons or age groups by a two‐sample test for equality of proportions with continuity correction. The duration of hospital stay was compared between seasons by Wilcoxon, Log‐Rank, and Log Likelihood tests.
Results
The characteristics of hospitalized patients with influenza diagnosis (total = 37 965) in the SDI database over time are summarized in Table 1. The 2009 (H1N1) influenza A pandemic was unique in commencing in the late spring, having a longer duration, and demonstrating two distinct waves; the number of hospitalized influenza cases was several times larger than that in previous influenza seasons.
Table 1.
Characteristics of hospitalized influenza patients from the SDI database by season
| Description | October 2006–April 2007 | October 2007–April 2008 | October 2008–April 2009 | May 2009–July 2009 | August 2009–March 2010 |
|---|---|---|---|---|---|
| Total diagnosed with influenza | 3453 | 9162 | 4138 | 3545 | 17 667 |
| Age group, n (%) | |||||
| 0–5 years | 1275 (37) | 2110 (23) | 1592 (38) | 971 (27) | 4076 (23) |
| 6–18 years | 386 (11) | 513 (6) | 529 (13) | 590 (17) | 2428 (14) |
| 19–64 years | 948 (27) | 2832 (31) | 1250 (30) | 1584 (45) | 9079 (51) |
| ≥65 years | 844 (24) | 3707 (40) | 767 (19) | 400 (11) | 2084 (12) |
| Duration of hospital stay, n (%) | |||||
| 1–2 days | 1496 (43) | 3295 (36) | 1786 (43) | 1448 (41) | 6999 (40) |
| 3–5 days | 1178 (34) | 3449 (38) | 1406 (34) | 1170 (33) | 6125 (35) |
| 6–9 days | 431 (12) | 1435 (16) | 542 (13) | 492 (14) | 2433 (14) |
| ≥10 days | 348 (10) | 983 (11) | 404 (10) | 435 (12) | 2110 (12) |
| Diagnostic test, n (%) | 2182 (63) | 5780 (63) | 2640 (64) | 2549 (72) | 11 247 (64) |
| Antiviral therapy, n (%)* | |||||
| Adamantanes | 59 (2) | 114 (2) | 338 (8) | 134 (4) | 215 (1) |
| NAIs | 1129 (33) | 3878 (42) | 1172 (28) | 1838 (52) | 12285 (70) |
| Peramivir | 0 | 2 | 0 | 0 | 10 |
| No antivirals | 2278 (66) | 5208 (57) | 2886 (70) | 1704 (48) | 5357 (30) |
NAI, neuraminidase inhibitor.
*Patients may have used more than one antiviral medication.
Patients in the 2009–2010 pandemic waves had a different age distribution than patients seen in the three preceding seasons (2006–2007: P = 0·0003; 2007–2008: P < 0·0001; 2008–2009: P < 0·0001). A higher proportion of patients in the 2009–2010 pandemic waves were in the 19–64 age group than in the three preceding seasons (2009–2010: 50% versus 2006–2007: 27%, 2007–2008: 31%, 2008–2009: 30%) and fewer were ≥65 years old (2009–2010: 12% versus 2006–2007: 24%, 2007–2008: 40%, 2008–2009: 24%). The duration of hospital stay was similar across influenza periods from 2006 to 2010 (P > 0·05) with 10–12% of stays ≥10 days (Table 1).
The proportion of hospitalized patients that received antiviral therapy increased from 34% in the 2006–2007 season to 70% during the second wave of the 2009 (H1N1) pandemic (Table 1, P < 0·0001). Antiviral therapy was comprised almost entirely of NAIs. During the two pandemic waves in 2009/2010, 7061 (33%) of the 21 212 hospitalized influenza patients received no antiviral treatment. Of the subset of influenza patients with a diagnostic test recorded, 71% were treated with antivirals compared to 58% of patients without a test, indicating that tested patients were 1·8 times more likely than untested to receive antiviral treatment (OR 1·8; 95% CI, 1·67–1·88; P < 0·0001). The proportion of hospitalized influenza patients that had a diagnostic test to confirm influenza remained fairly constant during the study period.
During the three previous influenza seasons, the rate of treatment with antivirals was greater for adults than for children (Figure 1). From 2006 to 2009, a higher proportion of adults ≥19 years of age hospitalized with seasonal influenza (52%) were treated with antivirals compared with children (15%, P < 0·001). This age‐related difference disappeared during the pandemic except for those patients 0–5 years (56%) compared with ≥6 years (70%, P = 0·046).
Figure 1.
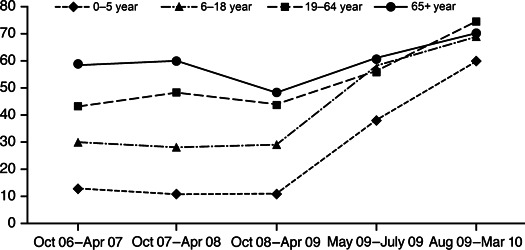
The proportion of hospitalized patients in each age group who were treated with antivirals by influenza season.
Discussion
Results from this study of recent influenza trends in a 20% sample of US hospitals show that substantially more patients were hospitalized with influenza during the 2009 (H1N1) influenza A pandemic than in three previous influenza seasons. This likely reflects the lack of pre‐existing immunity to the novel H1N1 strain in persons under 65 years and its tropism to the lower respiratory tract. 10 The age distribution of hospitalizations was younger than that of previous seasons, consistent with the “signature age shift” typically experienced with pandemic influenza. 11 These data are consistent with other reports from studies of 2009 pandemic influenza patients in the USA. 12 , 13 , 14
We found that treatment with antivirals, particularly NAIs, was more prevalent during the 2009 (H1N1) pandemic than in pre‐pandemic influenza seasons, possibly due to awareness of the pandemic, concerns about disease severity, and available guidelines that stressed early treatment of hospitalized patients. 6 In a case study of hospitalized US patients in the first wave of the pandemic, Jain et al. 2 reported that 75% of approximately 200 patients received antiviral treatment. We studied considerably more patients and found lower rates; 52% and 70% of patients in the first and second waves of the pandemic, respectively, were treated with antivirals; of these, only 7% and 2%, respectively, were treated with adamantanes. The low use of adamantanes may reflect early knowledge of the high rate of adamantane resistance and oseltamivir sensitivity of the 2009 pandemic H1N1 viruses and public health authority guidance. 13 As expected, absence of diagnostic influenza testing was associated with a lower rate of antiviral treatment.
Our data revealed that in previous seasons, older hospitalized patients were more likely to be treated with antivirals than younger patients, perhaps because of awareness of their higher mortality rates. In the 2009 (H1N1) pandemic, however, the proportion of patients who were treated rose in every age group so that >65% of patients in each of the 6–18 years, 19–64 years, and ≥65 years age groups were treated with antivirals.
The advantages of this electronic database include the timeliness of available data (lag period of only 1–2 months) and access to a large number of records for patients hospitalized with pandemic influenza. However, the study was limited by our use of ICD‐9 and influenza CPT codes to identify patients and the lack of information about the patients’ clinical course, mortality, detailed viral testing, and treatment prior to admission. Also, we did not have access to data on the delay from start of symptoms, diagnosis, and hospitalization to start of treatment, a key limitation given recent reports of the benefits of early oseltamivir treatment. 15 In addition, the reasons for lack of antiviral treatment are not known.
Conclusions
Significantly higher numbers of influenza patients were hospitalized during the 2009 (H1N1) influenza A pandemic and a higher proportion were treated with NAIs during their stay. Despite the increase in treatment for pandemic influenza, many hospitalized patients, especially young children, remained untreated, a potential missed opportunity and contrary to current public health guidelines. 6 Understanding the reasons for lack of treatment with NAIs is deserving of further study.
Acknowledgements
This study was conducted and the data analyzed by SDI Health, LLC, using their proprietary database, with additional statistical support from Stephanie Lepionka of PharPoint Research, Inc. The study and analysis were funded by BioCryst Pharmaceuticals, the manufacturer of an investigational influenza drug, peramivir. Financial support was also provided by HHS/BARDA Advanced Development Contract HHS0100200700032C. In the preparation of the manuscript, authors were assisted by a professional medical writer, Elizabeth Field, of Field Advantage Medical Communications, LLC, whose work was funded by BioCryst.
References
- 1. Johnson CE. Determination that a public health emergency exists. Available at http://www.flu.gov/professional/federal/h1n1emergency042609.html (Accessed 15 September 2010).
- 2. Jain S, Kamimoto L, Bramley AM et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April‐June 2009. N Engl J Med 2009; 361:1935–1944. [DOI] [PubMed] [Google Scholar]
- 3. Dominguez‐Cherit G, Lapinsky SE, Macias AE et al. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA 2009; 302:1880–1887. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . CDC estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States, April 2009–February 13, 2010. Available at http://www.cdc.gov/h1n1flu/estimates/April_February.htm (accessed 15 May 2010).
- 5. Viboud C, Miller M, Olson DR, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons [Internet]. Version 59. PLoS Curr 2010; PMC2843747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. Available at http://www.cdc.gov/h1n1flu/recommendations.htm (accessed 15 May 2010).
- 7. Uyeki T. Antiviral treatment for patients hospitalized with 2009 pandemic influenza A (H1N1). N Engl J Med 2009; 361:e110. [DOI] [PubMed] [Google Scholar]
- 8. Lee N, Choi KW, Chan PK et al. Outcomes of adults hospitalized with severe influenza. Thorax 2010; 65:510–515. [DOI] [PubMed] [Google Scholar]
- 9. McGeer A, Green KA, Plevneshi A et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568–1575. [DOI] [PubMed] [Google Scholar]
- 10. Shieh WJ, Blau DM, Denison AM et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 2010; 177:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009; 360:2595–2598. [DOI] [PubMed] [Google Scholar]
- 12. Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza . Clinical Aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708–1719. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Update: influenza activity – United States, 2009–10 season. MMWR Morb Mortal Wkly Rep 2010; 59:901–908. [PubMed] [Google Scholar]
- 14. Ross TM, Zimmer S, Burke D et al. Seroprevalence following the second wave of pandemic 2009 H1N1 influenza [Internet]. Version 30. PLoS Curr 2010: PMC2828126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuman A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother 2011; 66:959–963. [DOI] [PubMed] [Google Scholar]


