Abstract
Background Influenza A(H1N1)pdm09 virus has been circulating in human population for three epidemic seasons. During this time, monovalent pandemic and trivalent seasonal influenza vaccination against this virus have been offered to Finnish healthcare professionals. It is, however, unclear how well vaccine‐induced antibodies recognize different strains of influenza A(H1N1)pdm09 circulating in the population and whether the booster vaccination with seasonal influenza vaccine would broaden the antibody cross‐reactivity.
Objectives Influenza vaccine‐induced humoral immunity against several isolates of influenza A(H1N1)pdm09 virus was analyzed in healthcare professionals. Age‐dependent responses were also analyzed.
Methods Influenza viruses were selected to represent viruses that circulated in Finland during two consecutive influenza epidemic seasons 2009–2010 and 2010–2011. Serum samples from vaccinated volunteers, age 20–64 years, were collected before and after vaccination with AS03‐adjuvanted pandemic and non‐adjuvanted trivalent seasonal influenza vaccine that was given 1 year later.
Results Single dose of pandemic vaccine induced a good albeit variable antibody response. On day 21 after vaccination, depending on the virus strain, 14–75% of vaccinated had reached antibody titers (≥1:40) considered seroprotective. The booster vaccination 1 year later with a seasonal vaccine elevated the seroprotection rate to 57–98%. After primary immunization, younger individuals (20–48 years) had significantly higher antibody titers against all tested viruses than older persons (49–64 years) but this difference disappeared after the seasonal booster vaccination.
Conclusions Even a few amino acid changes in influenza A HA may compromise the vaccine‐induced antibody recognition. Older adults (49 years and older) may benefit more from repeated influenza vaccinations.
Keywords: Antibodies, humoral, immunity, influenza A, pandemic, vaccine
Introduction
Since the beginning of the influenza pandemic in 2009, two vaccinations against influenza A(H1N1)pdm09 virus have been offered to Finnish healthcare professionals, first a monovalent AS03‐adjuvanted pandemic influenza vaccine in October 2009 followed by a non‐adjuvanted trivalent seasonal influenza vaccine 1 year later. Both vaccines included A/California/7/2009 as a viral antigen. Recent studies indicate that one dose of the AS03‐adjuvanted pandemic influenza vaccine induces a strong humoral immune response in adults. 1 , 2 , 3 It has also been reported that vaccination with this vaccine may reduce the risk of influenza A(H1N1)pdm09 infection among healthcare professionals. 4 In children, a trivalent influenza vaccine given 1 year after the pandemic vaccine increased the seroprotection rate against the A/California/7/2009 virus from 46% to 98%, respectively. 5 However, there is little data on the persistence of humoral immunity induced by vaccination with the pandemic influenza vaccine in adults or the booster effect obtained by vaccination with seasonal influenza vaccine. Neither it is known how well the vaccine‐induced antibodies recognize different strains of influenza A(H1N1)pdm09 circulating in the population.
In this study, we analyzed in Finnish healthcare professionals the levels of antibodies induced by vaccination with a single dose of AS03‐adjuvanted pandemic influenza vaccine followed by one dose of trivalent non‐adjuvanted seasonal influenza vaccine 1 year later. As we recently observed that minor changes in the hemagglutinin of influenza viruses may have remarkable effects in antibody recognition, 6 we compared antibody responses against the vaccine strain and six other influenza A(H1N1)pdm09 viruses isolated in Finland during the 2009–2010 and 2010–2011 epidemic seasons. In addition, we evaluated age‐related differences in vaccine‐induced antibody responses.
Materials and methods
Participants
Clinically healthy volunteers were recruited from the personnel of the Department of Medicine, Helsinki University Hospital and the Virology Unit, National Institute for Health and Welfare. The participants, 14 men and 82 women (all Caucasian), were 20 to 64 years old (median 48 years) at the time of the pandemic vaccination in 2009. The study was approved by the Ethical Committee of the Helsinki‐Uusimaa Health District (Permissions 382/E5/07 §48/2008 and §289/2010 and 199/13/03/00/2009 §164) and received an European Union clinical trials database code of EudraCT 2010‐023313‐57. All participants gave their written informed consent before enrollment in the study.
Vaccines
Pandemrix TM (GlaxoSmithKline Biologicals, GSK, lots A81CA069A and A81CA072A) was given as a single dose of 0·5 ml containing 3·75 μg of hemagglutinin (HA) and AS03‐adjuvant according to the manufacturer’s instructions. 7 The seasonal influenza vaccine was a trivalent non‐adjuvanted vaccine Fluarix TM (GSK, lots AFLUA523AA, AFLU573AA, and AFLU574AA) containing the three WHO‐recommended influenza virus strains. Both vaccines were administered intramuscularly (deltoid muscle). Pandemrix vaccine was given on day 0 and the seasonal vaccine 1 year later (Figure 1). Thirteen volunteers were given also a second dose of Pandemrix on day 90 (and serum samples collected 21 days after the second vaccination). Serum samples were collected prior to vaccination on day 0 and the post‐vaccination serum specimens were collected on days 21, 90, 365 (day 0 for the seasonal influenza vaccine), and 21 and 90 days after the seasonal vaccination (days 386 and 455 from the beginning of the study) (Figure 1).
Figure 1.
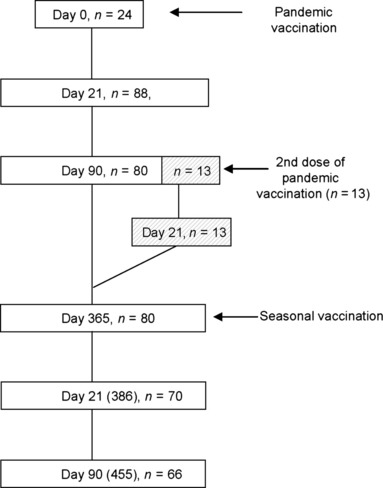
A schematic presentation of study design. The study included 96 clinically healthy healthcare professionals aged 20–64 years. The subject received monovalent Pandemrix vaccination at day 0 and follow‐up samples were collected at days 21, 90, and 365. A subgroup of vaccinees (n = 13), who were low responders to the initial Pandemrix vaccination received a second dose at day 90 and an additional follow‐up sample was collected 21 days later. At day 365, all subjects received a second trivalent non‐adjuvanted seasonal influenza vaccine and follow‐up samples were collected at days 21 and 90 after the booster vaccination. The numbers at each time point indicate the number of subjects of whom serum samples were collected.
Viruses
We have previously reported the circulation of four genetic groups of influenza A(H1N1)pdm09 viruses in Finland during the 2009–2010 pandemic wave and three groups during the 2010–2011 influenza season. 6 , 8 Representative viruses from these groups were selected for serological analyses: A/Finland/634/2009, A/Finland/686/2009, A/Finland/694/2009, A/Finland/6/2010, A/Finland/19/2010, and A/Finland/20/2010. In addition, the A/California/07/2009 vaccine virus was also included in the analyses. Phylogenetic analysis of the HA gene of selected Finnish viruses and reference strains was performed as described. 8 mega (Molecular Evolutionary Genetics Analysis) software version 4 9 was used in amino acid sequence comparison and the construction of the phylogenetic tree. The Neighbor‐joining method 10 with the maximum composite likelihood model 11 was used to generate the phylogenetic tree. Bootstrapping was performed with 1000 replicates. 12 Reference virus sequences for the phylogenetic tree were obtained from GISAID EpiFluTMDatabase (Table S1). The GenBank accession numbers of Finnish strains are HQ228083, HQ228125, HQ228133, JN601076, JN601088, and JN601089. The accession number for California/07/2009 is FJ966974.
The three‐dimensional structure of the HA molecule of influenza A(H1N1)pdm09 virus, A/California/04/2009 (RCSB Protein Bank accession number 3LZG) was used to locate amino acid differences between the Finnish A(H1N1)pdm09 viruses (seasons 2009–2010 and 2010–2011) and the A/California/07/2009 vaccine virus. The molecular models were constructed using RasMol Molecular Graphics software version 2.7.3. 13 Amino acid residues in the HA molecule are numbered without the signal peptide sequence. The clinical samples for isolation of viruses included in this study have been collected for routine viral diagnostic purposes. According to Finnish legislation, ethical permission is not required for specific microbiological diagnostics and further characterization of detected viruses. All viruses were propagated in MDCK cells and stored in aliquots at −80°C.
Serologic assays
All serum specimens were assayed by the hemagglutination inhibition (HI) test against the viral strains described previously. The HI tests were performed according to WHO guidelines using turkey erythrocytes (0·5% vol/vol). 14 , 15 For statistical analyses, serum specimens with HI titers <10 were assigned a titer value of 5.
Statistical analysis
Antibody levels were measured on days 0, 21, 90, and 365 after pandemic lvaccination and on days 0, 21, and 90 after vaccination with the seasonal influenza vaccine. Geometric mean titers (GMT) with 95% confidence intervals and presumable seroprotection rate (HI titer ≥1:40) for each virus were calculated. Statistically significant differences were calculated using Student’s t‐test (paired, two‐tailed) and the significance level was adjusted to P < 0·05.
Results
Vaccination with AS03‐adjuvanted pandemic influenza vaccine‐induced good antibody response
Altogether 96 adults were enrolled into the study. Antibody titers against seven influenza A(H1N1)pdm09 viruses were determined by the HI test. The selected viruses genetically resemble the WHO reference viral strains and cluster to corresponding genetic groups (Figure 2). The participants had practically no pre‐existing antibodies against any tested viruses (GMTs on day 0 between 5·0 and 6·7). We were able to collect only 24 pre‐vaccination samples (age range 27–62 years, median age 41 years) but according to previous reports by others and us 16 , 17 , 18 , 19 , 20 this age group lacked cross‐reactive antibodies against A(H1N1)pdm09 viruses before the pandemic and we therefore consider the baseline formed by these 24 samples applicable in the analysis.
Figure 2.
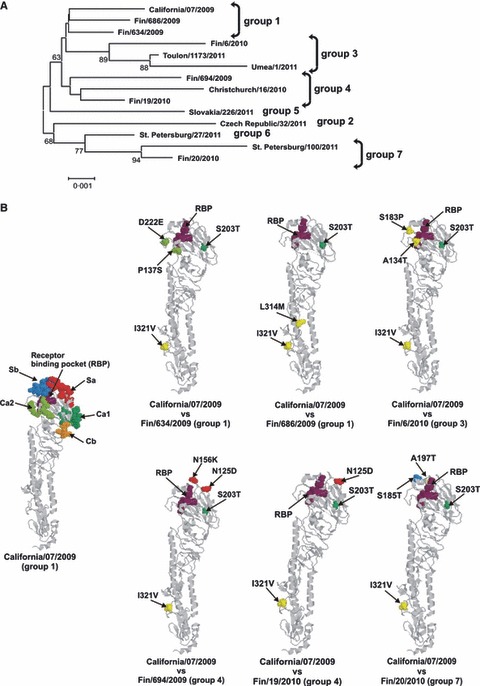
(A) Influenza A(H1N1)pdm09 strains isolated in Finland cluster in the same genetic groups with WHO reference strains in the phylogenetic tree of the HA. All sequences included in the phylogenetic tree constitute the entire 1698 nucleotide long coding region of HA. The horizontal lines are proportional to the number of nucleotide changes. (B) Schematic representation of amino acid differences in the HA molecule between the Finnish influenza A(H1N1)pdm09 viruses and the vaccine virus, A/California/07/2009. On the left, a side view of the monomeric structure of HA molecule of influenza A(H1N1(2009) (A/California/04/2009; RCSB Protein Bank accession number 3LZG) with previously identified H1 protein‐specific antigenic sites (Sa in red, Sb in blue, Ca1 in darker green, Ca2 in lighter green, and Cb in orange) of influenza A(H1N1) viruses and with the receptor binding pocket (RBP, purple) is shown. The amino acid changes of Finnish A(H1N1)pdm09 viruses compared with A/California/07/2009, the vaccine strain, are illustrated in the monomeric HA structure and colored as in A/California/07/2009 structure. Amino acid changes outside the antigenic sites are shown in yellow. Changes are illustrated by amino acid residue number and with serial number of virus where the respective amino acid change has been observed.
Three weeks after vaccination GMTs had risen to 10–108 depending on the viral strain (Table 1). Those remarkable differences associate with mutations in the HA of the tested viruses (Figure 2). Especially important are the mutations that locate to antigenic sites as in A/Finland/694/2009 viral strain, which showed clearly reduced antibody titers (GMT 9·8). This virus has mutations in antigenically important Sa sites (N125D and N156K), which affect its antigenic properties and lead to reduced antibody recognition (Figures 2B and 3). 6 On the other hand, the highest antibody titers were observed against the A/California/7/2009 vaccine virus and circulating viruses A/Finland/20/2010 and A/Finland/634/2009. A/Finland/20/2010, and A/Finland/634/2009, which have mutations in their Sb and Ca2 antigenic sites, respectively, (Figures 2B and 3). However, these mutations evidently did not affect the antibody recognition at least negatively. Thirteen volunteers (age 38 to 63 years, median 46 years) who had low antibody responses on day 21 (GMT for A/California/7/09 23·8, seroprotection rate 50%) received a second dose of pandemic vaccine on day 90. Serum samples were collected 21 days after this booster vaccination. Despite this second dose of pandemic vaccine, the antibody titers remained a lower level (not significantly) compared with those who received only one dose of pandemic vaccine. (data not shown). These low‐reacting samples were included in all analyses because exclusion would have biased this study.
Table 1.
Vaccine‐induced humoral immune responses against different influenza A(H1N1)pnd09 viral strains.
| California/7/09 | Finland/634/09 | Finland/686/09 | Finland/694/09 | Finland6/10 | Finland/19/10 | Finland/20/10 | |
|---|---|---|---|---|---|---|---|
| Geometric mean titer [95% CI] | |||||||
| Pandemic vaccine* | |||||||
| Day 0 (n = 24) | 5·3 [4·9–5·7] | 5·8 [5·0–6·7] | 5·6 [4·9–6·4] | 5·0 [5·0–5·0] | 5·8 [5·0–6·7] | 5·8 [4·9–6·8] | 6·7 [5·4–8·3] |
| Day 21 (n = 88) | 65·2 [48·7–87·3] | 82·6 [58·4–116·7] | 57·5 [40·9–80·7] | 9·8 [8·3–11·6] | 38·2 [27·0–53·8] | 43·6 [32·0–59·4] | 107·9 [75·8–153·6] |
| Day 90 (n = 80**) | 31·1 [23·5–41·2] | 39·0 [28·0–54·2] | 31·7 [23·4–42·9] | 7·8 [6·6–9·2] | 21·8 [15·8–30·1] | 23·0 [17·4–30·4] | 60·6 [43·9–83·7] |
| Seasonal vaccine*** | |||||||
| Day 365 (n = 80) 80 | 20·9 [16·2–26·9] | 29·5 [22·3–39·2] | 22·2 [17·2–28·6] | 7·5 [6·4–8·8] | 16·8 [13·1–21·7] | 19·5 [15·1–25·2] | 38·0 [28·3–50·9] |
| Day 21 (n = 70) | 127·4 [96·7–167·9] | 187·5 [142·4–246·9] | 150·8 [114·2–199·0] | 32·2 [24·8–41·7] | 106·6 [80·6–141·0] | 139·3 [104·4–185·8] | 307·6 [236·0–400·9] |
| Day 90 (n = 66) | 74·3 [56·2–98·3] | 108·5 [81·7–144·0] | 87·0 [65·9–114·8] | 18·8 [14·8–23·8] | 60·9 [45·8–80·9] | 72·0 [53·8–96·5] | 165·1 [124·2–219·6] |
| Seroprotection rate† | |||||||
| Pandemic vaccine | |||||||
| Day 0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 0·0 | 4·2 |
| Day 21 | 69·3 | 71·6 | 62·5 | 13·6 | 50·0 | 56·8 | 75·0 |
| Day 90 | 51·3 | 53·8 | 50·0 | 6·3 | 42·5 | 41·3 | 66·3 |
| Seasonal vaccine | |||||||
| Day 365 | 33·8 | 50·0 | 38·8 | 5·0 | 32·5 | 33·8 | 56·3 |
| Day 21 | 87·1 | 94·3 | 90·0 | 57·1 | 84·3 | 90·0 | 98·6 |
| Day 90 | 80·3 | 87·9 | 81·8 | 28·8 | 75·8 | 75·8 | 93·9 |
*One dose (3·75 μg HA) of AS03‐adjuvanted PandemrixTM vaccine produced by GSK Biologicals was given to healthy adults.
**On day 90, thirteen individuals were given a second dose of PandemrixTM vaccine, these are included in the analysis.
***One dose of non‐adjuvanted trivalent seasonal influenza vaccine FluarixTM produced by GSK Biologicals was given 1 year after the pandemic vaccination.
†Seroprotection rate: the percentage of subjects who have a post‐vaccination titer ≥1:40.
Depending on the viral strain, the seroprotection rates ranged from 14% to 75% on day 21 and declined to 6–66% on day 90, and to 5–56% on day 365 (Table 1). As expected, the seroprotection rate for A/Finland/694/2009 virus was clearly the lowest, while A/California/7/2009, A/Finland/20/2010, and A/Finland/634/2009 showed the highest seroprotection rates. Thus, depending on the virus strain, the HI titers varied considerably.
Revaccination with a trivalent seasonal influenza vaccine strongly boosted antibody responses against influenza A(H1N1)pdm09 viruses
One year after receiving Pandemrix, the antibody titers against influenza A(H1N1)pdm09 viruses had declined approximately 60% (GMTs 7·5–38·0) (Table 1). To analyze the effect of revaccination with A/California/07/09, volunteers were given on day 365 one dose of seasonal trivalent influenza vaccine containing A/California/07/2009 viral antigens. The geometric mean antibody titers against influenza A(H1N1)pdm09 viruses increased significantly to GMT values ranging from 32 to 308 (day 21 after the seasonal booster vaccination) depending on the viral strain (Figure 3). This increase indicates that revaccination with A/California/7/2009 induced a strong booster response.
Figure 3.
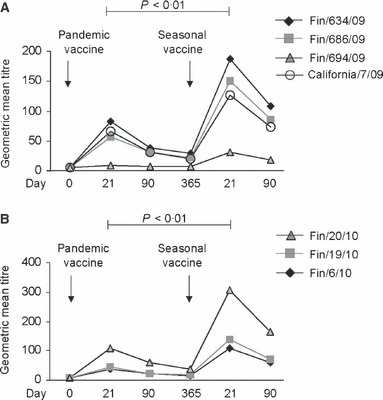
Antibody responses induced by vaccination with AS03‐adjuvanted pandemic influenza and non‐adjuvanted seasonal influenza vaccines. Antibody levels before and after vaccination with Pandemrix and seasonal influenza vaccines were analyzed by HI test using several influenza A(H1N1)pdm09 strains isolated during the 2009–2010 (A) or 2010–2011 (B) epidemic seasons. Serum samples were collected at six time points as indicated in the figure. Geometric mean titers were calculated for each viral strain and the significance of difference between post‐vaccination samples at day 21 and at day 365 were calculated using Student’s paired, two‐tailed t‐test. Statistically significant difference (P < 0·01) was observed to all viral strains.
Antibody response after influenza A(H1N1)pdm09 vaccination is age‐dependent
Vaccine‐induced antibody immune responses were analyzed in different age groups. The median age of the vaccinees was 48 years, and thus, the serum samples were evenly divided in two groups representing samples from younger and older vaccinees. Compared with older volunteers (49 to 64 years), the younger age group (20 to 48 years) showed significantly higher antibody levels against all analyzed viruses (Figure 4A). After vaccination with the seasonal influenza vaccine, antibody titers differed significantly only against two viruses: A/Finland/634/2009 and A/Finland/686/2009 (Figure 4B). When individual antibody titers against the vaccine virus on 21 day after the vaccination with Pandemrix were plotted against the age of the vaccinee, there was a significant (P = 0·006) negative correlation with age (Figure 4C). However, after revaccination with the seasonal influenza vaccine, this age‐dependent negative correlation was lost, even though a weak negative trend was still seen (Figure 4D).
Figure 4.
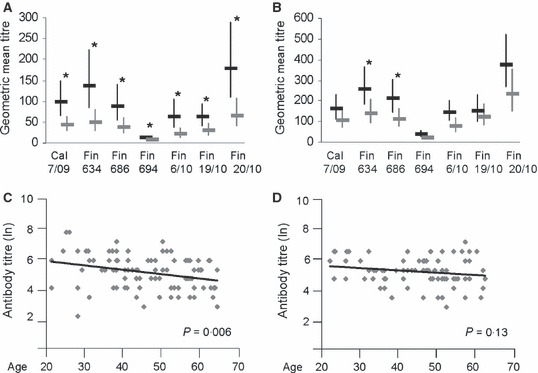
Vaccination‐induced antibody immune response correlates negatively with age. Analysis of antibody titers was performed separately for two age groups: younger adults aged 20–48 years (black bars), and older adults aged 49–64 years (gray bars). The geometric mean titers and 95% confidence intervals for the different age groups were calculated for the 21 day samples after the first vaccination (A) and the 21 day samples after the second booster vaccination (B). Statistical significances of differences between the groups were calculated using Student’s two‐tailed t‐test, *P < 0·05. The correlation between the age of the vaccines and the antibody titers (natural logarithms, ln) against the A/California/7/2009 vaccine virus are represented with scatter plots and trend lines 21 days after the first vaccination (C) and 21 days after the second booster vaccination (D), and the significances (P values) of correlation coefficients are indicated in the figure.
Discussion
In Finland, a monovalent Pandemrix vaccine and a trivalent Fluarix vaccine were used during the 2009–2010 and 2010–2011 influenza seasons, respectively, for protection against influenza A(H1N1)pdm09 viral infections. Here, we have analyzed the antibody responses in recipients of both vaccines against the A/California/07/09 vaccine virus and several A(H1N1)pdm09 viral strains isolated in Finland during two consecutive epidemic seasons in 2009–2010 and 2010–2011. The main objective of this study was to evaluate the antibody responses in healthcare professionals working with patients or with infective virus in the laboratory. Owing to this fact, we did not specifically ask for the possible underlying diseases from the participants. The viruses used in the analyses were selected based on their genetic and antigenic properties. 6 , 8 The selected viruses match with the WHO reference viral strains and cluster to the corresponding genetic groups. Serum samples taken before the Pandemrix vaccination showed practically no reactivity with any of the influenza A(H1N1)pdm09 viruses. It is noteworthy though that pre‐vaccination samples were collected only from 24 individuals in the age range between 27 and 62 years. In our earlier report, we describe that this age group of Finns lack cross‐reactive antibodies against A(H1N1)pdm09 virus. The reports from other countries also indicate that to a greater extent cross‐reactive antibodies against the A(H1N1)pdm09 viruses have only been found in individuals 60 years or older. 16 , 20 , 21 , 22 , 23 , 24 Exposure to the pandemic virus before the vaccination with Pandemrix is unlikely because the pandemic wave reached the Helsinki area several weeks after the vaccination. 25
AS03‐adjuvanted Pandemrix vaccine is capable of inducing strong antibody responses against the A/California/7/2009 vaccine virus. 1 , 2 , 3 However, at present, there is little information available on the persistence of antibody levels after the vaccination. Persistence of protective immunity not only depends on longevity of antibodies but also on the mutation rate of influenza viruses and the ability of antibodies to recognize different antigenic virus variants. Our data indicate that antibody titers against various viruses may differ significantly. We have previously reported that the mutation rate of the hemagglutinin of influenza A(H1N1)pdm09 viruses was maximally 1·4%/year during the first 2 years of circulation 8 and that already one to two amino acid changes in antigenically important sites can compromise antibody recognition significantly. 6
We also analyzed booster responses induced by the trivalent seasonal vaccine given 1 year after the pandemic vaccine. In addition, we evaluated the age‐related antibody levels after pandemic and seasonal booster vaccinations and found that younger individuals showed significantly higher antibody titers against all studied viruses. It is noteworthy that booster vaccination with the A/California/7/2009 virus without adjuvant increased the antibody levels significantly. Interestingly, the older age group seemed to benefit more from the seasonal booster vaccination; the differences in the mean antibody titers between the younger and older age groups decreased and the clear, age‐dependent statistically significant negative correlation disappeared after the second vaccination. It has previously been observed that especially elderly individuals respond more weakly to many vaccines, including influenza vaccines, owing to phenomenon called immunosenescence. 26 It was, however, surprising that in our study an age‐dependent reduction in antibody levels appeared to be linearly related with increasing age also among individuals less that 65 years of age (Figure 4C). It was also of interest that the seasonal booster vaccination did not improve or broaden the cross‐reactivity of antibodies against different viruses. In case, the circulating virus is mutating significantly in important antigenic sites, like in the A/Finland/694/2009 virus, booster vaccination with the original strain may not provide very good increase in seroprotection rate against already antigenically drifted viruses. In fact, even three immunizations with the same virus antigen (13 participants in this study) did not improve the cross‐reactivity. Thus, continuous surveillance of circulating influenza viruses and the selection of novel and prevalent antigenic variants for the seasonal vaccine are essential.
Supporting information
Table S1.Table of identification codes for the supplemental sequences for phylogenetic tree obtained from GISAID EpiFluTM Database..
Supporting info item
Acknowledgements
We thank Riitta Santanen, Anja Villberg, and Outi Rautio for expert technical assistance, and Mika Lahdenkari for helpful advice with statistical analysis. The study was supported by the funds from the National Institute for Health and Welfare (THL), the Ministry of Health and Social Affairs (Finland), and the Identification of Mechanisms Correlating with Susceptibility for Avian Influenza (IMECS) project (grant no 201169) by the European Commission, DG Research, and the participating member states. The funding agencies had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. We gratefully acknowledge the authors, originating and submitting laboratories of the sequences from GISAID’s EpiFluTMDatabase. All submitting laboratories may be contacted directly via the GISAID website http://www.gisaid.org.
References
- 1. Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A‐adjuvant: preliminary report of an observer‐blind, randomised trial. Vaccine 2010; 28:1740–1745. [DOI] [PubMed] [Google Scholar]
- 2. Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)‐Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 2010; 51:668–677. [DOI] [PubMed] [Google Scholar]
- 3. Roman F, Clement F, Dewe W et al. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costa JT, Silva R, Tavares M, Nienhaus A. High effectiveness of pandemic influenza A (H1N1) vaccination in healthcare workers from a Portuguese hospital. Int Arch Occup Environ Health 2011; DOI 10.1007/s00420‐011‐0714‐8 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilca V, De Serres G, Hamelin ME et al. Antibody persistence and response to 2010‐2011 trivalent influenza vaccine one year after a single dose of 2009 AS03‐adjuvanted pandemic H1N1 vaccine in children. Vaccine 2011; 30:35–41. [DOI] [PubMed] [Google Scholar]
- 6. Strengell M, Ikonen N, Ziegler T, Julkunen I. Minor changes in the hemagglutinin of influenza A(H1N1)2009 virus alter its antigenic properties. PLoS ONE 2011; 6:e25848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Medicines Agency . Pandemrix. Summary of product characteristics. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000832/WC500038121.pdf (Accessed 16 August 2012).
- 8. Ikonen N, Haanpaa M, Ronkko E et al. Genetic diversity of the 2009 pandemic influenza A(H1N1) viruses in Finland. PLoS ONE 2010; 5:e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007; 24:1596–1599. [DOI] [PubMed] [Google Scholar]
- 10. Saitou N, Nei M. The neighbor‐joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987; 4:406–425. [DOI] [PubMed] [Google Scholar]
- 11. Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor‐joining method. Proc Natl Acad Sci USA 2004; 101:11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hillis DM, Allard MW, Miyamoto MM. Analysis of DNA sequence data: phylogenetic inference. Methods Enzymol 1993; 224:456–487. [DOI] [PubMed] [Google Scholar]
- 13. Sayle RA, Milner‐White EJ. RASMOL: biomolecular graphics for all. Trends Biochem Sci 1995; 20:374. [DOI] [PubMed] [Google Scholar]
- 14. Kendal AP, Pereira MS, Skehel JJ. Concepts and Procedures for Laboratory‐Based Influenza Surveillance. WHO Collaborating Centers for Reference and Research on Influenza. Washington, D.C.: U.S. Dept. of Health and Human Services, 1982. [Google Scholar]
- 15. World Health Organization , Global Influenza Surveillance Network. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 16. Ikonen N, Strengell M, Kinnunen L et al. High frequency of cross‐reacting antibodies against 2009 pandemic influenza A(H1N1) virus among the elderly in Finland. Euro Surveill 2010; 15:19478. [PubMed] [Google Scholar]
- 17. Allwinn R, Geiler J, Berger A, Cinatl J, Doerr HW. Determination of serum antibodies against swine‐origin influenza A virus H1N1/09 by immunofluorescence, haemagglutination inhibition, and by neutralization tests: how is the prevalence rate of protecting antibodies in humans? Med Microbiol Immunol 2010; 199:117–121. [DOI] [PubMed] [Google Scholar]
- 18. Hardelid P, Andrews NJ, Hoschler K et al. Assessment of baseline age‐specific antibody prevalence and incidence of infection to novel influenza A/H1N1 2009. Health Technol Assess 2010; 14:115–192. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention (CDC) . Serum cross‐reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep 2009;58:521–524. [PubMed] [Google Scholar]
- 20. Rizzo C, Rota MC, Bella A et al. Cross‐reactive antibody responses to the 2009 A/H1N1v influenza virus in the Italian population in the pre‐pandemic period. Vaccine 2010; 28:3558–3562. [DOI] [PubMed] [Google Scholar]
- 21. Hancock KPD, Veguilla VMPH, Lu XMD et al. Cross‐Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med 2009; 361:1945–1952. [DOI] [PubMed] [Google Scholar]
- 22. Broberg E, Nicoll A, Amato‐Gauci A. Seroprevalence to influenza A(H1N1) 2009 virus‐‐where are we? Clin Vaccine Immunol 2011; 18:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross‐sectional serological study. Lancet 2010; 375:1100–1108. [DOI] [PubMed] [Google Scholar]
- 24. Gilbert GL, Cretikos MA, Hueston L, Doukas G, O’Toole B, Dwyer DE. Influenza A (H1N1) 2009 antibodies in residents of New South Wales, Australia, after the first pandemic wave in the 2009 southern hemisphere winter. PLoS ONE 2010; 5:e12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lyytikainen O, Kuusi M, Snellman M et al. Surveillance of influenza in Finland during the 2009 pandemic, 10 May 2009 to 8 March 2010. Euro Surveill 2011; 16:19908. [PubMed] [Google Scholar]
- 26. Cao W, Kim JH, Chirkova T et al. Improving immunogenicity and effectiveness of influenza vaccine in older adults. Expert Rev Vaccines. 2011; 10:1529–1537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Table of identification codes for the supplemental sequences for phylogenetic tree obtained from GISAID EpiFluTM Database..
Supporting info item


