Abstract
Background No studies of the clinical symptoms before starting therapy or of the effectiveness of neuraminidase inhibitors (NAIs) have been carried out of the 2009–2010 and 2010–2011 seasons that compare A(H1N1)pdm09 or the three circulating types of influenza virus.
Methods The clinical symptoms and duration of fever (body temperature ≥37·5°C) after the first dose of an NAI (oseltamivir, zanamivir, laninamivir) were analyzed. PCR was carried out for 365 patients with A(H1N1)pdm09 in the 2009–2010 season and for 388 patients with one of the three types of influenza circulating in the 2010–2011 season. IC50 for the three NAIs was also analyzed in 51 patients in the 2010–2011 season.
Results The peak body temperature was significantly higher in 2010–2011 than in 2009–2010 for patients under 20 years with A(H1N1)pdm09, and in the 2010–2011 season for children 15 years or younger with A(H1N1)pdm09 than for those with other virus types. The percentage of A(H1N1)pdm09 patients with loss of appetite or fatigue was significantly higher in 2010–2011 than in the previous season. The duration of fever was not affected by the kind of NAI or by age in multiple regression analysis. The percentage of patients afebrile at 48 hours after the first dose of NAI was significantly higher for A(H1N1)pdm09 than for A(H3N2) (laninamivir) or B (oseltamivir and laninamivir).
Conclusion Although the clinical symptoms of A(H1N1)pdm09 were slightly more severe in the 2010–2011 season, the effectiveness of the NAIs remained high in comparison with 2009–2010 and with other types of seasonal influenza.
Keywords: A(H1N1)pdm09, clinical symptom, IC50, laninamivir, neuraminidase inhibitor
Introduction
Influenza A(H1N1)pdm09 was highly prevalent in the 2009–2010 season, with few cases of A(H3N2) or B reported. 1 , 2 However, all three subtypes (types) spread widely and almost simultaneously in the 2010–2011 winter season. 1 , 3 , 4 Little study has been carried out of the differences in the clinical symptoms or the effectiveness of neuraminidase inhibitors (NAIs) between these two seasons for A(H1N1)pdm09 viruses or among the three influenza subtypes. A(H1N1)pdm09 was reported mainly in the autumn (mostly September–December) of the 2009–2010 season, but prevailed in winter (mostly January–March) in the 2010–2011 season, which is similar to the usual influenza season in Japan. 1 Therefore, we thought it would be interesting to determine how the clinical features of A(H1N1)pdm09 might have differed between these two seasons.
We have reported the usefulness of neuraminidase inhibitors (NAIs) almost annually 5 , 6 , 7 , 8 , 9 , 10 , 11 and have shown reduced effectiveness of oseltamivir in the 2008–2009 season, when the oseltamivir‐resistant (H275Y NA mutation) A(H1N1) viruses were highly prevalent, compared with the previous season and with zanamivir. 9 , 10 The new NAI, laninamivir, became available from the 2010–2011 season in Japan. 4 , 12 , 13 However, the effectiveness of NAIs, including laninamivir, against various types of influenza viruses, including A(H1N1)pdm09, has not been clinically compared in the same season.
In this report, we compare the clinical symptoms of A(H1N1)pdm09 patients in the 2009–2010 and 2010–2011 seasons and also among the A(H1N1)pdm09, A(H3N2), and B influenzas that were circulating in the 2010–2011 season. We analyzed the duration of fever ≥37·5°C after the first dose of oseltamivir or zanamivir in both seasons and for all three NAIs in the 2010–2011 season. 2 , 6 , 8 , 10 The IC50 (50% inhibitory concentration) of the three NAIs was determined for the three types of influenza virus in the 2010–2011 season. 7 , 9 , 14
Methods
Study procedures
Family doctors, pediatricians, and physicians at 13 clinics who belong to the Influenza Study Group of the Japan Physicians Association participated in the study. Patients were enrolled from August 11, 2009 through April 6, 2010 (median: November 11, 2009) in the 2009–2010 season and from November 18, 2010 through May 23, 2011 (median: January 31, 2011) in the 2010–2011 season. Patients who reported to any of our 13 clinics with an influenza‐like illness manifesting any two of the following symptoms: body temperature ≥37·5°C, rhinorrhea, sore throat, cough, general fatigue, loss of appetite, or headache were tested by commercial antigen detection kit. From all outpatients with influenza, diagnosed by antigen detection kit and without severe underlying diseases such as chronic obstructive pulmonary disease or chronic heart disease, those who received NAIs within 48 h after the onset of symptoms were registered in this study after providing informed consent.
Oseltamivir has been reported to be related to the neuropsychiatric symptoms of young adults and has been prohibited in Japan, in most cases, for use by patients aged from 10 to 19 years, and zanamivir and laninamivir are not recommended for patients with underlying respiratory disease or children under 5 years. Thus, intravenous peramivir was administered to a few patients. The symptoms of these patients were analyzed, but were excluded from the analysis of the duration of fever. The decision of which NAI to administer, oseltamivir, zanamivir, laninamivir, or peramivir, was left to the discretion of the patient’s physician, who followed the above guidelines and patient preference.
Specimens from nasal swabs, throat swabs, nasal aspirates, or blown nasal discharge were subjected to antigen detection and virus isolation. Of the commercially available antigen detection kits based on immunochromatography, Imuno Ace Flu [Touns], QuickNavi‐Flu [Denka Seiken], and Capilia FluA + B [Alfresa Pharma] were mainly used.
Oseltamivir (75 mg for adults and for children who weighed ≥37·5 kg and 2 mg/kg for children who weighed <37·5 kg) was taken orally twice per day for five days. Zanamivir (10 mg for adults and for children aged five years or over) was inhaled twice per day for five days. Laninamivir (20 mg for children <10 years old and 40 mg for adults or children 10 years and older) was inhaled at one sitting. 13 No antipyretics were administered, but acetaminophen was used temporarily in the case of emergency.
Age, sex, vaccination status, results of the antigen detection test kit, and body temperature were recorded for all patients. The date and time of the onset of fever, the date and time of administration of the NAI, and the resolution of fever were recorded by the physician, patient, or an attending family member. The first time point at which a patient reported a fever (temperature, 37·5°C) was defined as the time of onset. Patients were asked to measure body temperature at least three times per day (8:00 A.M., 2:00 P.M., and 8:00 P.M.). The time at which a body temperature of <37·5°C was attained and maintained for more than 24 hours was defined as the time the patient became afebrile. The highest body temperature during the course of the disease was also recorded. For clinical symptoms other than fever, the presence or absence of the following symptoms were noted by the doctor when influenza was diagnosed, cough, rhinorrhea, myalgia, loss of appetite, and fatigue.
All data were collected using an Internet‐based protocol based on a server located in a secure room at the Gifu City Medical Association. 15 The time from the initial administration of an NAI to the resolution of fever (the duration of fever after the first dose of NAI) was calculated automatically in the SQL database. 6 , 10 All study‐related documents and procedures were approved by the institutional review board at Hara‐Doi Hospital.
Influenza virus isolation
Clinical samples for viral isolation were obtained from nasal or pharyngeal swab, nasal aspiration, or self‐blown nasal discharge. Samples were suspended in a solution for virus preservation (M4‐RT medium) and sent to a central laboratory (Mitsubishi Chemical Medience Corporation) where they were kept at 4°C. The collected samples were cultured with Madin‐Darby canine kidney (MDCK) cells at 33°C.
Viral types and subtypes
The type and subtype of A(H3N2) or B were determined by RT‐PCR using subtype‐specific primers as described. 16 In brief, viral RNA was extracted from the viral culture supernatant, and then cDNA was synthesized using reverse transcriptase. PCR was carried out with cDNA using primer sets specific for the viral type and subtype. For the A(H1N1)pdm09 virus, the subtype was determined by real‐time RT‐PCR with a specific primer set and a fluorescent‐labeled probe. 17
Measurement of the IC50 of the NA inhibitors
IC50 to oseltamivir carboxylate, zanamivir, and laninamivir was determined by a fluorescence‐based neuraminidase inhibition assay, as described elsewhere 9 , 18 , with culture supernatants. Laninamivir and zanamivir were provided by Daiichi Sankyo Co., Ltd. Oseltamivir carboxylate was prepared from oseltamivir phosphate extracted from the commercial preparation Tamiflu® (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan).
Statistical analysis
The Student’s t‐test was used for between‐group comparisons of the peak body temperature, the duration of fever, age, the time from the onset to the first visit, and IC50. The chi‐square test was also performed to compare between‐group differences in the percentage of patients. Multiple regression analysis was performed to determine which factors affected the duration of fever, such as age, sex, vaccination status, the peak body temperature, the influenza type or subtype, the drug administered, and the time from the onset to the start of treatment. A P value <0·05 was considered statistically significant.
Results
Patient characteristics
A total of 442 patients were enrolled in the 2009–2010 season as were 415 in the 2010–2011 season. The complete data of 753 patients with influenza were available for analysis: 365 patients with A(H1N1)pdm09 aged 1 to 78 years old in the 2009–2010 season and 199 patients with A(H1N1)pdm09 aged 1 to 81 years old, 96 patients with A(H3N2) aged 1–74 years old, and 93 patients with B aged 3‐66 years old in the 2010–2011 season. The clinical characteristics of the patients are summarized in Table 1.
Table 1.
Baseline clinical characteristics and peak body temperature of patients 15 years or younger and over 15 years
| 2009–2010 | 2010–2011 | P value between | ||||||
|---|---|---|---|---|---|---|---|---|
| A(H1N1) pdm09 (a) | A(H1N1) pdm09 (b) | A(H3N2) (c) | B (d) | (a) and (b) | (b) and (c) | (c) and (d) | (b) and (d) | |
| Number of patients | 365 | 199 | 96 | 93 | ||||
| Age, mean years ± SD (range) | 19·0 ± 13·6 (1–78) | 25·7 ± 18·4 (1–81) | 19·2 ± 19·5 (1–74) | 14·9 ± 11·9 (3–66) | <0·001 | <0·01 | NS | <0·001 |
| Male/female | 188/177 | 105/94 | 58/38 | 39/54 | NS | NS | <0·05 | NS |
| Vaccination* | 74/286/5 | 45/151/3 | 31/58/7 | 27/60/6 | NS | NS | NS | NS |
| Positive/negative/unknown | ||||||||
| Time from the onset | 16·3 ± 11·3 | 15·4 ± 10·8 | 15·3 ± 10·8 | 16·5 ± 11·2 | NS | NS | NS | NS |
| To the first visit at clinic (hours) | ||||||||
| Peak body temperature (°C) | 39·0 ± 0·7 | 39·0 ± 0·7 | 38·9 ± 0·7 | 38·9 ± 0·5 | NS | NS | NS | NS |
| ≤15 years (n) | 39·1 ± 0·7 (200) | 39·3 ± 0·6 (74) | 39·0 ± 0·7 (66) | 38·9 ± 0·5 (66) | <0·05 | <0·01 | NS | <0·001 |
| >15 years (n) | 38·8 ± 0·6 (165) | 38·9 ± 0·7 (125) | 38·7 ± 0·7 (30) | 38·9 ± 0·5 (27) | NS | NS | NS | NS |
*Vaccination for seasonal influenza.
() number of patients.
The mean age was significantly higher for A(H1N1)pdm09 in the 2010–2011 season (25·7 ± 18·4 years) than in the 2009–2010 season (19·0 ± 13·6 years, P < 0·001) and for A(H3N2) and B in the 2010–2011 season (19·2 ± 19·5 years, P < 0·01, and 14·9 ± 11·9 years, P < 0·001, respectively). More female than male patients had influenza B. No significant differences were found in vaccination status or time from the onset to the first visit at a clinic.
Peak body temperature
No significant differences in peak body temperature were found in the age group analysis or for adults over 15 years. However, in children 15 years or younger, the peak body temperature was significantly higher in A(H1N1)pdm09 in the 2010–2011 season (39·3 ± 0·6°C) than in A(H1N1)pdm09 in the 2009–2010 season (39·1 ± 0·7°C, P < 0·05) and in A(H3N2) and B (39·0 ± 0·7°C, P < 0·01 and 38·9 ± 0·5°C, P < 0·001, respectively). (Table 1)
In comparison with the peak body temperature to A(H1N1)pdm09 in both seasons of patient groups 0–9, 10–19, 20–39, and 40 years or over, the temperatures of the 0–9 and 10–19 years’ age groups (P < 0·01 and P < 0·05, respectively) were significantly higher in the 2010–2011 than in the 2009–2010 season (Figure 1)
Figure 1.
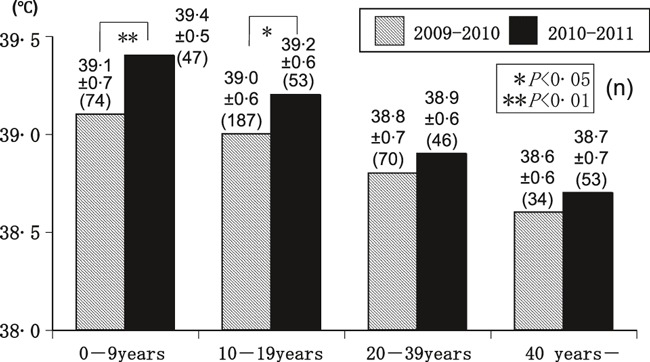
The peak body temperature (°C) of patients with A(H1N1)pdm09 in the 2009–2010 and 2010–2011 seasons, by age. The peak body temperature was significantly higher in the 2010–2011 than the 2009–2010 seasons in the 0–9 and 10–19 years’ age groups.
Other clinical symptoms
The symptoms at the first visit to the clinic, except for fever, are shown in Table 2. The percentages of patients with cough, rhinorrhea, myalgia, loss of appetite, and fatigue were significantly higher for patients with A(H1N1)pdm09 infection in the 2010–2011 than in the 2009–2010 season. This was also true for A(H3N2), except for loss of appetite. No significant differences in the percentages were found for A(H3N2) and B infection.
Table 2.
Percentage of patients with each clinical symptoms at first visit to clinics
| 2009–2010 | 2010–2011 | P value between | ||||||
|---|---|---|---|---|---|---|---|---|
| A(H1N1) pdm09 (a) | A(H1N1) pdm09 (b) | A(H3N2) (c) | B (d) | (a) and (b) | (b) and (c) | (c) and (d) | (b) and (d) | |
| Number of patients | 365 | 199 | 96 | 93 | ||||
| % of patients with each symptom | ||||||||
| Cough | 82·7 | 90·5 | 82·3 | 82·8 | <0·05 | <0·05 | NS | NS |
| Rhinorrhea | 49·6 | 59·8 | 81·3 | 71 | <0·05 | <0·001 | NS | NS |
| Myalgia | 27·4 | 46·7 | 18·8 | 25·8 | <0·001 | <0·001 | NS | <0·001 |
| Loss of appetite | 23·3 | 49·2 | 56·3 | 44·1 | <0·001 | NS | NS | NS |
| Fatigue | 44·1 | 75·4 | 61·5 | 62·4 | <0·001 | <0·05 | NS | <0·05 |
Between‐season comparison of children (≤15 years) and adults (>15 years) with A(H1N1)pdm09 showed the percentages of all five symptoms to be significantly higher for adults in the 2010–2011 than in the 2009–2010 season (Figure 2). For children, the percentage of patients with loss of appetite or fatigue was significantly higher in the 2010–2011 than in the 2009–2010 season.
Figure 2.
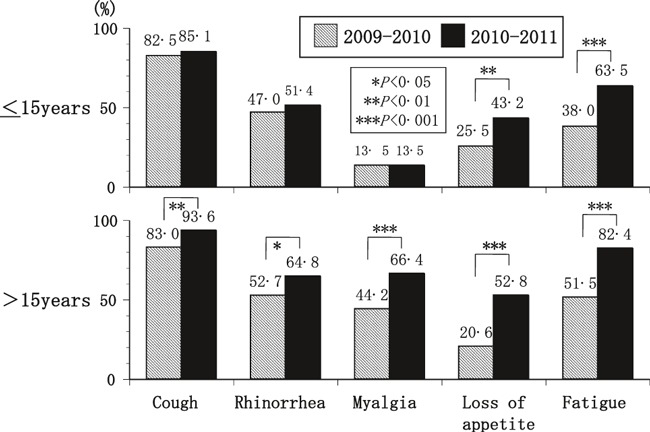
The percentages of the symptoms suffered by patients with A(H1N1) pdm09 infection, by season. The percentage of patients with loss of appetite or fatigue was significantly higher in the 2010–2011 season than in the previous season in children 15 year or younger. The percentage of patients with cough, rhinorrhea, myalgia, loss of appetite, or fatigue was significantly higher in the 2010–2011 season than in the previous season in adults over 15 years.
Effectiveness of NAIs
The duration of fever after the first dose of oseltamivir, zanamivir, or laninamivir is shown for 365 patients in 2009–2010 and 374 patients in 2010–2011 season. (Table 3) Fourteen patients (5 with A(H1N1)pdm09, 7 with A(H3N2), and 2 with B) to whom peramivir was administered in the 2010–2011 season were excluded from this analysis.
Table 3.
The effectiveness of neuraminidaze inhibitors in the 2009–2010 and 2010–2011seasons evaluated by duration of fever
| Duration of fever after the first dose, hour | 2009–2010 | 2010–2011 | P value between | |||||
|---|---|---|---|---|---|---|---|---|
| A(H1N1) pdm09 (a) | A(H1N1) pdm09 (b) | A(H3N2) (c) | B (d) | (a) and (b) | (b) and (c) | (c) and (d) | (b) and (d) | |
| Oseltamivir | 23·1 ± 12·0 (158) | 26·5 ± 10·6 (30) | 32·0 ± 19·8 (36) | 35·7 ± 25·7 (36) | NS | NS | NS | NS |
| Zanamivir | 26·6 ± 15·0 (207) | 29·6 ± 18·2 (59) | 33·0 ± 22·1 (22) | 30·9 ± 16·8 (19) | NS | NS | NS | NS |
| Laninamivir | n.a | 25·0 ± 15·0 (103) | 30·9 ± 21·1 (33) | 38·5 ± 26·3 (36) | NS | NS | <0·01 | |
() number of patients.
Fourteen patients [5 with A(H1N1)pdm09, 7 with A(H3N2), and 2 with B] to whom peramivir was administered in the 2010–2011 season were excluded from this analysis.
The duration tended to be shorter for A(H1N1)pdm09 in both seasons than for A(H3N2) or B in the 2010–2011 season. No significant differences in the duration were found among oseltamivir, zanamivir, and laninamivir for A(H1N1)pdm09, A(H3N2), and B in the 2010–2011 season. For A(H1N1)pdm09 infection, the duration of fever after starting oseltamivir or zanamivir therapy was slightly, but not significantly, longer in the 2010–2011 season than in the 2009–2010 season.
Multiple regression analysis that included the type of virus and the peak body temperature showed significant relationships with the duration of fever (P = 0·00055 and 0·00033, respectively). No significance was found for the duration of fever after the first dose of an NAI with the NAI administered, age, sex, vaccination status, or the time from the onset to the start of treatment (Table 4).
Table 4.
Results of multiple regression analysis to determine which factors influenced the duration of fever after the first dose
| Factor | P value |
|---|---|
| Age | NS |
| Sex | NS |
| Vaccination status | NS |
| Peak body temperature | 0·00033 |
| Influenza type or subtype | 0·00055 |
| Drug administered | NS |
| Time from the onset to the start of treatment | NS |
There was no significant difference between the two seasons in the percentage of patients with A(H1N1)pdm09 afebrile at 48 hours after the first dose of oseltamivir or zanamivir (Figure 3).
Figure 3.
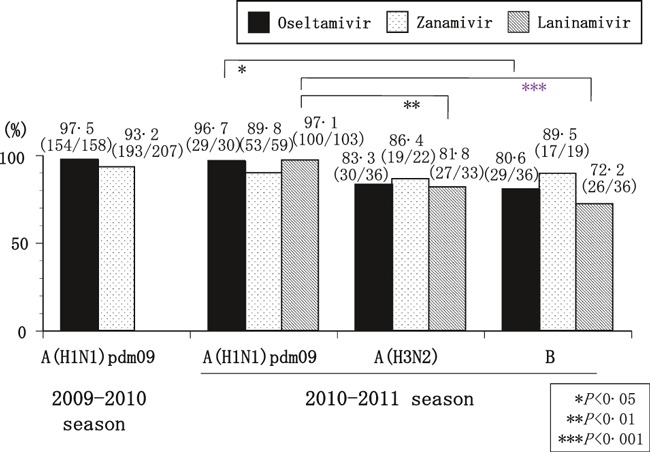
The percentage of patients afebrile at 48 hours after the first dose of each neuraminidase inhibitor. The percentage of patients afebrile at 48 hours after the first dose was significantly higher for A(H1N1)pdm09 than for A(H3N2) (laninamivir) or B (oseltamivir and laninamivir). No significant between‐season difference in A(H1N1)pdm09 was found.
In the 2010–2011 season, the percentage of patients afebrile at 48 hours after the first dose of laninamivir was significantly higher for A(H1N1)pdm09 (97·1%) than for A(H3N2) and B (81·8%; P < 0·01 and 72·2%; P < 0·001, respectively) (Figure 3). The percentage after the first dose of oseltamivir was significantly higher for A(H1N1)pdm09 than for B (96·7% and 80·6%, P < 0·05). However, no significant difference of duration from the onset to the first dose of an NAI was found between the afebrile and febrile patient groups at 48 hours after the first dose (afebrile and febrile group: 17·1 ± 11·1 and 19·6 ± 14·9 hours in A(H1N1)pdm09, 16·8 ± 11·2 and 18·2 ± 12·1 hours in A(H3N2), and 19·0 ± 10·6 and 16·1 ± 12·5 hours in B, respectively).
In vitro, the IC50s of zanamivir and laninamivir were significantly lower for A(H1N1)pdm09 (0·86 ± 0·32 and 1·77 ± 0·78 nm, respectively) than for A(H3N2) (1·94 ± 0·43 and 3·9 ± 1·6 nm, respectively) or B (12·3 ± 4·0 and 21·3 ± 6·9 nm, respectively). (Table 5) The IC50 of oseltamivir was lowest for A(H3N2) (0·74 ± 0·13 nm) and highest for B (44·5 ± 13·6 nm) (Table 5).
Table 5.
Pre‐treatment IC50 values for each neuraminidase inhibitor used in the 2010–2011 season
| IC50 before starting therapy, nm | A(H1N1) pdm09 (a) | A(H3N2) (b) | B (c) | P value between | ||
|---|---|---|---|---|---|---|
| (a) and (b) | (b) and (c) | (a) and (c) | ||||
| Oseltamivir | 0·97 ± 0·48 (31) | 0·74 ± 0·13 (9) | 44·5 ± 13·6 (11) | <0·05 | <0·001 | <0·001 |
| Zanamivir | 0·86 ± 0·32 (31) | 1·94 ± 0·43 (9) | 12·3 ± 4·0 (11) | <0·001 | <0·001 | <0·001 |
| Laninamivir | 1·77 ± 0·78 (31) | 3·9 ± 1·6 (9) | 21·3 ± 6·9 (11) | <0·001 | <0·001 | <0·001 |
*Duration of fever after the first dose () number of patients.
Discussion
Cao et al. reported that the majority of patients with A(H1N1)pdm09 infection had a mild illness. 19 We also reported that the clinical symptoms of outpatients with A(H1N1)pdm09 infection in the 2009–2010 season tended to be more mild than those of seasonal A(H1N1) in the 2007–2008 and 2008–2009 seasons. 2
In this study, the peak body temperature was significantly higher in A(H1N1)pdm09 in the 2010–2011 season than in A(H3N2) or B in children 15 years or younger and in A(H1N1)pdm09 in the 2009–2010 season in patients <20 years. The percentage of patients with loss of appetite or fatigue were also higher in the 2010–2011 than in the 2009–2010 season for A(H1N1)pdm09 virus infection in both the ≤15 years and >15 years’ age groups. These results suggest that the severity of symptoms to A(H1N1)pdm09 is increasing as the virus changes from pandemic to seasonal occurrence.
The reason the symptoms to the A(H1N1)pdm09 virus have become slightly more severe is unclear. The percentage of H275Y mutation of A(H1N1)pdm09 in the 2010–2011 season was only 1·1% (2/185) in another of our studies. 4 The virus titer and/or cytokine level may have been increased in this season compared with the previous season. Further study will be necessary. Differences in the season or climate when the A(H1N1)pdm09 was circulating (autumn in the 2009–2010 and winter in the 2010–2011) may also be related to our findings.
We have already reported that oseltamivir was more effective against A(H1N1)pdm09 than against seasonal A(H1N1) in the 2007–2008 and 2008–2009 seasons. 2 We also reported previously that the duration of fever after the first dose of an NAI is significantly correlated, by multiple regression analysis, with the type of virus and peak body temperature, but that there is no correlation with age or the kind of anti‐influenza drug. 5 In addition, the effectiveness of vaccination on the duration of fever, as reported in our previous studies, was not confirmed in this study. 5 , 20
In this study, the duration of fever and the percentage of patients afebrile at 48 hours after the first dose of oseltamivir or zanamivir did not change significantly from the previous season. However, the duration of fever was significantly shorter for A(H1N1)pdm09 than for B in patients treated with laninamivir, and the percentage of patients afebrile at 48 hours was significantly higher for A(H1N1)pdm09 than for A(H3N2) (laninamivir) or B (oseltamivir and laninamivir).
In our previous study of the 2006–2007 season, the percentages of patients afebrile at 48 hours were 83·1% and 86·7% against influenza A and 55·6% and 80·2% against influenza B for oseltamivir and zanamivir therapy, respectively. 8 In the 2006–2007 season, A(H3N2) was responsible for 90·5% (95/105) of the influenza A cases. 8 The percentage of patients with influenza A(H3N2) afebrile (83·3% and 86·4%, for oseltamivir and zanamivir, respectively) in this study were similar to the data from the 2006–2007 season.
The duration of fever after the first dose of a drug was analyzed to evaluate the clinical effectiveness of these NAIs because it is difficult to evaluate the clinical effectiveness of drugs in outpatient clinics by estimating the mortality rate or incidence of hospitalization. There is a limit to the findings of our study in that it was performed in a general practice setting and not in the context of a rigorous clinical protocol. The body temperature of our outpatients was obtained from reports self‐recorded by the patient or a family member. In our previous analysis using this method or virus shedding, oseltamivir was less effective for influenza B than for influenza A and was less effective for A(H1N1) with than without H275Y mutation, especially in children but not so in adults. 9 , 10 Also, in this study, the duration of fever after oseltamivir therapy tended to be longer in influenza B than in A(H1N1)pdm09 or A(H3N2). However, the difference in the duration between influenza A and B was smaller than in our previous study. The effectiveness of oseltamivir for influenza B compared with A may differ with season. Further study, especially for influenza B, will be necessary.
In this study, we did not compare NAI and non‐NAI therapy groups. In Japan, it is unusual to not use an NAI for patients with influenza diagnosed by commercial antigen detection kit. The usefulness of NAIs is wide, and NAI therapy is supported by the public medical insurance system. We previously reported that the duration of fever was shorter in NAI therapy than in non‐NAI therapy in patients with seasonal influenza. 6 , 12 We have also reported that the usefulness of oseltamivir and zanamivir for A(H1N1)pdm09 is equal to or higher than for seasonal A(H1N1) without H275Y NA mutation. 2
The severity of the first and second influenza A(H1N1)pdm09 waves was compared in England. 21 , 22 , 23 Keramarou et al. 21 reported more hospital admissions (n = 379) and deaths (n = 26) in Wales in the second wave (peaked in late October, 2009) than in the first wave (n = 44 and only one, respectively; peaked in late July, 2009). Higher mortality rates in the second (September–February) than in the first (June–August) wave were also reported by Presanis et al., (0·025% and 0·015% of patients with A(H1N1)pdm09, respectively) and Mytton et al. (5·5 and 1·6 deaths per million population, respectively). 22 , 23 Our results may coincide with these results; however, accurate comparison is difficult because NAIs are more commonly used in Japan than in England. To our knowledge, no comparison of the severity of A(H1N1)pdm09 virus infection in the first or second waves of the 2009–2010 season and the 2010–2011 season has been reported.
Laninamivir octanoate is inhaled, then converted to laninamivir in the lung, and the binding of laninamivir to virus NA is relatively more stable and lasts longer than has been observed for other NAIs. 13 , 24 In this study, laninamivir was almost equally as effective as oseltamivir or zanamivir, estimated clinically by the duration of fever; nevertheless, the IC50 of laninamivir tended to be higher than that of the other NAIs. Kubo, et al. recently reported that 6 days after intranasal administration of 236 μg/kg laninamivir octanoate, the concentration of laninamivir in the lungs of mice was maintained about 730‐fold the IC50 for A(H1N1)pdm09, 77‐fold that of A(H3N2), and 70‐fold that of B. 22 In another of our studies, the persistence rates of virus culture 4–6 days after the start of laninamivir therapy were 2·3% (2/86) for A(H1N1)pdm09, 10·5% (2/19) for A(H3N2), and 29·4% (5/17) for B in the 2010–2011 season (Unpublished data by Kawai N, Ikematsu H and Kashiwagi S). Thus, laninamivir has been shown to be more effective against A(H1N1)pdm09 than against either A(H3N2) or B in both in vitro and in vivo studies. In addition, laninamivir is very convenient to use in outpatient clinics because it can be administered in a single sitting.
In conclusion, although the fever of patients with A(H1N1) pdm09 infection improved quickly with NAI therapy in the 2010–2011 season, the clinical symptoms were more severe than in the 2009–2010 season and more severe than for A(H3N2) or B virus infection. It is notable that the effectiveness of oseltamivir and zanamivir for A(H1N1)pdm09 virus infection has not changed since emergence in 2009 and that the effectiveness of laninamivir for A(H1N1)pdm09 was also high. These NAIs should continue to be recommended, especially for A(H1N1)pdm09 virus infection.
Acknowledgements
We thank Drs Osame Tanaka, Shinro Matsuura, Kenichi Kawamura, Satoshi Yamauchi, Ken‐ichi Doniwa, and Kunio Kondou for their support in this study.
References
- 1. Infectious disease Surveillance Center . Weekly reports of influenza virus isolation/detection. Available at https://hasseidoko.mhlw.go.jp/Byogentai/Pdf/data2e.pdf.
- 2. Kawai N, Ikematsu H, Tanaka O et al. Comparison of the clinical symptoms and the effectiveness of neuraminidase inhibitors for patients with pandemic influenza H1N1 2009 or seasonal H1N1 influenza in the 2007–2008 and 2008–2009 seasons. J Infect Chemother 2011; 17:375–381. [DOI] [PubMed] [Google Scholar]
- 3. WHO . Summary review of the 2010–2011 northern hemisphere winter influenza season. Distribution of virus subtypes by influenza transmission zone (October 2010‐April 2011). Available at http://www.who.int/influenza/2010_2011_seasonal_review_map_main.jpg.
- 4. Ikematsu H, Kawai N, Kashiwagi S. In vitro neuraminidase inhibitory activities of four neuraminidase inhibitors against influenza viruses isolated in the 2010‐2011 season in Japan. J Infect Chemother. 2012 Feb 28. [DOI] [PubMed] [Google Scholar]
- 5. Kawai N, Ikematsu H, Iwaki N et al. Factors influencing the effectiveness of oseltamivir and amantadine for the treatment of influenza: a multicenter study from Japan of the 2002–2003 influenza season. Clin Infect Dis 2005; 40:1309–1316. [DOI] [PubMed] [Google Scholar]
- 6. Kawai N, Ikematsu H, Iwaki N et al. A Comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis 2006; 43:439–444. [DOI] [PubMed] [Google Scholar]
- 7. Kawai N, Ikematsu H, Iwaki N et al. Longer virus shedding in influenza B than in influenza A among outpatients treated with oseltamivir. J Infect 2007; 55:267–272. [DOI] [PubMed] [Google Scholar]
- 8. Kawai N, Ikematsu H, Iwaki N et al. A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza A and B. J Infect 2008; 56:51–57. [DOI] [PubMed] [Google Scholar]
- 9. Kawai N, Ikematsu H, Iwaki N et al. Clinical effectiveness of oseltamivir for influenza A(H1N1) virus with H274Y neuraminidase mutation. J Infect 2009; 59:207–212. [DOI] [PubMed] [Google Scholar]
- 10. Kawai N, Ikematsu H, Hirotsu N et al. Clinical effectiveness of oseltamivir and zanamivir for treatment of influenza A virus subtype H1N1 with the H274Y mutation. A Japanese multicenter study of the 2007–2008 and 2008–2009 Influenza Seasons. Clin Infect Dis 2009; 49:1825–1835. [DOI] [PubMed] [Google Scholar]
- 11. Kawai N, Ikematsu H, Iwaki N et al. Persistence of pandemic influenza H1N1 virus in young patients after oseltamivir therapy in the 2009‐2010 season: a comparison with seasonal H1N1 with or without H275Y mutation. J Infect Chemother 2012; 18:180–186, DOI 10.1007/s10156‐011‐0314‐2. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y, MARVEL Study Group . Long‐acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: A double‐blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175. [DOI] [PubMed] [Google Scholar]
- 13. Ikematsu H, Kawai N. Laninamivir octanoate: a new long‐acting neuraminidase inhibitor for the treatment of influenza. Expert Rev Anti Infect Ther 2011; 9:851–857. [DOI] [PubMed] [Google Scholar]
- 14. Boivin G, Goyette N. Susceptibility of recent Canadian influenza A and B virus isolates to different neuraminidase inhibitors. Antiviral Res 2002; 54:143–147. [DOI] [PubMed] [Google Scholar]
- 15. Kawai N, Ikematsu H, Iwaki N et al. A prospective, Internet‐based study of the effectiveness and safety of influenza vaccination in the 2001–2002 influenza season. Vaccine 2003; 21:4507–4513. [DOI] [PubMed] [Google Scholar]
- 16. Stockton J, Ellis JS, Saville M, Clewley JP, Zambon MC. Multiplex PCR for typing and subtyping influenza and respiratory syncytial viruses. J Clin Microbiol 1998; 36:2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC protocol of realtime RT‐PCR for influenza A (H1N1). Available at http://www.who.int/csr/resources/publications/swineflu/realtimeptpcr/en/index.html.
- 18. Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubo S. CS‐8958, a prodrug of the new neuraminidase inhibitor R‐125489, shows long‐acting anti‐influenza virus activity. Antimicrob Agents Chemother 2008; 53:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao B, Li XW, Mao Y et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med 2009; 361:2507–2517. [DOI] [PubMed] [Google Scholar]
- 20. Ikematsu H, Takeuchi Y, Rosenlund M et al. The post‐infection outcomes of influenza and acute respiratory infection in patients above 50 years of age in Japan: an observational study. Influenza Other Respi Viruses 2012; 6:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keramarou M, Cottrell S, Evans MR et al. Two waves of pandemic influenza A(H1N1) 2009 in Wales‐‐the possible impact of media coverage on consultation rates, April‐December 2009. Euro Surveill 2011; 16:pii: 19772. [PubMed] [Google Scholar]
- 22. Presanis AM, Pebody RG, Paterson BJ et al. Changes in severity of 2009 pandemic A/H1N1 influenza in England: a Bayesian evidence synthesis. BMJ 2011; 343:d5408, doi: 10.1136/bmj.d5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mytton OT, Rutter PD, Mak M, Stanton EA, Sachedina N, Donaldson LJ. Mortality due to pandemic (H1N1) 2009 influenza in England: a comparison of the first and second waves. Epidemiol Infect 2012; 140:1533–41. [DOI] [PubMed] [Google Scholar]
- 24. Kubo S, Tokumitsu A, Tomozawa T, Kakuta M, Yamashita M. High and continuous exposure of laninamivir, an anti‐influenza drug, may work suppressively to generate low‐susceptibility mutants in animals. J Infect Chemother 2012; 18:69–74. [DOI] [PubMed] [Google Scholar]


