Abstract
Background To date, little is known about the role of behavioral risk factors for influenza transmission as well as hygiene behavior in the household setting during the influenza pandemic (H1N1) 2009. In a household‐based study conducted during 2008/2009, we identified several behavioral risk factors for influenza transmission; 30% of index patients and 30% of household contacts reported increased hand cleaning frequency in the week after symptom onset of the index patient. We conducted another household‐based study during the pandemic season 2009/2010.
Methods We identified index patients with laboratory confirmed influenza infection and interviewed household members after illness day 8 of the index patient. Outcome was influenza‐like illness (ILI) in a household contact.
Results We included 108 households. Overall secondary attack rate was 10·1% (27/267) and decreased with increasing age. Apart from being in close daily proximity with the index patient for at least 9 hours, no other behavioral risk factor was associated with secondary ILI. Of all index patients and household contacts, 49% and 55%, respectively, cleaned their hands more often in the week after symptom onset of the index patient (in comparison with 2008/2009 P‐value for both <0·01).
Conclusions While the study was hampered by its relatively limited size, data suggest that a significantly larger proportion of influenza households practiced good hand hygiene compared to the last pre‐pandemic season. This may have led to a different risk factor profile and a delay of the time threshold necessary for transmission among household members with close contact.
Keywords: Behavioral risk factors, household transmission, hygiene behavior, influenza, pandemic influenza
Introduction
Being a member of a household with an influenza case carries the highest risk for infection. 1 , 2 However, household settings have the advantage that they can be readily investigated and provide a clearly defined epidemiological unit where a biased ascertainment of secondary cases can be largely excluded. 3 Because of these two characteristics, households are frequently used to study important questions in influenza epidemiology, such as antiviral effectiveness or viral transmission properties in general.
To identify determinants of influenza transmission in household settings, studies have been carried out during seasonal influenza 4 , 5 and during the pandemic (H1N1) 2009 3 , 6 season. Only Cowling et al. 7 have examined the comparative epidemiology of pandemic (H1N1)pdm09 virus and seasonal influenza viruses in household settings focusing on the crude secondary attack rates (SAR), shedding patterns and the clinical course of illness. In general, risk factors associated with transmission of influenza in households can be divided into behavioral risk factors that are modifiable or even preventable and those that are not. Studies have consistently found that children have higher SAR compared to adults, 4 , 8 but findings on other risk factors, such as household size, remain controversial. While household size or age are risk factors that cannot be modified, the following two questions are particularly relevant to public health purposes: (i) What are the differential risks for different positions in the family, for example, sibling, mother and father? (ii) Which behaviors contribute to virus transmission? Experience regarding behavioral risk factors is limited and was, to our knowledge, only systematically examined by France et al. 6
In the context of the pandemic (H1N1) 2009, it is also of interest to know how and to which extent recommendations of public health authorities on hygiene behavior, such as intensified hand hygiene, have been accepted and were implemented on household level in response to the appearance of the new virus and to public service announcements.
In 2008/2009, we conducted a first study where we investigated preventable risk factors, non‐preventable risk factors, the prevalence of hygiene behavior in influenza households and their impact on secondary influenza infections. 9 The onset of the pandemic gave us the opportunity to study again – under pandemic conditions – these risk factors and to compare results of this study with those of the pre‐pandemic season 2008/2009.
We conducted a retrospective cohort study during the pandemic (H1N1) 2009. The aims of this study were (i) to identify risk factors that contribute to influenza transmission, (ii) to better understand behavioral risk factors that may be associated with differential risks of family members (i.e. sibling, mother, father), and (iii) to measure to which extent recommendations on hygiene behavior have been implemented by household members.
Methods
Study population
To identify influenza patients eligible for this study, we used the database of the German National Reference Center for Influenza (NRCI). National Reference Center for Influenza conducts virological surveillance on circulating influenza viruses by means of approximately 140 sentinel physicians (67% general practitioners or specialists in internal medicine, 33% pediatricians) who send respiratory samples of patients with influenza‐like illness (ILI) to NRCI accompanied by a short patient questionnaire. Samples are tested by real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) for influenza virus.
A household was defined as a social unit composed of those living together in the same dwelling. We included households with at least two persons. The index patient had to be at least 2 years old. Children were defined as persons aged <14 years. Households were excluded if any household member reported an acute respiratory illness in the preceding 14 days before symptom onset of the index patient, had a potential co‐primary case (with illness onset on the same day as the index patient), or if any person (index patient or household member) was hospitalized in the week after symptom onset of the index case. We conducted the telephone interviews with each household member 8 days after symptom onset of the index patient (which was defined as day 1) or later. Parents answered the questions for their children. For the analysis of attitudes/perceptions of index patients and household contacts toward prevention of secondary infections through hygiene behavior, only answers of adult participants were used.
Ethical considerations and data protection
This study was conducted as part of the public health management of the pandemic (H1N1)2009. All index patients were swabbed routinely within the context of the national virological influenza surveillance and no additional samples were collected for the purpose of this study. Upon presentation at the physicians’ office oral consent to be contacted was obtained from all index patients or from their legal guardians and documented in written. When we contacted the index patients by telephone he/she and all household members were asked whether they consented with giving the interview.
If the index patient or the household contact was children, their parents or legal guardians were asked to provide oral consent on their behalf. Individual information at the time of data collection was de‐identified at the time of analysis and an institutional data protection officer assured adherence with data protection laws.
Questionnaire and variables
The variables asked in the questionnaire were divided into three categories:
-
1
Non‐preventable risk factors: age, household size, relationship with the index patient (e.g. father, mother, sibling), pre‐existing chronic diseases (cardiovascular, respiratory, immune impairment).
-
2
Preventable risk factors:
-
•
Behavior or behavioral changes during a 7‐day period after symptom onset of the index patient; all variables were calculated as average values per day.
-
•
Time spent in close contact (i.e. <2 m) with the index patient during day time (hours).
-
•
Having provided care for the index patient (frequency per day).
-
•
Having eaten meals together with the index patient (frequency per day).
-
•
Having slept in the same room with the index patient (never, sometimes, mostly, or always).
-
•
Fomite exposure: having touched items the index patient had contact with (i.e. towels or clothes) (never, sometimes, mostly, or always).
-
•
Hygiene behavioral changes because of the presence of the influenza case in the household:
-
•
Having cleaned hands more frequently than before (in general) (yes, no).
-
•
Having cleaned hands in special situations (e.g. after having had physical contact with the index patient, after having touched items used by the index patient before, or in other situations) (never, sometimes, mostly, or always).
-
•
Having adopted other measures to prevent secondary infections (e.g. having kept distance to the index patient) (yes, no).
-
•
Cleaning hands was defined as either washing or disinfecting hands and questions in this study were identical to those used in the previous season, with one exception: in the season 2008/2009, “washing” was used instead of “washing or disinfection” in the one question that asked about hand hygiene in general.
-
3
Pharmacological protective measures:
-
•
Vaccination against influenza.
-
•
Antiviral therapy.
-
•
Study period
The interviews were conducted between November, 12, 2009, and January, 7, 2010, and started shortly before the peak of the pandemic influenza wave.
Outcome measures
The main outcome measure was the occurrence of ILI in any household member during the time period between the first and the seventh day after symptom onset of the index patient (i.e. between day 2 and 8). We defined ILI as [fever (i.e. body temperature of 38·0°C or higher) or shivering] and (cough or sore throat).
Serial interval
We calculated the serial interval as the number of days between symptom onset of the index patient and the first day of ILI symptoms in any household member. Any ILI occurring in other household members after this day was excluded from the calculation of the serial interval.
Statistical analysis
We used Student’s t‐test for numerical and chi‐squared tests for categorical variables. We conducted univariable analyses for all binary or categorical exposure variables and calculated odds ratios (OR) and 95% confidence intervals (95% CI). Exact logistic regression was used for variables with a frequency of 0 in one of the exposure levels. We included variables with a P‐value of <0·1 in univariable analysis for multivariable modeling. In both univariable and multivariable analysis, we took household clustering into account using mixed effect models with household as random variable. To understand how age in years of the household contact was associated with the occurrence of ILI we used multivariable fractional polynomial models to test which type of model fits best the association between these two variables. Hypothesis tests were performed two‐sided, and a P‐value of <0·05 was considered statistically significant. We used the statistical software package stata, version 11 (STATA Corp., College Station, TX, USA).
Results
Response rate
Overall, 213 index patients with laboratory confirmed influenza infection who met the inclusion criteria consented to participate in the study. One hundred and five households were excluded because household members declined to be interviewed (n = 65), could not be contacted after repeated attempts (n = 36), or the index patient was hospitalized (n = 4). Age and sex were not significantly different between index patients of included and excluded households. Finally, 108 index patients and 267 household contacts were included in the study and were interviewed on a median of 16 days (range, 8–51) after symptom onset of the index patient (Figure 1).
Figure 1.
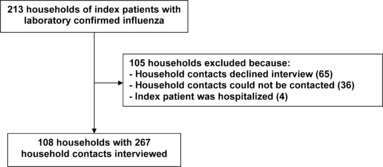
Breakdown of households according to exclusion criteria; pandemic season (H1N1) 2009.
Baseline characteristics
Age of index patients ranged from 2 to 58 years (median 11 years), 66 (61·1%) of 108 index patients were children. Fifty‐four (50·0%) were male and 25 of 106 (23·6%) received antiviral treatment within 2 days after symptom onset. All index patients were infected with influenza A (H1N1)pdm09. Household size ranged from 2 to 6 (mean 3·5) (Table 1).
Table 1.
Demographic and baseline characteristics of index patients and household contacts
| Variable | n/N (%) |
|---|---|
| Index patients (n = 108) | |
| Age‐group | |
| 0–4 | 9/108 (8·3) |
| 5–13 | 57/108 (52·8) |
| 14–34 | 25/108 (23·1) |
| 35–49 | 8/108 (7·4) |
| ≥50 | 9/108 (8·3) |
| Sex (male) | 54/108 (50·0) |
| Chronic underlying disease | 25/107 (23·4) |
| Smoker | 11/102 (10·8) |
| Vaccinated against pandemic influenza | 1/108 (0·9) |
| Antiviral treatment | 25/106 (23·6) |
| Household size | |
| 2–3 | 53/108 (49·1) |
| ≥4 | 55/108 (50·9) |
| Household contacts (n = 267) | |
| Age‐group | |
| 0–4 | 18/258 (6·6) |
| 5–13 | 47/258 (17·8) |
| 14–34 | 56/258 (21·7) |
| 35–49 | 115/258 (44·6) |
| ≥50 | 24/258 (9·3) |
| Sex (male) | 129/267 (48·3) |
| Chronic underlying disease | 40/266 (15·0) |
| Smoker | 66/260 (25·4) |
| Vaccinated against pandemic influenza | 29/267 (10·9) |
| Relationship to index patient | |
| Father | 73/261 (28·0) |
| Mother | 83/261 (31·8) |
| Sibling | 67/261 (25·7) |
| Other | 38/261 (14·6) |
Age of household contacts ranged from 1 to 82 years (median 36 years), 65 of 267 (24·4%) were children and 29 (10·9%) were vaccinated.
Secondary attack rates
Twenty‐seven of 267 (10·1%) household contacts developed secondary ILI and occurred in 23/108 (21·3%) households: 19 households (17·6%) reported one secondary case, 4 (3·7%) reported two, and none reported three or more.
The serial interval ranged from 1 to 6 days, with a median of 3 days (interquartile bounds = 1·5–4).
Vaccination and antiviral therapy
Vaccination was associated with an OR below 1 (OR = 0·3, 95% CI = 0·04–2·2); however, it was not statistically significant. Antiviral therapy of the index patient that was started within 2 days after symptom onset was not statistically significant associated with reduced SAR (OR = 1·7, 95% CI = 0·7–4·1).
Age and family relationship
Secondary attack rate increased with younger age. However, this trend excluded the youngest age group (0–4 years) which had the lowest attack rate of all age groups [1/17 (5·9%; 95% confidence interval (CI) = 0·1–28·7)] (Figure 2).
Figure 2.
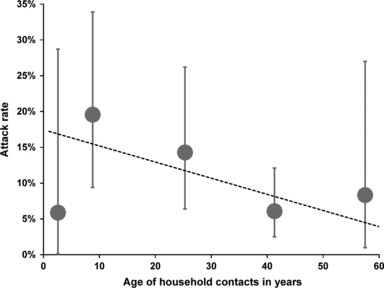
Secondary attack rate (in % with 95% CI) by age of household contact; points on the x‐axis are plotted at the median of the participants contained in the respective age group; pandemic season (H1N1) 2009. Ages were divided into five groups: 0–4; 5–13; 14–34; 35–49; and ≥50 years.
A fractional polynomial model that examined the type of association of household contacts’ age (in years) and the chance for ILI indicated that a linear decline in SAR fitted best the age dependency of household contacts. For each additional year of age, the chance of secondary ILI was calculated to be reduced by 2% (OR = 0·98, 95% CI = 0·95–1·0, P = 0·05; Figure 2, dotted line).
Regarding the family position of the household contact to the index patient, siblings had the highest SAR followed by the mother and the father of the index patient (disregarding “other” relationships; Table 2). Using father as reference, there was a significantly higher chance for a sibling to develop secondary ILI (OR = 4·1, CI = 1·1–15·6, P = 0·04). Household size or sex was not statistically significantly associated with secondary ILI.
Table 2.
Secondary attack rates (SARs) and univariable associations of preventable and non‐preventable risk factors with secondary ILI
| Variable | Category | Household contacts (n = 267) with ILI/total (SAR in %) | OR (95% CI) | P |
|---|---|---|---|---|
| Non‐preventable risk factors | ||||
| Age‐group | 0–4 | 1/17 (5·9) | 0·9 (0·1–8·4) | 0·97 |
| 5–13 | 9/46 (19·6) | 3·7 (1·3–10·8) | 0·02 | |
| 14–34 | 8/56 (14·3) | 2·6 (0·9–7·5) | 0·08 | |
| 35–49 | 7/115 (6·1) | Ref | NA | |
| ≥50 | 2/24 (8·3) | 1·4 (0·3–7·2) | 0·69 | |
| Sex | Female | 14/138 (10·1) | Ref | NA |
| Male | 13/129 (10·1) | 1·0 (0·4–2·2) | 0·99 | |
| Smoker | No | 19/194 (9·8) | Ref | NA |
| Yes | 8/66 (12·1) | 1·3 (0·5–3·1) | 0·59 | |
| Relationship to index patient | Father | 3/73 (4·1) | Ref | NA |
| Mother | 7/83 (8·4) | 2·1 (0·5–8·6) | 0·28 | |
| Sibling | 10/67 (14·9) | 4·1 (1·1–15·6) | 0·04 | |
| Other | 5/38 (13·2) | 3·5 (0·8–15·7) | 0·10 | |
| Vaccination and antiviral therapy | ||||
| Vaccinated against pandemic influenza | No | 26/238 (10·9) | Ref | NA |
| Yes | 1/29 (3·4) | 0·3 (0·04–2·2) | 0·24 | |
| Antiviral therapy of the index patient within 2 days | No | 19/211 (9·0) | Ref | NA |
| Yes | 8/56 (14·3) | 1·7 (0·7–4·1) | 0·25 | |
| Preventable risk factors | ||||
| Close contact* with index patient | 0–1 hours | 6/ 81 (7·4) | Ref | NA |
| 2–4 hours | 10/103 (9·7) | 1·3 (0·5–3·9) | 0·58 | |
| 5–8 hours | 3/51 (5·9) | 0·8 (0·2–3·3) | 0·74 | |
| ≥9 hours | 8/30 (26·7) | 4·5 (1·4–14·5) | 0·01 | |
| Ate meals with index patient** | No | 7/111 (6·3) | Ref | NA |
| Yes | 20/155 (12·9) | 2·2 (0·9–5·4) | 0·09 | |
| Provided care for index patient** | No | 24/230 (10·4) | Ref | NA |
| Yes | 3/37 (8·1) | 0·8 (0·2–2·6) | 0·66 | |
| Slept in the same room regulary *** | No | 20/216 (9·3) | Ref | NA |
| Yes | 7/50 (14·0) | 1·6 (0·6–4·0) | 0·41 | |
| Shared items with index patient regulary*** (towels, clothes) | No | 16/161 (9·9) | Ref | NA |
| Yes | 11/104 (10·6) | 1·1 (0·5–2·4) | 0·87 | |
| Preventive behaviour | ||||
| Cleaned hands more often in general | No | 10/116 (8·6) | Ref | NA |
| Yes | 16/144 (11·1) | 1·3 (0·6–3·0) | 0·51 | |
| Cleaned hands regularly*** | No | 2/19 (10·5) | Ref | NA |
| …after physical contact with index patient | Yes | 1/29 (3·4) | 0·3 (0·03–3·6) | 0·34 |
| Cleaned hands regularly*** | No | 21/202 (10·4) | Ref | NA |
| …after contact with items used by the index patient before | Yes | 5/54 (9·3) | 0·9 (0·3–2·4) | 0·81 |
| Other measures to prevent infection | No | 17/169 (10·1) | Ref | NA |
| Yes | 9/92 (9·8) | 1·0 (0·4–2·3) | 0·94 | |
CI, confidence interval; ILI, influenza‐like illness; OR, odds ratio.
*Close contact: <2 m to index patient.
**More than once per day.
***Regularly: always or mostly.
Preventable risk factors
Behavioral risk factors were not statistically significantly associated with secondary ILI, even after stratifying for the respective family relationships (father, mother, sibling; data not shown). The only exception was the duration of close contact to the index patient where we found a statistically significant association when the contact time exceeded 8 hours (Table 2, Figure 3).
Figure 3.
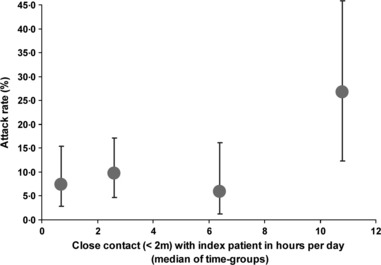
Secondary attack rate (in % with 95% CI) by category of close contact time with the index patient (in hours); points on the x‐axis are plotted at the median of the participants contained in the respective intervals; pandemic season (H1N1) 2009. Time groups are defined as: 0–1; 2–5; 5–8; and 9 or more hours.
Hygiene behavior
Twenty‐five (69·4%) of 36 adult index patients and 144 (80·9%) of 178 adult household contacts were convinced that hand cleaning could reduce the risk of a secondary influenza infection; 52 (49·1%) of 106 index patients and 144 (55·4%) of 260 household contacts stated that they had cleaned their hands more often in the 7‐day period after symptom onset of the index patient (Table 3). Twenty‐nine (60·4%) of 48 and 54 (26·7%) of 202 household contacts reported that they had cleaned their hand regularly after contact with the index patients or with potentially contaminated household items, respectively. About half of the index patients (52/108) stated that they had cleaned their hands regularly after coughing or sneezing.
Table 3.
Attitudes toward hygiene and reported hygiene behavior of index patients and household contacts
| Variable | Yes | All | % |
|---|---|---|---|
| Household contacts (n = 267) | |||
| Do you think, hand cleaning could reduce secondary infection?* | 144 | 178 | 80·9 |
| Cleaned hands more often in general | 144 | 260 | 55·4 |
| Cleaned hands regularly** after physical contact with index | 29 | 48 | 60·4 |
| Cleaned hands regularly** after contact with household items | 54 | 202 | 26·7 |
| Cleaned hands regularly** in other situations | 39 | 258 | 15·1 |
| Adopted other measures to prevent infection | 92 | 261 | 35·2 |
| Do you think, face mask wearing could reduce secondary infection?* | 70 | 149 | 47·0 |
| Worn face mask at some time? | 6 | 267 | 2·3 |
| Index patients (n = 108) | |||
| Do you think, hand cleaning could reduce secondary infection?* | 25 | 36 | 69·4 |
| Cleaned hands more often in general | 52 | 106 | 49·1 |
| Cleaned hands regularly** before contact with household items | 20 | 107 | 18·7 |
| Cleaned hands regularly** in other situations | 19 | 108 | 17·6 |
| Cleaned hands regularly** after coughing/sneezing | 52 | 108 | 48·2 |
| Adopted other measures to prevent secondary infection | 51 | 103 | 49·5 |
| Do you think, face mask wearing could reduce secondary infection?* | 23 | 34 | 67·7 |
| Worn face mask at some time? | 7 | 99 | 6·6 |
*Only adult household contacts.
**Regularly: always or mostly.
Twenty‐three (67·7%) of 34 adult index patients and 70 (47·0%) of 149 adult household contacts believed that wearing face masks could reduce the risk of secondary infection; however, only 6 (2·3%) of 267 household contacts and 7 (7·1%) of 99 index patients reported that they have worn a face mask at some time. None of the variables coding for hygiene behavior were statistically significantly associated with secondary ILI (Table 2).
Multivariable analysis
After the adjustment for age and the variables “relationship to the index patient,”“ate meals with index patient,” and “close contact with index patient” in the final mixed effect model age and contact time of at least 9 hours remained statistically significant risk factors for secondary ILI (Table 4).
Table 4.
Crude and adjusted OR for variables associated with influenza‐like illness among household contacts of index patients
| Variable | Category | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjusted P |
|---|---|---|---|---|
| Age of household contact | Risk per year | 0·98 (0·95–1·0) | 0·97 (0·94–1·0) | 0·04 |
| Close contact* with index patient | 0–1 hours | Ref | Ref | NA |
| 2–4 hours | 1·3 (0·5–3·9) | 1·6 (0·5–5·3) | 0·42 | |
| 5–8 hours | 0·8 (0·2–3·3) | 0·8 (0·2–3·7) | 0·76 | |
| ≥9 hours | 4·5 (1·4–14·5) | 5·5 (1·4–21·4) | 0·02 |
CI, confidence interval; OR, odds ratio.
*Close contact: <2 m to index patient.
Discussion
To our knowledge, this is the first study that examined in one study the effect of preventable and non‐preventable risk factors on secondary household ILI as well as the frequency of hygiene behavior in influenza households during the pandemic (H1N1) 2009. The overall attack rate was 10%. The length of time in close proximity to the index patient was an important factor that influenced influenza transmission in households; we could not identify other behavioral risk factors that were statistically significant, a relatively small sample size may have prevented us from finding risk factors with a small to moderate effect. About half of influenza households reported that they have practiced hygiene behavior as recommended through public service announcements.
To understand behavioral, that is, modifiable or “preventable,” risk factors is important because they determine influenza transmission in the household setting. France et al. found during the early phase of the pandemic that in households with a school age, index patient parents who provide care to the index patient, who sleep in the same room as the index patient or siblings, who watch television or play video games with the index patient were at increased risk for secondary ILI. In our study that we conducted during the last pre‐pandemic season 2008/2009, we identified similar risk factors as France et al. 9 In that study, we found out that the odds for a secondary ILI was significantly higher for all contact persons (parents and siblings) when they slept in the same room as the index patients, the odds was more than tenfold higher among mothers when they provided care for the index child more than once per day, and for fathers and siblings if they had contact regularly to items that the index patient also has had contact to. In this study, none of these behaviors showed a significant effect, although the observed differences in respect to family relationship were still likely influenced by behavior, contact pattern, and/or age. Being in close contact to the index patient for at least 9 hours per day was the only independent risk factor. In comparison, in our 2008/2009 study, the threshold contact time necessary to reach a plateau in the SAR was only 2 hours. However, because of the relatively small sample size, an indirect comparison should be interpreted with caution.
One could hypothesize that the differences in risk factors identified in the 2008/2009 pre‐pandemic and the pandemic season are associated with differences in hygiene behavior. The prevalence of good hygiene practice as reported by household members was substantially lower in the pre‐pandemic season 2008/2009: 9 only 30% (32/119) of index patients and 30% (81/271) of household contacts reported that they had cleaned their hands more frequently during the week after symptom onset of the index patient. However, in this study, that is, during the pandemic season, the prevalence for the same variable was 49% and 55% for index patients and household contacts, respectively, a difference that was statistically significant [P‐value = 0·001 (index patients) and P‐value < 0·001 (household contacts)]. Moreover, in the pre‐pandemic season 2008/2009, only 18% (21/120) of index patients cleaned their hands regularly after coughing or sneezing, and in the pandemic season, the proportion rose to 48% (52 of 108, P‐value < 0·001). Unfortunately, France et al. did not measure the prevalence of hand hygiene in their study. We are not aware of any other direct comparisons between pandemic and pre‐pandemic hygiene behavior in the literature. During the pandemic, public service announcements provided recommendations for individuals how to reduce the likelihood of influenza transmission (e.g. to clean hands more often in general or to avoid close contact to infected persons). 10 Public service announcements may have aided in the adoption of better hygiene practice, and this is supported by studies in other countries. 11 , 12 Because of these behavioral changes, we assume that the household members succeeded in “postponing” transmission. However, as Figure 3 suggests, at a certain point of cumulative contact time with the index patient, transmission occurred nevertheless.
The importance of hand cleaning and the impact of fomite transmission remained unclear for the transmission of influenza. While influenza viruses survive on surfaces for hours or even days 13 and contact transmission of influenza is thought to be possible, its impact on infection is unknown 14 , 15 and may be of secondary importance compared to droplet or droplet nuclei transmission. One other study found that hand washing within households reduced influenza contamination of household surfaces; however, this did not have an influence on secondary infection rates. 16 Nevertheless, the relevance of hand cleaning in reducing influenza transmission is indicated by two recent studies 5 , 17 and one systematic review pointing out the importance of hand cleaning to reduce the spread of respiratory viruses in general. 18
Previous studies have consistently found that ILI increased with younger age of household contacts both during pre‐pandemic influenza seasons 4 , 5 and during the pandemic (H1N1) 2009. 3 , 7 , 8 , 19 , 20 Therefore, it is notable that our data showed that household contacts aged 0–4 years had the lowest attack rate of all age groups. However, using a multivariable fractional polynomial model, the association between age and secondary ILI could be best described as linear. Thus, the lower SAR among 0–4 year olds might just be due to chance. Conversely, if it is real, it would be in line with findings from a study by Charu et al. 21 who analyzed the mortality burden in different age groups in Mexico. Although 0‐ to 4‐year‐olds are in general an extremely vulnerable age group for influenza, parents may have gone out of their way to protect this frail population.
Our study has several limitations. First, the relatively small size of the study may have limited the power to identify (behavioral) risk factors with a small to moderate effect on ILI rates. Second, we used a syndromic definition for secondary cases without laboratory confirmation of influenza infection. Third, although community transmission is possible in principle, we assumed that any secondary infection was acquired within the household. Fourth, using an ILI case definition for index patients may have led to an overestimation of the transmission potential of the virus. As influenza infections often result in a milder form than ILI and symptom severity is positively associated with the degree of viral shedding, 20 index patients in our study may have been more infectious than the “average” influenza patient. Fifth, we excluded households only if there had been respiratory infections within the 2 weeks before symptom onset of the index patient. We cannot rule out that influenza infections had occurred before, which would have rendered these household members (partially) immune or at least not fully susceptible. Sixth, when parents were answering questions on behalf of their children, they may have projected their own behavior or expectation into the answers “for” their children. Seventh, the retrospective study design may have influenced the accuracy of answers in either direction, because of imperfect recall or because answers reflect public health recommendations rather than actual behavior. However, we believe that these inaccuracies are likely non‐differential.
In conclusion, SAR in our households was 10%, and the most important risk factor associated with secondary ILI was 9 hours or more daily time of close proximity to the index patient. We could not detect other statistically significant behavioral factors, perhaps due to the increased prevalence of good hygiene behavior compared to the 2008/2009 pre‐pandemic season.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
None.
Acknowledgements
The authors thank all contacted patients, their families, and the physicians who participated in the study as well as the students for conducting the telephone interviews.
References
- 1. Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwok KO, Leung GM, Riley S. Modelling the proportion of influenza infections within households during pandemic and non‐pandemic years. PLoS ONE 2011; 6:e22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cauchemez S, Donnelly CA, Reed C et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med 2009; 361:2619–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Viboud C, Boelle PY, Cauchemez S et al. Risk factors of influenza transmission in households. Br J Gen Pract 2004; 54:684–689. [PMC free article] [PubMed] [Google Scholar]
- 5. Cowling BJ, Chan KH, Fang VJ et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med 2009; 151:437–446. [DOI] [PubMed] [Google Scholar]
- 6. France AM, Jackson M, Schrag S et al. Household transmission of 2009 influenza A (H1N1) virus after a school‐based outbreak in New York City, April–May 2009. J Infect Dis 2010; 201:984–992. [DOI] [PubMed] [Google Scholar]
- 7. Cowling BJ, Chan KH, Fang VJ et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med 2010; 362:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgan OW, Parks S, Shim T et al. Household transmission of pandemic (H1N1) 2009, San Antonio, Texas, USA, April–May 2009. Emerg Infect Dis 2010; 16:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert Koch Institute . Influenza‐Infektion: Vergleich von Risikofaktoren in Haushalten während der Saisons 2008/09 und 2009/10 in Deutschland. Epidemiologisches Bulletin 2011; 49:Available at http://edoc.rki.de/documents/rki_fv/reziH188SfNLQ/PDF/29S4Dl68GanrI.pdf (Accessed 12 December 2011). [Google Scholar]
- 10. Robert Koch Institute and Bundeszentrale fuer gesundheitliche Aufklaerung . Wir‐gegen‐Viren. Available at http://www.wir‐gegen‐viren.de/ (Accessed 4 July 2011).
- 11. Park JH, Cheong HK, Son DY, Kim SU, Ha CM. Perceptions and behaviors related to hand hygiene for the prevention of H1N1 influenza transmission among Korean university students during the peak pandemic period. BMC Infect Dis 2010; 10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin GJ, Amlot R, Page L, Wessely S. Public perceptions, anxiety, and behaviour change in relation to the swine flu outbreak: cross sectional telephone survey. BMJ 2009; 339:b2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis 1982; 146:47–51. [DOI] [PubMed] [Google Scholar]
- 14. Influenza Team ECfDPaC . Influenza transmission: research needs for informing infection control policies and practice. Euro Surveill 2007; 12:E0705101. [PubMed] [Google Scholar]
- 15. Vukotich CJ Jr, Coulborn RM, Aragon TJ et al. Findings, gaps, and future direction for research in nonpharmaceutical interventions for pandemic influenza. Emerg Infect Dis 2010; 16:e2. [DOI] [PubMed] [Google Scholar]
- 16. Simmerman JM, Suntarattiwong P, Levy J et al. Influenza virus contamination of common household surfaces during the 2009 influenza A (H1N1) pandemic in Bangkok, Thailand: implications for contact transmission. Clin Infect Dis 2010; 51:1053–1061. [DOI] [PubMed] [Google Scholar]
- 17. Aiello AE, Murray GF, Perez V et al. Mask use, hand hygiene, and seasonal influenza‐like illness among young adults: a randomized intervention trial. J Infect Dis 2010; 201:491–498. [DOI] [PubMed] [Google Scholar]
- 18. Jefferson T, Del Mar C, Dooley L et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2009; 339:b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papenburg J, Baz M, Hamelin ME et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory‐confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis 2010; 51:1033–1041. [DOI] [PubMed] [Google Scholar]
- 20. Suess T, Buchholz U, Dupke S et al. Shedding and transmission of novel influenza virus A/H1N1 infection in households – Germany, 2009. Am J Epidemiol 2010; 171:1157–1164. [DOI] [PubMed] [Google Scholar]
- 21. Charu V, Chowell G, Palacio Mejia LS et al. Mortality burden of the A/H1N1 pandemic in Mexico: a comparison of deaths and years of life lost to seasonal influenza. Clin Infect Dis 2011; 53:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]


