Abstract
Objective Describe the influenza A(H1N1) pandemic in Bhutan.
Design Observational study from sentinel surveillance sites.
Setting Bhutan remains isolated, with only one to two flights a day at the lone airport, no trains, and only three major roads that enter from India.
Main outcome measures PCR positive human respiratory samples
Results The first case of A(H1N1)pdm09 infection was detected in Bhutan in July 2009, 3 months after the virus was first reported in Mexico in April 2009. During the official WHO pandemic period (11 June 2009 to 8 August 2010), a total of 2149 samples were collected and tested by RT‐PCR of which 22.7% (487) were confirmed A(H1N1)pdm09; H3N2, H1N1, and B were positive in 2.2%, 1.1%, and 7.2%, respectively. The highest rate of A(H1N1)pdm09 cases (57.4%) was detected in the 6‐20 year‐old age group. Importantly, Bhutan increased from 3 sentinel sites in April 2009 to 11 a year later, and in April 2010 established PCR capability for influenza.
Conclusions Despite relative isolation, the A(H1N1)pdm09 reached Bhutan within 3 months of identification in Mexico. The H1N1 pandemic has made Bhutan more prepared for epidemics in the future.
Keywords: Bhutan, H1N1, influenza, pandemic
Introduction
Bhutan is an extremely rugged and mountainous country located in the eastern Himalayas between the Tibetan Plateau and the Indian plains. It covers an area of 38 394 square kilometers (about the size of Switzerland) with an elevation ranging from 160 m in the south to more than 7500 m in the north. The population was 634 928 in 2005 with growth rate of 1·3% and median age of 22·3 years. Around 30% live in urban areas and rest in rural areas. 1
Essential health service is free in Bhutan and is guaranteed by the constitution. There are 30 hospitals including one national referral hospital, two regional referral hospitals, 181 basic health units (BHU) and 518 outreach clinics scattered throughout the country. The referral hospitals have medical specialties and high‐end diagnostic services, while district hospitals have general consultation and basic diagnostic services. The BHU cater to only minor ailments, assist normal deliveries, and administer prevention and sanitation activities within the communities. People can access any health center and patients are referred both to a higher level and back to communities for monitoring and rehabilitative measures. 2
Respiratory disease is one of the top ten public health diseases in the country every year. However, there was no surveillance system in place to ascertain respiratory disease burden and etiology until 2008. In 2008, the Public Health Laboratory (PHL) located in the capital city (Thimphu), in collaboration with Department of Virology, Armed Force Research Institute for Medical Sciences (AFRIMS) based in Bangkok, Thailand, initiated influenza virology surveillance. The surveillance was initially restricted to only three sites that were relatively close to PHL. This was done because sample shipment and logistics are difficult in the rugged terrain. However, the World Health Organization (WHO) declared a health emergency on April 29, 2009, and PHL added six more sites in May 2009. A year later, two more sampling sites were added. In April 2010, influenza PCR capability was established.
This study summarizes epidemiological data on influenza collected from sentinel surveillance sites in the country from November 2008 to 2011, including the pandemic period of A(H1N1)pdm09. This is the first description of influenza data from Bhutan.
Materials and methods
Bhutan is broadly divided into three regions: west, central, and east. Representative sentinel sites were selected from each region based on strategic geographical location, climatic conditions, population size, and referral of patients from health centers (Figure 1). The first three sites (Thimphu, Paro, and Punakha hospitals) were opened in November 2008. During the pandemic, six additional sites (Phuentsholing, Trongsa, Tsirang, Gelephu, Mongar, and Trashigang hospitals) were added in May 2009 and last two sites (Samtse and SamdrupJongkhar hospitals) in May–June 2010.
Figure 1.
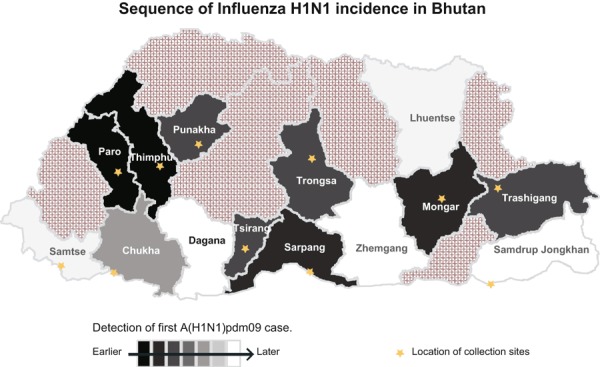
Location of sentinel sites and progression A(H1N1)pdm09 progression in the country.
A suspect case for influenza was defined as a person with fever (temperature ≥38°C) or history of fever within 72 hours and cough or sore throat. Cases included outpatients and inpatients of any age. Surveillance also required every site to report death cases because of suspected influenza or pneumonia.
Nasal and throat swab samples were collected from cases after obtaining informed consent, clinical and epidemiological information. The nasal swab was used for rapid testing (QuickVue) in each site to aid clinicians for treatment and management. The throat swab was put in viral transport media (VTM) tube and shipped to PHL within 72 hours in cold box at 2–8°C. Samples were stored at −70°C in PHL and shipped to Department of Virology, AFRIMS every month in dry ice. Later (April 2010) with establishment of real time PCR in PHL, throat swab samples in VTM were aliquoted into two: one for testing in PHL and one for referral to Department of Virology, AFRIMS.
Samples were first tested for universal influenza A and B; all influenza A positive were tested for seasonal A/H3 and A/H1 during the pre‐pandemic phase. During the pandemic phase, all influenza A positive samples were first tested for A(H1N1)pdm09 followed by seasonal A/H3 and A/H1 if the sample was A(H1N1)pdm09 negative. Primers and probes sequence used for all the tested influenza virus and its subtypes were reference sequence from CDC (Table 1).
Table 1.
Sequences of primers and probes for PCR and real time RT‐PCR
| Primer and probe | Sequence | Reference |
|---|---|---|
| FluA Forward | GAC CRA TCC TGT CAC CTC TGA C | CDC |
| FluA Reverse | AGG GCA TTY TGG ACA AAK CGT CTA | CDC |
| FluA Probe | TGC AGT CCT CGC TCA CTG GGC ACG | CDC |
| FluB Forward | TCC TCA AYT CAC TCT TCG AGC G | CDC |
| FluB Reverse | CGG TGC TCT TGA CCA AAT TGG | CDC |
| FluB Probe | CCA ATT CGA GCA GCT GAA ACT GCG GTG | CDC |
| H1 Forward | AAC TAC TAC TGG ACT CTR CTK GAA | CDC |
| H1 Reverse | CCA TTG GTG CAT TTG AGK TGA TG | CDC |
| H1 Probe | TGA YCC AAA GCC “T”CT ACT CAG TGC GAA AGC | CDC |
| H3 Forward | AAG CAT TCC YAA TGA CAA ACC | CDC |
| H3 Reverse | ATT GCR CCR AAT ATG CCT CTA GT | CDC |
| H3 Probe | CAG GAT CAC ATA TGG GSC CTG TCC CAG | CDC |
| pH1 Forward | GTG CTA TAA ACA CCA GCC TYC CA | CDC |
| pH1 Reverse | CGG GAT ATT CCT TAA TCC TGT RGC | CDC |
| pH1 Probe | CA GAA TAT ACA “T” CC RGT CAC AAT TGG ARA A | CDC |
The significance of bold value indicates the TaqMan probes are labeled at the 5'‐end with the reporter molecule 6‐carboxyfluorescein (FAM) and quenched internally at a modified "T" residue with BHQ1, with a modified 3'‐end to prevent probe extension by Taq polymerase.
The database was maintained and analyzed in Excel (Microsoft). Surveillance data analyzed were from June 11, 2009 to August 8, 2010 (WHO pandemic period). Differences between mean ages of other influenza subtypes against A(H1N1)pdm09 were performed using independent sample t‐test.
Results
Prior to pandemic period, the strains circulating were A/H1 and A/H3. A(H1N1)pdm09 was first detected on July 20, 2009 and slowly dominated the seasonal strains during the pandemic period. After the pandemic period, A(H1N1)pdm09 still remained a dominant strain for almost a year later replaced by A/H3 (Figure 2). Influenza B continued to be present through pre‐pandemic, pandemic, and post‐pandemic periods.
Figure 2.
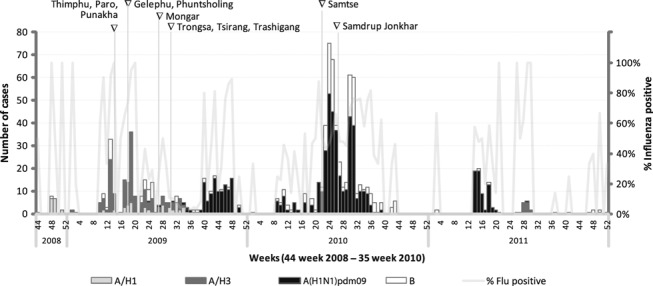
Influenza virus subtypes November 2008–2011; sites added are labeled across the top.
During the pandemic period, a total of 2149 samples were collected and tested: 22·7% (487) samples were positive for A(H1N1)pdm09, 1·1% (23) for A/H1, 2·2% (47) for A/H3, and 7·2% (154) for influenza B. The rapid test sensitivity during pandemic period was 43% for influenza A and 59% for influenza B compared to PCR; the specificity was 99% for both A and B.
The mean ages of A/H1, A/H3, and B were 27·2, 22·4, and 16·4 years, respectively. The most common age group affected by A(H1N1)pdm09 was 6–20 years (57·4%), and the mean age of those with A(H1N1)pdm09 was 19·7 years, significantly younger than those with A/H1 (P = 0·04), significantly older than those with B (P = 0·002), but with no significant difference compared to A/H3 (P = 0·18; see Table 2).
Table 2.
Positivity of samples by age and virus from June 11, 2009 to August 8, 2010
| Subtype | Age median (in years) | Total cases | Age group, % | ||||
|---|---|---|---|---|---|---|---|
| 0–5 | 6–20 | 21–35 | 36–50 | >50 | |||
| A/H1 | 25·0 | 23 | 17·4 | 4·3 | 47·8 | 17·4 | 13·0 |
| A/H3 | 22·5 | 47 | 11·4 | 31·8 | 45·5 | 6·8 | 4·5 |
| A(H1N1)pdm09 | 18·0 | 487 | 6·2 | 57·4 | 27·7 | 7·1 | 1·5 |
| Flu B | 15·0 | 154 | 12·9 | 60·5 | 21·8 | 2·7 | 2·0 |
| Total | 18·0 | 711 | 8·4 | 54·6 | 28·3 | 6·5 | 2·2 |
The first cases detected were in Thimphu and Paro and subsequently in Punakha and Gelephu. The detection date of A(H1N1)pdm09 in each sentinel site and district is given in Figure 1. During the pandemic period, the first institutional outbreak of A(H1N1)pdm09 was in two schools from the eastern district of Trashigang in May 2010. In a span of 2 months, 20 outbreaks were reported in schools and institutes across the country. Out of 20 outbreaks, 16 were confirmed as A(H1N1)pdm09 and one as influenza B; three outbreaks could not be confirmed because they were located far from sentinel sites, and sample transportation was blocked by road closure caused by continuous raining.
Discussion
Bhutan had minimal influenza surveillance prior to pandemic. During the pre‐pandemic phase, the predominant seasonal strain was A/H1 followed A/H3. After the detection of A(H1N1)pdm09, transmission took 12 weeks to dominate and replace both the seasonal influenza A/H1 and A/H3. This is in contrast to most countries of northern hemisphere including India where transmission was rapid. 3 , 4 We believed that one factor for slow transmission was the timing of virus entry coinciding with the summer season which is less conducive for transmission. A(H1N1)pdm09 was the predominant strain during pandemic period and even after the WHO pandemic period for 11·5 months before being replaced by A/H3. However, influenza B continued to present throughout the pre‐pandemic, pandemic, and post‐pandemic periods. We found that the change of influenza strain in Bhutan was in concordance with the global trend of influenza, especially with that of the South‐East Asian Region (SEAR). 5 One particular influenza A strain dominating over the other strains at a particular period of time could be attributed to the cross‐protection to an already exposed strain of influenza. Influenza B virus, on the other hand, is said to mutate at a much lower rate. This slower and more‐erratic viral evolution may be the driving force behind both the less‐frequent and the less‐periodic emergence of influenza B virus capable of infecting large numbers of people. 6 , 7
The first A(H1N1)pdm09 cases were confirmed on July 20, 2009 by report received from the Department of Virology, AFRIMS; the samples were collected on June 16 and 18, 2009 from Thimphu, the capital. Case confirmation was delayed because at that time PHL lacked testing capability and samples were stored and shipped to the Department of Virology, AFRIMS in Bangkok every month. We found that none of the first two confirmed cases or any family members who had contact with cases had travel history outside the country. Therefore, it was concluded that transmission was indigenous. Considering the incubation period of virus and number of contacts, we hypothesize that A(H1N1)pdm09 was probably introduced in the country in the first week of June 2009, shortly after neighboring countries reported A(H1N1)pdm09 outbreaks. It was not possible to precisely follow A(H1N1)pdm09 progression in the country. However, Figure 1 shows the date of detection of A(H1N1)pdm09 in each district and suggests the first cases in 2009 occurred in Paro (the location of the airport) and Thimphu (1·5 hours from the airport), with subsequent movement to Punakha, a common adjacent destination. Another entry point was likely Gelephu (Sarpang) on the border with India with movement to Trongsa and Tsirang.
The first real wave of A(H1N1)pdm09 virus infection was during the usual season (October–November 2009). The second wave was toward the end of May 2010 with outbreaks across the country. The attack rates in the country are unknown because proper serological surveys have not been conducted. From the information from the field, the attack rates seemed very high in schools. 8 , 9 The basic reproduction number for A(H1N1)pdm09 has been documented as high as 3·0–3·6 in schools. 9
After the detection of first two cases on July 20, 2009, media hype and public anxiety contributed to a dramatic increase in sample collection. We believe increased visits to sites by those who reported fever that may not have had it likely resulted in the low percentage of confirmed cases in July and August (2009), and also in May and June (2010) when first A(H1N1)pdm09 institutional outbreaks was reported. The rapid test (QuickVue) positivity rate for A(H1N1)pdm09 cases was 48%, which is lower than others found. 10 We believed that this could be associated with low technical expertise especially with the quality of nasal sample collection because the rapid test was newly introduced in the sites.
Most illness caused by A(H1N1)pdm09 virus in humans has been self‐limited with the highest attack rate reported among children and young adults. 6 , 11 , 12 This was also observed in Bhutan with highest attack rate (56·8%) in those 6–20 years old. The relative sparing of older adults is presumably due to the exposure of this age group to antigenically related influenza virus resulting in cross‐protective antibodies. 12 , 13
The attack rate in the country is unknown because proper serological surveys have not been conducted. However, according to the information obtained from the fields, the attack rate seemed to be very high in schools. 8 , 9 The attack rate of A(H1N1)pdm09 has varied in different countries, but the overall attack rate was estimated at 11% 9 The overall case fatality rate of A(H1N1)pdm09 has been <0·5% globally with a wide range of estimates (0·0004–1·5%) reflecting uncertainty regarding case ascertainment and the denominator of infections. 14 , 15 , 16 In Bhutan, there is no documented case fatality of A(H1N1)pdm09 virus. However, there were 22 death cases reported as influenza and pneumonia deaths during the pandemic, but none of them had samples collected for laboratory confirmation.
Influenza surveillance in Bhutan started only two and half years ago. The reliability of the sample collection has not been evaluated. As the surveillance program matures, it will need evaluation for the comprehensiveness and stability of data collection.
Despite relative isolation, Bhutan was part of the pandemic within 2 months of the confirmation of A(H1N1)pdm09. Owing to the A(H1N1)pdm09 pandemic, the government was obliged to set up PCR facilities at Public Health Laboratory and expedite the establishment of sentinel surveillance for influenza. The country now has influenza sentinel surveillance in place and laboratory capability to test influenza virus including H5N1. In addition, PHL analyzes surveillance data every month and shares monthly reports to sentinel sites and relevant stakeholders in the Ministry of Health. The sharing of surveillance reports has helped the national influenza program and Department of Public Health in planning activities associated with influenza including pandemic preparedness planning for the country. PHL is now in the process of establishing National Influenza Center (NIC) in the country by the global WHO Influenza Programme.
The A(H1N1)pdm09 pandemic demonstrates that the influenza virus requires little time to reach even the most remote and isolated country like Bhutan. Surveillance is indispensible to monitor any novel influenza viruses as well as seasonal strains.
Financial sources
None.
Conflict of interest
None.
Disclaimer
The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the official views of the United States Army or the United States Department of Defense.
Acknowledgement
The authors thank clinicians and paramedics involved in the surveillance and Angkana Huang for her assistance with data management and graphics.
References
- 1. Office of the Census Commissioner . Royal government of Bhutan, results of population and housing census, 2005.
- 2. Tobgay T, Dorji T, Pelzom D, Gibbons RV. Progress and delivery of health care in Bhutan, the land of the thunder dragon and gross national happiness. Trop Med Int Health 2011; 16:731–736. [DOI] [PubMed] [Google Scholar]
- 3. Leo YS, Lye DC, Barkham T et al. Pandemic (H1N1) 2009 surveillance and prevalence of seasonal influenza, Singapore. Emerg Infect Dis 2010; 16:103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic influenza (H1N1) 2009 is associated with severe disease in India. PLoS ONE 2010; 5:e10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narian JP, Kumar R, Bhatia R. Pandemic (H1N1) 2009 epidemiological, clinical and prevention aspects Natl Med J India 2009 Sept-Oct; 22:242–247. [PubMed] [Google Scholar]
- 6. Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med 2000; 51:407–421. [DOI] [PubMed] [Google Scholar]
- 7. Lofgren E, Fefferman NH, Naumov YN et al. Influenza seasonality: underlying causes and modeling theories. J Virol 2007; 81:5429–5436. Epub 2006 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Witkop CT, Duffy MR, Macias EA et al. Novel influenza A (H1N1) outbreak at the U.S. AirForce academy: epidemiology and viral shedding duration. Am J Prev Med 2010; 38:121–126. [DOI] [PubMed] [Google Scholar]
- 9. Lessler J, Reich NG, Cummings DA et al. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med 2009; 361:2628–2636. [DOI] [PubMed] [Google Scholar]
- 10. Velasco JM, Montesa‐Develos ML, Jarman RG et al. Evaluation of QuickVue influenza A+ B rapid test for detection of pandemic influenza A/H1N1 2009. J Clin Virol 2010; 48:120–122. Epub 2010 Apr 15. [DOI] [PubMed] [Google Scholar]
- 11. Kelly H, Grant K, Williams S, Smith D. H1N1 swine origin influenza infection in the United States and Europe in 2009 may be similar to H1N1 seasonal influenza infection in two Australian states in 2007 and 2008. Influenza Other Respi Viruses 2009; 3:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reed C, Angulo FJ, Swerdlow DL et al. Estimates of the prevalence of pandemic (H1N1)2009, United States, April–July 2009. Emerg Infect Dis 2009; 15:2004–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Novel Swine‐Origin Influenza A (H1N1) Virus Investigation Team . Emergence of a novel swine‐origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605–2615. [Erratum, N Engl J Med 2009; 361:102.] [DOI] [PubMed] [Google Scholar]
- 14. Donaldson LJ, Rutter PD, Ellis BM et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009; 339:b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson N, Baker NG. The emerging influenza pandemic: estimating the case fatality ratio. Euro Surveill 2009; 14. [PubMed] [Google Scholar]
- 16. Perez‐Padilla R, De la Rosa‐Zamboni D, Ponce de Leon S et al. Pneumonia and respiratory failure from swine‐origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680–689. [DOI] [PubMed] [Google Scholar]


