Abstract
Background Information on influenza virology and epidemiology from Lao PDR is limited and the seasonal patterns of influenza have not been previously described.
Objectives To describe epidemiological and virologic characteristics of influenza in Lao PDR to recommend public health interventions, including improvements in surveillance and response.
Patients/Methods We performed a descriptive analysis of samples taken from patients with influenza‐like‐illness (ILI) (fever >38°C with cough and/or sore throat) presenting at seven sentinel hospitals in three regions of Lao PDR, January 2008–December 2010. A nasopharyngeal (NP) swab or combined nasal with oropharyngeal swab was collected from patients with ILI. Samples were tested for influenza by either Luminex RVP, conventional reverse transcriptase PCR (RT‐PCR) (January 2008–2009), or by real‐time PCR (rRT‐PCR) using US CDC reagents (February 2009 onward).
Results Of 2346 samples tested from patients with ILI, 523 (22%) were positive for influenza. The median age of those positive was 12 years (range, <1–60 year). The percentage of samples that were influenza positive was similar over the 3 years (20–23%). Each year 3–4 types/subtypes cocirculated with differing predominant type/subtype. Influenza was detected year‐round with the highest proportion of positive specimens in the 3rd and 4th quarter.
Conclusions Similar to other countries in the region, we found that influenza is present year‐round and has a peak activity from July to December. Dominant types or subtypes vary by year. A large proportion of patients with ILI are not influenza positive. ILI surveillance is critical for weighing disease burden, both morbidity and mortality, against the costs of advancing influenza vaccine delivery strategy.
Keywords: Epidemiology, influenza, Lao PDR, virology
Introduction
Influenza is a viral infection and common seasonal illness causing epidemics worldwide. It is estimated that about 10–20% of the world’s population is affected by seasonal influenza each season, with an average of 250 000–500 000 deaths annually. 1 The majority of fatal cases related to influenza in developed countries occur among people aged 65 years or older. 2 In addition, in temperate climates, there is a distinct influenza seasonal pattern, typically occurring in the colder winter months. 3 , 4 In tropical countries, however, the epidemiology is less well defined, with available data suggesting that seasonal influenza viruses circulate throughout the year with one or two peaks, particularly during the rainy season. 3 , 5 , 6 Other information on influenza, including seasonality, prevalence, and estimates of disease burden, is limited in tropical countries. 7 , 8 Such data are essential to better inform national and international prevention strategies.
Lao PDR occupies part of the Mekong Region of Southeast Asia and falls just within the northern hemisphere (18° latitude and 105° longitude), straddling both tropical and semi‐tropical zones. More than 70% of the 5·6 million population live in rural areas. 9 The climate is subtropical with monsoon rains from May to October. Lao PDR has limited capacity for influenza surveillance and response. However, in response to avian influenza A (H5N1) and the 2009 pandemic (influenza A (H1N1)pdm09), Lao PDR has been building capacity to respond to emerging infectious diseases, including strengthening surveillance and laboratory capacity.
Laboratory confirmation of influenza has been available at the National Centre for Laboratory and Epidemiology (NCLE) in Vientiane since 2006, and Influenza‐like‐Illness (ILI) virological surveillance has been ongoing since 2007. Prior to this, data on confirmed influenza infections were scarce in Lao PDR, as laboratory confirmation was only conducted on samples referred to NCLE on an ad hoc basis. Three years after establishing sentinel surveillance, NCLE was recognized as a National Influenza Center by WHO on 6 August 2010 10 and participates in the WHO Global Influenza Surveillance and Response System (GISRS). 11 Seasonal influenza vaccine is not routinely available in public healthcare facilities in Lao PDR, but a national influenza control strategy has been in existence since 2009. Since November 2009, 289 971 doses of donated pandemic (influenza A (H1N1)pdm09) vaccine have been administered, targeting high‐risk groups via a national vaccination campaign.
This study reviews the data from ILI sentinel virologic surveillance by NCLE during the period 2008–2010. We describe the epidemiological and virologic characteristics of influenza based on our results.
Methods
Surveillance data were analyzed from surveillance conducted during the period January 2008–December 2010 in seven government‐run hospital out‐patient departments (OPD) and emergency rooms (ER) in three regions of Lao PDR. Sentinel sites included three central hospitals (Vientiane Capital) in 2008 and then two Provincial hospitals in the south of the country (Savannakhet and Champasack Provinces), and one Provincial and one military hospital in the north (Luang Prabang Province) since 2009.
Data were collected on patients who presented for care at participating health facilities and who met the WHO ILI case definition—any person who has fever >38°C with cough and/or sore throat, in the absence of other diagnoses. ILI case data were received on the specimen form from all sentinel sites and included age, sex, onset date, admission date, number of days of illness, and ward. Weekly aggregated data were received only from the three central hospitals and included (i) the total number of outpatients and (ii) the number of outpatients with ILI.
Descriptive analyses of aggregate data and case data from patients with ILI were conducted in stata 10.0 (Statacorp, College Station, TX, USA). Data were compared using the chi‐squared test or Wilcoxon signed‐rank test, where appropriate. A two‐tailed P‐value < 0·05 was considered significant.
Some data from 2008 have previously been presented in an early report on the laboratory‐based influenza surveillance in Lao PDR. 12
Specimen collection
An NP swab or combined nasal swab with oropharyngeal swab was collected from all patients with ILI on one (central) or two (provincial) days a week from each site. Once samples were collected, swabs were put in Viral Transport Media, stored at 4°C, and then transported to the NCLE within 24 hours. Case data, including demographic and clinical information, were collected on a laboratory form from all patients from whom a swab was collected.
From January 2008 to January 2009, samples were tested for influenza virus by either Luminex RVP or conventional reverse transcriptase‐polymerase chain reaction (RT‐PCR). From February 2009 onward, samples were tested for influenza A and B viruses by real‐time reverse transcriptase PCR (rRT‐PCR) using US CDC reagents.
RNA from throat and nasal swab specimens from each patient was extracted using the QIAamp Viral RNA mini commercial kit (Qiagen Inc., Mettmann, Hilden, Germany) according to manufacturer’s instructions. The SuperScript III Platinum one‐step quantitative RT‐PCR system (Invitrogen, Carlsbad, CA, USA) was used for single‐step rRT‐PCR according to US CDC protocol. The primers and probes for the influenza virus (H1N1, H3N2, A (/H1N1) pdm09, H5N1, and influenza B) were derived from US CDC (as a WHO Influenza Collaborating Center) protocols 13 and shared by US CDC through the WHO National Influenza Centers’ (NICs) Network.
From 15 May 2009 onward, positive influenza A specimens were further subtyped for H1, H3, and A (H1N1)pdm09. If the specimens tested positive for influenza A/H1, H3, or A (H1N1)pdm09, then they were also inoculated into MDCK cells, 14 , 15 and isolates were identified by the haemagglutination inhibition assay (HIA) using the WHO or National Institute of Infectious Diseases (NIID), Japan influenza diagnostic kit. If the result of the specimen subtyping for H1, H3, and A (H1N1)pdm09 was negative, then subtyping was conducted for H5.
The influenza virus isolates presented in Table 2 come from specimens of a number of sources: patients who meet the ILI and severe acute respiratory infection (SARI) case definition (a person who meets the ILI case definition plus shortness of breath or difficulty breathing and who requires hospital admission), outbreak investigation cases, and any other ad hoc samples received for influenza testing including from enhanced surveillance during the A(/H1N1) pdm09 pandemic. The isolates were identified by the haemagglutination inhibition assay (HIA) using the WHO or NIID influenza diagnostic kit. Further characterization required the use of specific ferret antiserum.
Results
On average, ILI cases account for 10% (50 390/509 313) of all patients presenting to OPD and ER of hospitals in Vientiane Capital from 2008 to 2010. The highest monthly numbers of ILI presented to surveillance sites between May and September, before and during the rainy season (Figure 1). On the basis of aggregate data, patients with ILI were younger than all OPD/ER patients (64% being under 18 years of age versus 28%) (Figure 2). Patients from whom respiratory samples were obtained and tested had similar age distribution compared with all patients presenting with ILI (Figure 2). For Vientiane Capital, only 3% (1511/50 390) of all patients with ILI were sampled and tested for influenza.
Figure 1.
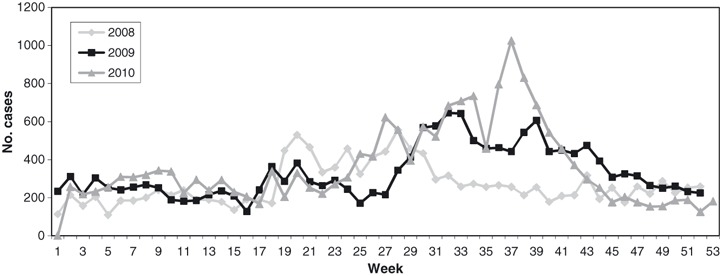
Patients meeting ILI case definition, three central hospitals in Vientiane Capital, 2008–2010. ILI, influenza‐like‐illness.
Figure 2.
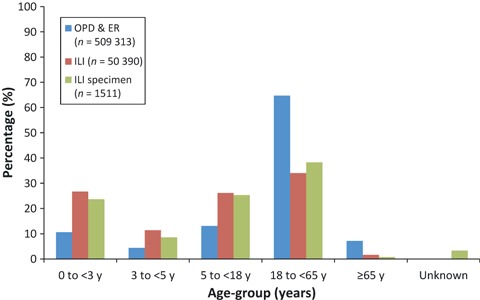
Age group of patients presenting to three central hospitals, Vientiane Capital, 2008–2010.
For the 3‐year period, 2346 samples from patients with ILI presenting to all sentinel healthcare facilities were received in good condition and tested. The majority of samples (65%) were from hospitals in the central region, with 22% from the north and 13% from the south. The majority of samples were tested in 2010 (50%), with 36% in 2009 and 14% in 2008. The median age of patients with ILI tested was 7 years (range, <1–89 years; IQR = 1·6–21 years) and the male‐to‐female ratio was 1:1·1. Of these samples, results from testing of eight samples were uninterpretable and not repeated, mostly during 2008 (n = 7), and therefore excluded from further analysis.
Of the 2338 samples tested from patients with ILI, 523 (22%) samples tested positive for influenza virus. The proportion of specimens positive for influenza virus was similar in the three surveillance years (Table 1). The proportion of samples testing positive for influenza varied geographically, with 23% of samples testing positive in the central region, 16% positive from north, and 29% in the south (P < 0·05). Overall, the positivity rate was slightly higher in males (24%) than females (21%) (P < 0·05). The median age of influenza‐positive patients was 12 years (range, <1–60 years; IQR = 5–23 years). Children 5–17 years of age were the highest percent positive for influenza among those with ILI (33%) followed by the 18–64 years of age group (28%). Only a small proportion (10%) of children aged <3 years were positive for influenza virus (Table 1). No adults aged 65 years or older tested positive for influenza. Influenza‐infected patients were significantly older than those who tested negative for influenza viruses (median, 12 versus 5 years; P < 0·001). There were no differences in age or gender distribution of positive cases when comparing data year to year.
Table 1.
The number samples tested and proportion positive for influenza viruses by year, geographical site and demographic characteristics; influenza‐like illness (ILI) surveillance, Lao PDR, 2008–2010
| No. samples tested | No. (%) influenza positive | No. (%)* positive for influenza type/subtype | |||||
|---|---|---|---|---|---|---|---|
| A/H1 | A (H1N1) pdm09 | A/H3 | B | Other** | |||
| Year | |||||||
| 2008 | 331 | 69 (20·9) | 20 (29) | 0 (0) | 1 (1·4) | 46 (66·7) | 2 (2·9) |
| 2009 | 833 | 184 (22·1) | 25 (13·6) | 54 (29·4) | 93 (50·5) | 5 (2·7) | 7 (3·8)*** |
| 2010 | 1174 | 270 (23·0) | 0 (0) | 143 (53) | 35 (12·9) | 91 (33·7) | 1 (0·4) |
| Total | 2338 | 523 | 45 (8·6) | 197 (37·7) | 129 (24·7) | 142 (27·2) | 10 (1·9) |
| Region (n = 2338) | |||||||
| Central | 1511 | 349 (23·1) | 44 (12·6) | 121 (34·7) | 92 (26·4) | 82 (23·5) | 10 (2·9) |
| North | 516 | 83 (16·1) | 1 (1·2) | 25 (30·1) | 20 (24·1) | 37 (44·6) | 0 (0) |
| South | 311 | 91 (29·3) | 0 (0) | 51 (56·0) | 17 (18·7) | 23 (25·3) | 0 (0) |
| Gender (n = 2337) | |||||||
| Male | 1210 | 291 (24·1) | 24 (8·3) | 105 (36·1) | 71 (24·4) | 86 (29·6) | 5 (1·7) |
| Female | 1127 | 231 (20·5) | 20 (8·7) | 92 (39·8) | 58 (25·1) | 56 (24·2) | 5 (2·2) |
| Age group (n = 2281) | |||||||
| 0 to <3 | 782 | 78 (10·0) | 7 (9·0) | 37 (47·4) | 15 (19·2) | 17 (21·8) | 2 (2·6) |
| 3 to <5 | 211 | 48 (22·8) | 3 (6·3) | 14 (29·2) | 13 (27·1) | 18 (37·5) | 0 (0) |
| 5 to <18 | 543 | 178 (32·8) | 14 (7·9) | 69 (38·8) | 45 (25·3) | 48 (27·0) | 2 (1·1) |
| 18 to <65 | 726 | 203 (28·0) | 21 (10·3) | 69 (34·0) | 52 (25·6) | 55 (27·1) | 6 (3·0) |
| >65 | 19 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
*Denominator is total positive specimens.
**Coinfection/untypable.
***Note: a random sample of three specimens that were initially positive but untypable at National Centre for Laboratory and Epidemiology was found to be negative when retested by WHO Collaborating Centre.
Influenza was detected year‐round with the highest proportion of positive specimens in the 3rd and 4th quarter (Figure 3). A bi‐modal seasonal pattern was seen in 2008 when there were peaks in September (39% positivity) and again in December (62%), but the sample size was small. In 2009 and 2010, the highest proportion of specimens that were influenza positive occurred in August–September (Figure 3).
Figure 3.
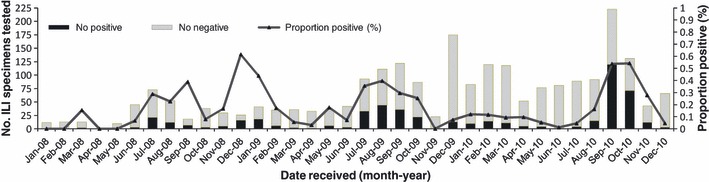
No. ILI specimens tested and proportion positive for influenza in Lao PDR, 2008–2010 (n = 2338). ILI, influenza‐like‐illness
Each year, influenza A and B viruses cocirculated (Table 1, Figure 4). In 2008, the dominant type was influenza B. In 2009, influenza A (H3N2) viruses predominated until A (H1N1)pdm09 emerged in July 2009, after which both subtypes cocirculated. In 2010, A (H1N1)pdm09 and influenza B were the most common types identified (Figure 4) and the temporal pattern of influenza seasonality by subtype shows a peak dominated by A (H1N1)pdm09 in weeks 37–43 (Figure 5). One specimen in 2010 was positive for both A (H1N1)pdm09 and influenza B. Nine specimens were subtype A but non‐subtypable (two in 2008, seven in 2009). These samples had low viral loads that were below the threshold of detection with subtype‐specific reagents. The highest proportion of influenza B specimens were reported from the north of the country (45%) and the highest proportion of A (H1N1)pdm09 from the south (56%).
Figure 4.
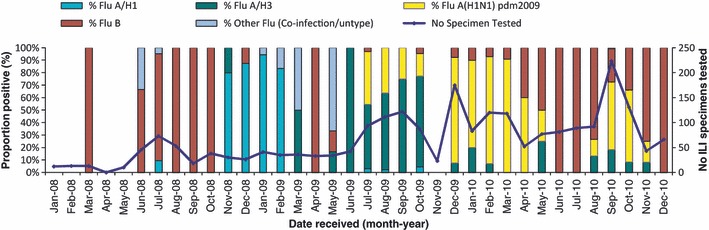
Proportion of influenza subtypes by month in Lao PDR, 2008–2010 (n = 2338).
Figure 5.
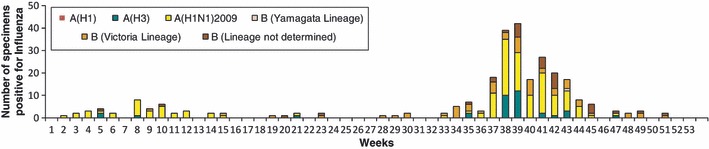
Number of specimens positive for influenza by subtype and week, Lao PDR, 2010 (n = 270).
Virus isolation was successful for 176 of 268 influenza‐positive samples (all sources) (Table 2). Hemagglutination inhibition assays identified seven isolates as “A/Brisbane/59/2007 (H1N1)‐like” viruses in 2008. There were no A/H3N2 or B isolates in this year. In 2009 and 2010, with the emergence of A (H1N1)pdm09, “A/California/7/2009‐like” was isolated in both years. In 2009 and 2010, the majority of (H3N2)‐like viruses isolates were “A/Perth/16/2009‐like” and the majority of B isolates were “B/Brisbane/60/2008‐like.” Only one B isolate was Yamagata lineage, in 2010.
Table 2.
Antigenic characterization of influenza virus isolates (all sources: including influenza‐like‐illness, SARI and outbreaks) in Lao PDR (n = 176)
| Year | Influenza virus A H1N1 | Influenza virus A H3N2 | Influenza virus A pdmH1N1‐2009 | Influenza Virus B | ||||
|---|---|---|---|---|---|---|---|---|
| Number* | Virus | Number* | Virus | Number* | Virus | Number* | Virus | |
| 2008** | 20, 7 | “A/Brisbane/59/2007‐like” | 1, 0 | 0 | 46, 0 | |||
| 2009*** | 47, 8 | “A/Brisbane/59/2007‐like” | 264, 29 | “A/Perth/16/2009‐like” | 295, 20 | “A/California/7/2009‐like” | 14, 3 | “B/Brisbane/60/2008‐like”† |
| 2010†† | 0 | 46, 33 | “A/Perth/16/2009‐like” | 199, 14 | “A/California/7/2009‐like” | 110, 60 | “B/Brisbane/60/2008‐like”† | |
| , 1 | “B/Taiwan/55/2009‐like”†,††† | |||||||
| , 1 | “B/Wisconsin/1/2010‐like”‡ | |||||||
| Total | 67, 15 | 311, 62 | 494, 34 | 170, 65 | ||||
*Number PCR‐positive, number antigenically characterized.
**In 2008 only northern hemisphere vaccine strain selection (recommendation made for 2008–2009 winter), included “A/Brisbane/59/2007 (H1N1)‐like” virus.
***In 2009 both the northern (recommendation made for 2009–2010 winter) and southern vaccine (recommendation made for 2009) contained “A/Brisbane/59/2007 (H1N1)‐like” virus but only the northern vaccine contained “B/Brisbane/60/2008‐like” virus. Neither vaccine contained “A/Perth/16/2009‐like” or “A/California/7/2009‐like” that were isolated in Lao PDR.
†This strain is Victoria lineage.
††In 2010, both southern (recommendation made for 2010) and northern (recommendation made for 2010–2011 winter) vaccine strain selections were the same and contained “A/California/7/2009 (H1N1)‐like” virus, “A/Perth/16/2009 (H3N2)‐like” virus and “B/Brisbane/60/2008‐like” virus.
†††B/Taiwan/55/2009 is an antigenic variant against B/Brisbane/60/2008. B/Brisbane/60/2008 is a representative virus of genetic clade 1 of HA gene phylogenetic tree, but B/Taiwan/55/2009 fall into clade 5, which is a minor antigenic variant group of B/Victoria lineage.
‡This strain is Yamagata lineage.
Discussion
From the analysis of 3 years of sentinel surveillance data, we found that influenza was detected year‐round in Lao PDR with a season from July to December. This is the first study that has provided information about the seasonality of influenza in Lao PDR and provides useful data for recommendations on seasonal influenza vaccination.
We confirmed that a large proportion of ILI was not caused by influenza virus infection, particularly in younger children. This was also found in our provisional analysis of ILI surveillance results from 2007 to 2008 where influenza was most commonly identified but a large proportion of younger patients were found to be infected with viruses such as adenovirus, rhinovirus, and metapneumovirus. 12
Trends in influenza circulation in Lao PDR share many similarities with our neighboring countries and the region. Influenza positivity during this period (2008–2010) was 22%, which is similar to that found for ILI surveillance in Vietnam (19%) 16 and for febrile outpatients in other countries in east and southeast Asia (11–26%). 7 , 17 In Lao PDR, the median age of patients with influenza infection was 12 years, and older children were the highest percent positive for influenza among those with ILI (positivity rate 32·8% for 5–<18 years). In neighboring Cambodia, the median age of influenza cases was 9 years old, and children aged 5–14 years were the highest percent positive for influenza (12·1%) among those with ILI. 18 , 19 Health‐seeking behavior study results from Lao PDR show reported respiratory illnesses to be significantly higher in children aged ≤15 years than in adults (4·9 versus 2·7%; P < 0·001). 20 Other countries in the region, including Thailand, 21 , 22 have found a large proportion of hospitalized influenza pneumonia cases are children. We expect that this may also be the case in Lao PDR. Of note, among adults over the age of 65 years, only 19 were tested and none were positive for influenza. Older patients may be less likely to be included in the surveillance because they are often referred immediately to the inpatient department for monitoring.
Influenza‐like‐illness cases were seen to increase from around May each year confirming that ILI appears before influenza season (July–December), suggesting the contribution of other respiratory pathogens. 12 Our finding of year‐round circulation with a peak of influenza activity during the rainy season is similar to Thailand and Northern Vietnam. 16 , 23 Many tropical countries, including Vietnam, report a bimodal pattern. 3 , 16 , 24 This was observed in Lao PDR in 2008 but not following the emergence of A (H1N1)pdm09, after which a single‐peak around August to September was observed. Cambodia and Myanmar recently also reported the peak of influenza activity occurring during the wet season, from June to December 18 , 19 , 25 but did not detect influenza year‐round. Factors driving seasonality of transmission are not well defined but likely include a combination of climatic conditions, susceptibility of the population, and virus characteristics. 26 , 27 As such, influenza activity is likely to vary year to year, and there is a need to collect and analyze multiple years of data to determine trends.
We found variability in the circulating subtypes with a different predominant strain each year. Our results clearly show the transition from A (H1N1)pdm09 into a mix of seasonally occurring viruses. Of interest, seasonal A/H1N1 has not been isolated since the emergence of A (H1N1)pdm09 in July 2009. Influenza B was not seen in quarter three and four of 2009 after emergence of A (H1N1)pdm09, but returned in 2010. Of note, in 2008 and 2009, Lao‐circulating influenza strains, when compared to the most recent vaccine recommendations, suggest a closer match to the vaccine strain selection for the northern hemisphere for 2008–09 and 2009–10 respectively (Table 2) 28 . Vaccine strain selection was the same for the northern and southern hemisphere in 2010. 28 The temporal pattern by subtype for 2010 showed a peak dominated by A (H1N1)pdm09 in the second half of 2010, which resembled the pattern of seasonality of the southern hemisphere. 29
Our findings are subject to several limitations. First, our surveillance data focuses only on patients with ILI from sentinel hospital sites, which may not be representative of the entire population of Lao PDR. Furthermore, a convenience sample on 1–2 set days of the week should be taken for testing, but this requirement is not always followed by hospitals (as shown by only 3% of ILI cases in Vientiane Capital having been sampled). As the catchment populations of the sites are unknown, extrapolation of results for estimates of disease burden cannot be made. Nevertheless, samples are collected regularly from all sites and from age‐group analysis of central site data, patients from which samples are collected appear to be representative of the ages of all patients with ILI presenting to the sentinel sites. Lao PDR has historically had a limited capacity for surveillance and response, and this surveillance was only recently established and has expanded substantially since it began in 2007. As a result, over 50% of the samples were collected in 2010, data are mostly available from the three central sites (65%), and there is some missing information on age and date of onset. Owing to specimen storage and transport difficulties at provincial sites, specimens are not always received within 24 hour and may arrive in suboptimal condition.
Influenza‐like‐illness surveillance needs to continue to be strengthened to provide more years of data in order to better understand the epidemiology and impact of influenza in Lao PDR. The relatively recent recognition of seasonal influenza patterns in Lao PDR combined with future data, including from SARI surveillance, will be important for weighing disease burden, both morbidity and mortality, against the costs of advancing influenza vaccine delivery strategy.
Addendum for authorship
Ms Bouaphanh Khamphaphongphanh: lead author in writing the paper and for design of paper; Pakapak Ketmayoon: data analysis and presentation of virological data; Hannah Lewis: interpretation of data and critical writing of paper; Darouny Phonekeo: surveillance coordinator and assisted in writing methodology/results; Sisouk Thongchanh: surveillance coordinator and assisted in writing methodology/results; Sinakhone Xayadeth: lead for analysis of virological samples and contributed to writing of paper; Somvay Ongkhamme: data analysis and presentation of epidemiological data; Phengta Vongphrachanh: contributed to concept/design of paper and final approval for publication for NCLE; Reiko Tsyuoko: contributed to concept/design of paper and final approval for publication for WHO CO Lao PDR; Ann Moen: revision of intellectual content; Andrew Corwin: concept of paper, revision of intellectual content and coordinator for all input into the paper.
Acknowledgements
The authors wish to thank all the healthcare providers and technicians working at ILI hospital sites, including Dr Khamla Choumlyvong, Dr Sanong Thongsana, Dr Simmaly Phongmany, Dr Bounthan Phaithanavan, Dr. Soudamany Ahmarathithada, and Dr Valy Phongsavath who are working in sample and data collection, processing, and logistics. Also to all laboratory technicians at the National Center for Laboratory and Epidemiology (NCLE), in the testing of specimens, and Dr Onechanh for his considerable support and guidance. We also thank the WHO Collaborating Centre for Reference and Research on Influenza at the National Institute of Infectious Diseases in Japan. Sonja Olson and Ms. Carrie Reed from the Centers for Disease Control and Prevention (US CDC) who assisted in early preparations of this manuscript during a “manuscript writing workshop”. Importantly Emerging Disease Surveillance and Response team, WHO Lao Country Office who tirelessly worked to support NCLE in advancing Laboratory Diagnostic Capabilities and ILI Surveillance in Lao PDR. Mr Jeffrey Partridge and Ms Emma Field of WHO Western Pacific Regional Office, and Giovanna Gutierrez (European Public Health Microbiology Training Programme fellow) for their comment on the final draft. Lastly, we greatly appreciate the expertise and kind financial and logistic support through the collaboration of the World Health Organization‐US CDC, and from SISEA Project of Institute Pasteur (2007–2010).
© 2012 Blackwell Publishing Ltd. The World Health Organization retains copyright and all other rights in the manuscript of this article as submitted for publication.
Institution where the work has been conducted: National Center for Laboratory and Epidemiology (NCLE), Ministry of Health, Lao People’s Democratic Republic.
References
- 1. WHO . Influenza: Report by the Secreteriat. Geneva: World Health Organization, 2003. [Google Scholar]
- 2. Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186. [DOI] [PubMed] [Google Scholar]
- 3. Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine 1999; 17(Suppl. 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 4. Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS ONE 2007; 2:e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev 2003; 4:105–111. [DOI] [PubMed] [Google Scholar]
- 6. Simmerman JM, Chittaganpitch M, Levy J et al. Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005–2008. PLoS ONE 2009; 4:e7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simmerman JM, Uyeki TM. The burden of influenza in East and South‐East Asia: a review of the English language literature. Influenza Other Respi Viruses 2008; 2:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med 2006; 3:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Statistics Centre . Population census 2005. National Statistics Centre of the Lao PDR. 2005.
- 10. WHO . National influenza centres. Available at http://www.who.int/influenza/gisrs_laboratory/national_influenza_centres/en/ (accessed 16 August 2011).
- 11. WHO . Global influenza surveillance and response system. Available from: http://www.who.int/influenza/gisrs_laboratory/en/ (accessed 16 August 2011).
- 12. Vongphrachanh P, Simmerman JM, Phonekeo D et al. An early report from newly established laboratory‐based influenza surveillance in Lao PDR. Influenza Other Respi Viruses 2010; 4:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO Collaborating Centre for influenza . CDC Protocol of Realtime RTPCR for Influenza A (H1N1). Atlanta, GA: Centers for Disease Control and Prevention, 2009. [Google Scholar]
- 14. Kendal AP, Pereira MS, Skehel J. Concepts and Procedures for Laboratory‐Based Influenza Surveillance. Geneva: World Health Organization, 1982. [Google Scholar]
- 15. WHO . Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Geneva: World Health Organization, 2011. [Google Scholar]
- 16. Nguyen HT, Dharan NJ, Le MT et al. National influenza surveillance in Vietnam, 2006–2007. Vaccine 2009; 28:398–402. [DOI] [PubMed] [Google Scholar]
- 17. Blair PJ, Wierzba TF, Touch S et al. Influenza epidemiology and characterization of influenza viruses in patients seeking treatment for acute fever in Cambodia. Epidemiol Infect 2010; 138:199–209. [DOI] [PubMed] [Google Scholar]
- 18. Sreng B, Touch S, Sovann L et al. A description of influenza‐like illness (ILI) sentinel surveillance in Cambodia, 2006–2008. Southeast Asian J Trop Med Public Health 2010; 41:97–104. [PubMed] [Google Scholar]
- 19. Mardy S, Ly S, Heng S et al. Influenza activity in Cambodia during 2006–2008. BMC Infect Dis 2009; 9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. University of Health Sciences . Respiratory illness health seeking behavior assessment in peri‐urban and rural areas in the Lao People’s Democratic Republic. University of Health Sciences Lao PDR, National Centre for Laboratory and Epidemiology, CARE International., US CDC, WHO, 2010.
- 21. Dawood FS, Fry AM, Muangchana C et al. A method for estimating vaccine‐preventable pediatric influenza pneumonia hospitalizations in developing countries: Thailand as a case study. Vaccine 2011; 26:4416–4421. [DOI] [PubMed] [Google Scholar]
- 22. Olsen SJ, Thamthitiwat S, Chantra S et al. Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol Infect 2000; 138:1811–1822. [DOI] [PubMed] [Google Scholar]
- 23. Simmerman JM, Thawatsupha P, Kingnate D, Fukuda K, Chaising A, Dowell SF. Influenza in Thailand: a case study for middle income countries. Vaccine 2004; 23:182–187. [DOI] [PubMed] [Google Scholar]
- 24. Hampson AW. Epidemiological data on influenza in Asian countries. Vaccine 1999; 17(Suppl 1):S19–S23. [DOI] [PubMed] [Google Scholar]
- 25. Dapat C, Saito R, Kyaw Y et al. Epidemiology of human influenza A and B viruses in Myanmar from 2005 to 2007. Intervirology 2009; 52:310–320. [DOI] [PubMed] [Google Scholar]
- 26. Lofgren E, Fefferman NH, Naumov YN, Gorski J, Naumova EN. Influenza seasonality: underlying causes and modeling theories. J Virol 2007; 81:5429–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 2007; 3:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. WHO . Recommendations for influenza vaccine composition. Available at http://www.who.int/influenza/vaccines/vaccinerecommendations1/en/index.html (accessed 20 January 2012).
- 29. WHO . Global influenza surveillance and response system flunet charts. Available at http://www.who.int/csr/disease/influenza/influenzanetwork/flunet/charts/en/index.html (accessed 18 August 2011).


