Abstract
Background Human parainfluenza viruses (HPIVs) are an important cause of acute respiratory illness in young children but little is known about their epidemiology in the tropics.
Methods From 2003–2007, we conducted surveillance for hospitalized respiratory illness in rural Thailand. We performed reverse‐transcriptase polymerase chain reaction on nasopharyngeal specimens and enzyme immunoassay on paired sera
Results Of 10,097 patients enrolled, 573 (5%) of all ages and 370 (9%) of children <5 years of age had evidence of HPIV infection (HPIV1=189, HPIV2=54, HPIV3=305, untyped=27). Average adjusted annual incidence of HPIV‐associated hospitalized respiratory illness was greatest in children aged <1 year (485 per 100,000 person years).
Conclusions In Thailand, HPIV caused substantial illnesses requiring hospitalization in young children.
Keywords: Human parainfluenza viruses, pneumonia, surveillance, Thailand
Introduction
Human parainfluenza viruses (HPIVs) are a leading cause of respiratory tract infection and hospitalization among children under 5 years. 1 , 2 HPIV infection causes a spectrum of illness, ranging from the common cold to croup, bronchiolitis, and pneumonia. 2 In the United States, a temperate climate country, the most common HPIV types associated with respiratory illness are HPIV‐1 and HPIV‐3, and HPIV‐2 and HPIV‐4 are less commonly detected. 1 HPIV‐3 circulates annually with seasonal peaks in spring and summer months, and HPIV‐1 peaks biennially in the fall during odd‐numbered years. 1 Little is known about the epidemiology or disease burden of HPIV in countries with tropical climates. In this study, we used molecular and serological methods to describe 4 years of incidence and seasonality of hospitalized HPIV‐1‐, HPIV‐2‐, and HPIV‐3‐associated acute lower respiratory tract illness (LRTI) from two provinces in rural Thailand.
Methods
Patient enrollment
As part of a comprehensive respiratory pathogen study in Thailand, 3 , 4 , 5 , 6 we prospectively enrolled patients with acute LRTI who were admitted to any of eight hospitals in Sa Kaeo Province from September 1, 2003, to December 31, 2007, and any of 12 hospitals in Nakhon Phanom Province from January 1, 2005, to December 31, 2007. Patients were eligible for enrollment at admission to hospital if they had evidence of active infection and at least one sign or symptom of respiratory disease, as described previously 3 ; had no hospital admission for acute LRTI within the previous 14 days; and had a chest radiograph (CXR) ordered by the physician within 48 hours after admission. Patients (or parents of children aged <18 years) were consented before enrollment. An independent panel of board‐certified radiologists in Bangkok reviewed the CXRs using standard criteria as previously described. 7
Clinical specimens and laboratory investigation
We collected nasopharyngeal swab specimens from all patients, and during the first 2 years of the study (September 1, 2003, to August 30, 2005), we obtained acute‐ and convalescent‐phase paired sera three to 6 weeks apart. Specimen aliquots collected during the first two study years were sent to the Centers for Disease Control and Prevention (CDC) in Atlanta, where they were tested for HPIV‐1, HPIV‐2, and HPIV‐3 RNA by RT‐PCR assays 8 , 9 and for HPIV‐1, HPIV‐2, and HPIV‐3 IgG antibodies by indirect enzyme immunoassay. 10 Serology was used for diagnosis, not to describe immunological characteristics. For the last 2 years, specimens were tested at the National Institute of Health (NIH) in Thailand using only real‐time RT‐PCR (rRT‐PCR) assays. We did not test for HPIV‐4. In addition, during September 1, 2003, to August 30, 2005, virus isolation was performed at NIH using previously described methods. 11
Total nucleic acids were extracted from swab specimens at CDC using the QIAamp Virus BioRobot MDx Kit on a BioRobot MDx Workstation (Qiagen, Valencia, CA, USA); at NIH, Thailand, extractions were performed manually using the QIAamp MinElute Virus Spin Kit (Qiagen). Recovered nucleic acids were tested by two different RT‐PCR methods: samples collected during the first two study years were tested by GeneScan RT‐PCR assays as previously described, 9 and samples from subsequent years were tested by newly developed rRT‐PCR assays described here. Briefly, multiple primer/probes targeting type‐specific conserved regions of each of the HPIV‐1, HPIV‐2, and HPIV‐3 hemagglutinin‐neuraminidase genes were designed from alignments of sequences available from GenBank and CDC databases using Primer Express (version 3.0, Applied Biosystems, Carlsbad, CA, USA) and Beacon Designer software (version 5.0, Premier Biosoft International, Palo Alto, CA, USA). Primers/probes that gave the best performance in rRT‐PCR assays for each virus were selected for the subsequent study (Table 1). Amplification conditions and reagents were identical to those previously described. 4 All swab specimens were also tested by RT‐PCR for human GAPDH or RNase P housekeeping genes as a control for specimen quality.
Table 1.
Human parainfluenza virus real‐time RT‐PCR primer and probe sequences
| Assay, primer/probe | Final concentration (nm) | Accession no.* | Location* | Sequence (5′>3′) |
|---|---|---|---|---|
| HPIV‐1 forward | 500 | AF457102 | 8036‐8063 | AGT TGT CAA TGT CTT AAT TCG TAT CAA T |
| HPIV‐1 reverse | 500 | 8111–8134 | TCG GCA CCT AAG TAA TTT TGA GTT | |
| HPIV‐1 probe** | 50 | 8077–8109 | ATA GGC CAA AGA “T”TG TTG TCG AGA CTA TTC CAA | |
| HPIV‐2 forward | 750 | NC_003443 | 7447–7471 | GCA TTT CCA ATC TAC AGG ACT ATG A |
| HPIV‐2 reverse | 750 | 7571–7536 | ACC TCC TGG TAT AGC AGT GAC TGA AC | |
| HPIV‐2 probe** | 50 | 7475–7506 | CCA TTT ACC “T”AA GTG ATG GAA TCA ATC GCA AA | |
| HPIV‐3 forward | 750 | NC_001796 | 7693–7720 | TGG YTC AAT CTC AAC AAC AAG ATT TAA G |
| HPIV‐3 reverse | 500 | 7793–7815 | TAC CCG AGA AAT ATT ATT TTG CC | |
| HPIV‐3 probe** | 200 | 7762–7792 | CCC RTC TG”T” TGG ACC AGG GAT ATA CTA CAA A |
HPIV, human parainfluenza viruse.
*GenBank accession numbers of reference sequences for locating primers and probes.
**Hydrolysis probes labeled as previously described [ 4 , 2007] 5′ = FAM “T” = BHQ1‐dT 3′ = Phosphorylated.
We considered patients as having evidence of a recent HPIV infection if they had a positive RT‐PCR/rRT‐PCR result or HPIV virus isolation or showed a ≥fourfold increase in IgG antibody titer to HPIV‐1, HPIV‐2, and HPIV‐3. Because of known serologic cross‐reactions among HPIVs, 2 we considered samples with a negative RT‐PCR result, but positive by serology for more than one HPIV type, to be positive for HPIV but untyped. All swab specimens were also tested for influenza viruses, respiratory syncytial virus (RSV), human metapneumovirus, and adenovirus by RT‐PCR. 11 Detailed descriptions of the detection of these viruses have been published. 11 , 12 , 13 , 14 , 15
Statistical analysis
We calculated crude and adjusted age‐specific incidence for HPIV‐associated hospitalized respiratory illness. Denominators were mid‐year population estimates from Thailand’s National Economic and Social Development Board. 16 Adjustments were made for enrollment by assuming that the proportion positive for HPIV and CXR‐confirmed pneumonia was the same in enrolled and eligible non‐enrolled patients. Similarly, for patients whose CXRs were not reviewed by the radiology panel, we assumed that the proportion with radiographic confirmation of pneumonia was the same as for patients whose CXRs were reviewed. We calculated confidence intervals for adjusted age‐specific incidence rates using the 95% confidence intervals of the proportion of HPIV‐positive tests assuming a binomial distribution. We described seasonal patterns by calculating the proportion of enrolled patients with HPIV detection for each month. We performed all analyses using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
This study was approved by the Ethical Review Committee of the Thailand Ministry of Health and an institutional review board of CDC.
Results
HPIV‐1, HPIV‐2, and HPIV‐3 Real‐time RT‐PCR assays
Optimized HPIV‐1, HPIV‐2, and HPIV‐3 rRT‐PCR assays were specific with high‐titer cultures of HPIV‐1 (C‐35), HPIV‐2 (Greer), and HPIV‐3 (C‐243) and were non‐reactive with a broad range of other respiratory pathogens, including influenza viruses A and B, RSV, HPIV‐4A and HPIV‐4B, mumps virus, human metapneumovirus, adenovirus, rhinovirus, human coronaviruses 229E and OC43, and human bocavirus. Pooled nasal wash specimens from healthy persons were also negative by all assays. In direct comparisons with the GenScan RT‐PCR assays 9 , the rRT‐PCR assays showed comparable sensitivity with serially diluted HPIV‐1, HPIV‐2, and HPIV‐3 RNA and gave identical results with a collection of positive and negative specimens of HPIV‐1, HPIV‐2, and HPIV‐3 from CDC archives and the second year of this study.
Study results
From September 1, 2003, through December 31, 2007, there were 41 761 hospital admissions owing to LRTI in Sa Kaeo and Nakhon Phanom Provinces. Of the 22 857 (55%) patients with CXRs and who were eligible for enrollment, 10 097 (49%) were enrolled, of whom 573 (5%) had evidence of HPIV infection. Of 573 patients with evidence of HPIV infection, 189 (33%) had HPIV‐1, 54 (9%) had HPIV‐2, 305 (53%) had HPIV‐3, and 27 (5%) were untyped; two patients had coinfections with 2 HPIV serotypes (HPIV‐1 + ‐2 and HPIV‐2 + ‐3). Of the 404 patients in the 2 years with both RT‐PCR and serology results, 76% were detected by RT‐PCR only, 7·4% by serology only, and 17% by both methods. 17 Five HPIV specimens were detected by only virus isolation. Mixed viral infections were identified in 48 (8·4%) patients with HPIV detection: 4% with influenza viruses, 3% with adenovirus, and 2% with RSV.
Of 468 HPIV patients with CXRs evaluated, 85% had radiographic evidence of pneumonia (interstitial infiltrates 63%, alveolar infiltrates 21%, consolidation 9%). Among the 573 patients with evidence of HPIV infection, the most common signs and symptoms of respiratory infection were fever (69%, 51% with temperature ≥38 and 18% with reported fever only), cough (98%), sputum production (62%), and tachypnea (54%). Other signs and symptoms included rhonchi (48%), rales/crepitation (41%), wheezing (31%), chest pain (6%), and hemoptysis (2%). One hundred and 89 (33·2%) HPIV‐positive patients required oxygen therapy, and six needed mechanical ventilation. There was no marked difference in frequency of signs and symptoms at admission by HPIV type. Of the 573 HPIV‐positive patients, six (1·0%) died, two aged 68 and 71 years with HPIV‐1, and four aged 39, 58, 69, and 76 years with HPIV‐3. Of the six deaths, one also had an influenza virus detected.
From January 1, 2004, to December 31, 2007, the proportion of enrolled patients with HPIV was higher for infants aged <1 year (9%) and children aged 1–4 years (9%) than for children aged 5–9 years (6%) and 10–19 years (3%) and adults aged 20–64 years (2%) and ≥65 years (3%) (Table 2). Fifty‐nine percent of HPIV‐infected patients were male. Infants aged <1 year had the highest rate of HPIV‐associated hospitalized respiratory disease (486 per 100 000 person‐years) and radiographically confirmed pneumonia (229 per 100 000 person‐years). Rates decreased among older age groups. Persons aged 10–19 years had the lowest rates of HPIV‐associated hospitalized respiratory disease (4 per 100 000 person‐years) and radiographically confirmed pneumonia (2 per 100 000 person‐years). The rate of HPIV‐associated hospitalized respiratory disease among males was 15 per 100 000 person‐years and among females was 11 per 100 000 person‐years.
Table 2.
Average crude and adjusted incidence for hospitalized patients with HPIV infections, Sa Kaeo and Nakhon Phanom Provinces, Thailand, September 1, 2003, to December 31, 2007
| Age group (in years) | Person‐years of Population under Surveillance | Enrolled/eligible patients (% of eligible) | Enrolled patients with CXR‐confirmed pneumonia (% of enrolled) | Enrolled patients with HPIV (% of enrolled) | CXR‐confirmed pneumonia with HPIV (% of patients with CXR pneumonia) | Crude incidence HPIV hospitalization | Incidence/100 000 person‐years | ||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted* incidence HPIV hospitalization (95% CI) | Crude incidence CXR‐confirmed HPIV pneumonia | Adjusted* incidence CXR‐confirmed HPIV pneumonia (95% CI) | |||||||
| <1 | 58 683 | 1343/3216 (42) | 619 (46) | 119 (8·9) | 56 (9·0) | 203 | 486 (402–569) | 95 | 229 (171–286) |
| 1–4 | 244 897 | 2810/6232 (45) | 1356 (48) | 251 (8·9) | 129 (9·5) | 102 | 227 (200–254) | 53 | 117 (98–136) |
| 5–9 | 376 315 | 610/1299 (47) | 240 (39) | 36 (5·9) | 18 (7·5) | 9·6 | 20 (14–27) | 4·8 | 10 (5·7–15) |
| 10–19 | 828 310 | 477/979 (49) | 158 (33) | 16 (3·4) | 8 (5·1) | 1·9 | 4·0 (2·1–5·9) | 1·0 | 2·0 (0·6–3·3) |
| 20–64 | 2 684 669 | 3212/7867 (41) | 1414 (44) | 72 (2·2) | 37 (2·6) | 2·7 | 6·6 (5·1–8·1) | 1·4 | 3·4 (2·3–4·4) |
| ≥65 | 277 618 | 2643/6669 (40) | 1228 (46) | 79 (3·0) | 37 (3·0) | 28 | 72 (56–87) | 13 | 34 (23–44) |
| Total | 4 470 492 | 11 095/26 262 (42) | 5015 (45) | 573 (5·2) | 285 (5·7) | 13 | 30 (28–33) | 6 | 15 (13–17) |
CXR, chest radiograph; CI, confidence intervals; HPIV, human parainfluenza viruse.
*Adjustments were made for enrollment by assuming that the proportion positive for HPIV was the same in enrolled and eligible non‐enrolled patients.
Among specific HPIV serotypes, most infections were detected in children 1–4 aged years for all three serotypes (Table 3). Among children aged <1 year, infection with HPIV‐3 [69/118 (58%)] was more common than that with HPIV‐1 [41/118 (35%)]. Similarly, among children aged 1–4 years, HPIV‐3 [140/243 (58%)] infection was more common than HPIV‐1 [85/243 (35%)]. HPIV‐1 and HPIV‐3 infections were equally represented among children aged 5–9 years [HPIV‐3: 15/31 (48%) and HPIV‐1: 11/31 (35%)].
Table 3.
Proportion of HPIV serotypes* among hospitalized pateints with HPIV infections
| Age group | HPIV‐1 (n = 189) No. (%) | HPIV‐2 (n = 54) No. (%) | HPIV‐3 (n = 305) No. (%) |
|---|---|---|---|
| <1 year | 41 (22) | 8 (15) | 69 (23) |
| 1–4 years** | 85 (45) | 19 (35) | 140 (46) |
| 5–9 years | 11 (5·8) | 5 (9·3) | 15 (4·9) |
| 10–19 years | 8 (4·2) | 4 (7·4) | 2 (0·7) |
| 20–64 years | 22 (12) | 11 (21) | 36 (12) |
| ≥65 years*** | 22 (12) | 7 (13) | 43 (14) |
HPIV, human parainfluenza viruse.
*excludes untyped HPIV.
**1 patient aged 1 year with HPIV‐1 and HPIV‐2 detected by PCR.
***1 patient aged 74 years with HPIV‐2 and HPIV‐3 detected by PCR.
Seasonal pattern
HPIV‐1 and HPIV‐3 viruses could be detected throughout the year and often cocirculated (Figure 1). HPIV‐3 was detected more frequently each year from January until May–July. HPIV‐1 appeared to circulate more frequently from October 2003 through July 2005 and October 2005 through July 2006. However, HPIV‐1 circulated in low numbers every year.
Figure 1.
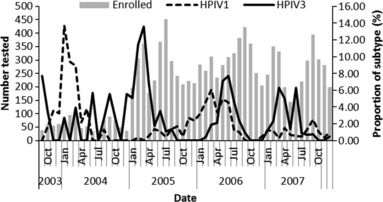
Proportion of patients hospitalized with acute lower respiratory tract illness with Human parainfluenza viruse1 (HPIV‐1) or HPIV‐3 in Sa Kaeo (September 1, 2003, to December 31, 2007) and Nakhon Phanom (January 1, 2005, to December 31, 2007), Thailand.
Discussion
In Thailand, HPIV was an important virus associated with hospitalized acute LRTI particularly among young children. HPIV infection was associated with 9% of hospitalized CXR‐confirmed pneumonia and 9% of hospitalized LRTI among children aged below 5 years in Thailand. The incidence of HPIV‐associated hospitalized LRTI was highest in infants and children aged 2–4 years, similar to other reports. 2 , 18 , 19 Targeting HPIV for new interventions in these age groups would have an impact on respiratory hospitalizations in Thailand.
Few published studies report the incidence of hospitalized HPIV infection. The incidence of hospitalized HPIV‐associated LRTI in Thailand was slightly higher than that in a report from the United States (age <1 year: 240/100 000, 1 year 150/100 000, 2–4 years 40/100 000). 11 , 19 While the incidence of hospitalized HPIV‐associated LRTI was lower than that of RSV‐associated LRTI among children aged below 5 years in Thailand, 11 , 14 HPIV still accounted for a substantial proportion of hospitalizations. Also, the incidence of HPIV‐associated hospitalized pneumonia was slightly higher in males compared with females, similar to other reports. 20 , 21 HPIV‐3 was more frequently detected than HPIV‐1 (53% versus 33%), and together these serotypes were responsible for 86% of patients hospitalized with HPIV‐associated acute LRTI.
It is difficult to prove that detection of HPIV from the upper respiratory tract was associated with LRTI. However, a concurrent study only rarely found HPIV from nasal pharyngeal swabs taken from a comparison group of persons without respiratory illness. From August 2004 through December 2007, a sample of patients without fever, diarrhea, or respiratory symptoms in the previous 3 days was selected from the outpatient clinics of the hospitals participating in surveillance (H Baggett, personal comm). Overall, 1647 asymptomatic controls were tested with rRT‐PCR for HPIV‐1, HPIV‐2, or HPIV‐3; 12 (0·73%) were positive, 5 were HPIV‐1, 2 were HPIV‐2, and 5 were HPIV‐3. Three (25%) of 12 HPIV‐positive controls were aged <5 years. Thus, it seems likely that HPIV detected from hospitalized patients was associated with their current illness.
Similar to other respiratory viruses in Thailand, HPIV circulated at low levels throughout the year. 11 However, the annual periodicity of HPIV types was similar to that in the United States. 1 There was a seasonal pattern whereby the proportion of HPIV‐positive cases tended to be highest between January and April but not always limited to a single type. Previous studies of HPIV infection in Southeast Asia found seasonal peaks; a 2‐year study in Thailand found a February–March peak, and a 4‐year study in Singapore found a February–May peak. 22 , 23 In the United States, HPIV‐3 prevalence increases in spring and summer months, while HPIV‐1 prevalence increases in the fall. 1 , 18 HPIV‐1 circulates biennially during odd‐numbered years, and it is uncommon to have both serotypes cocirculating. It has been proposed that cross‐protection of antibodies from previous HPIV infection influences the predominance of circulating HPIV type. 21 During our 4 years of surveillance in Thailand, HPIV‐1 circulated every year, but there appeared to be increased circulation beginning in October of two of three odd‐numbered years. Also, it was common for both serotypes to cocirculate, at least at low levels.
Our study had several limitations. Even though prospective enrollment of hospitalized patients in a well‐defined population with independent radiographic confirmation of pneumonia provided a robust estimate of HPIV‐associated pneumonia incidence, our study included only hospitalized patients with CXR performed, which only represents one part of the severe end of the disease spectrum. As HPIV infection also causes other syndromes that can require hospitalization, such as croup and asthma exacerbation, as well as less severe disease, HPIV infection burden in Thailand likely exceeds our estimates. In the third year of our study, we used only RT‐PCR to detect HPIV without serology and likely failed to identify a few patients. However, approximately 90% of infections for each HPIV type were detected by RT‐PCR in the first 2 years when both methods were used. We also found that 8·4% of patients with HPIV had coinfection with other viral respiratory pathogens, and it is not possible to conclusively determine which of these pathogens was the primary cause of the pneumonia.
In Thailand, HPIV infection was an important cause of hospitalized LRTI and pneumonia, especially among infants and children. Most infections were caused by types HPIV‐1 and HPIV‐3. As HPIVs contribute to a substantial proportion of childhood pneumonia in Thailand and possibly other tropical countries, improved prevention strategies such as vaccines 24 or antiviral agents 25 deserve greater attention and economic evaluation in the tropics.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
Conflicts of interest
No commercial or other conflict of interest declared.
Acknowledgements
We thank Dr Ryan Dare (CDC) for his assistance. This study was funded by the Center for Disease Control and Prevention.
The findings reported here were presented at the meeting of the American Society for Tropical Medicine and Hygiene, December 9, 2008, New Orleans, USA.
References
- 1. Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clin Infect Dis 2006; 43:1016–1022. [DOI] [PubMed] [Google Scholar]
- 2. Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev 2003; 16:242–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olsen SJ, Laosiritaworn Y, Siasiriwattana S, Chunsuttiwat S, Dowell SF. The incidence of pneumonia in rural Thailand. Int J Infect Dis 2006; 10:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real‐time reverse‐transcription polymerase chain reaction assays. J Infect Dis 2007; 196:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fry AM, Lu X, Chittaganpitch M et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 2007; 195:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phares CR, Wangroongsarb P, Chantra S et al. Epidemiology of severe pneumonia caused by Legionella longbeachae, Mycoplasma pneumoniae, and Chlamydia pneumoniae: 1‐year, population‐based surveillance for severe pneumonia in Thailand. Clin Infect Dis 2007; 45:e147–e155. [DOI] [PubMed] [Google Scholar]
- 7. Javadi M, Subhannachart P, Levine S et al. Diagnosing pneumonia in rural Thailand: digital cameras versus film digitizers for chest radiograph teleradiology. Int J Infect Dis 2006; 10:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberg GA, Erdman DD, Edwards KM et al. Superiority of reverse‐transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis 2004; 189:706–710. [DOI] [PubMed] [Google Scholar]
- 9. Erdman DD, Weinberg GA, Edwards KM et al. GeneScan reverse transcription‐PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol 2003; 41:4298–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marx A, Gary HE Jr, Marston BJ et al. Parainfluenza virus infection among adults hospitalized for lower respiratory tract infection. Clin Infect Dis 1999; 29:134–140. [DOI] [PubMed] [Google Scholar]
- 11. Olsen SJ, Thamthitiwat S, Chantra S et al. Incidence of respiratory pathogens in persons hospitalized with pneumonia in two provinces in Thailand. Epidemiol Infect 2010; 138:1811–1822. [DOI] [PubMed] [Google Scholar]
- 12. Simmerman JM, Lertiendumrong J, Dowell SF et al. The cost of influenza in Thailand. Vaccine 2006; 24:4417–4426. [DOI] [PubMed] [Google Scholar]
- 13. Simmerman JM, Chittaganpitch M, Levy J et al. Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005–2008. PLoS ONE 2009; 4:e7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fry AM, Chittaganpitch M, Baggett HC et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS ONE 2010; 5:e15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fry AM, Lu X, Olsen SJ et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS ONE 2011; 6:e17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Economic and Social Development Board (NESDB) . National Economic and Social Development Board (NESDB) of Thailand Population Projections of Thailand 2000–2030. Available at http://www.nesdb.go.th/temp_social/pop.zip. (Accessed: 5 August 2008).
- 17. Pongpun S, Chittaganpitch M, Hall H et al. Serology as an adjunct to polymerase chain reaction assays for surveillance of acute respiratory virus infections. Clin Infect Dis 2012; 54:445–446. [DOI] [PubMed] [Google Scholar]
- 18. Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001; 344:1917–1928. [DOI] [PubMed] [Google Scholar]
- 19. Weinberg GA, Hall CB, Iwane MK et al. Parainfluenza virus infection of young children: estimates of the population‐based burden of hospitalization. J Pediatr 2009; 154:694–699. [DOI] [PubMed] [Google Scholar]
- 20. Marx A, Torok TJ, Holman RC, Clarke MJ, Anderson LJ. Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis 1997; 176:1423–1427. [DOI] [PubMed] [Google Scholar]
- 21. Glezen P, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med 1973; 288:498–505. [DOI] [PubMed] [Google Scholar]
- 22. Suwanjutha S, Chantarojanasiri T, Watthana‐kasetr S et al. A study of nonbacterial agents of acute lower respiratory tract infection in Thai children. Rev Infect Dis 1990; 12(Suppl 8):S923–S928. [DOI] [PubMed] [Google Scholar]
- 23. Chew FT, Doraisingham S, Ling AE, Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol Infect 1998; 121:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karron RA, Belshe RB, Wright PF et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J 2003; 22:394–405. [DOI] [PubMed] [Google Scholar]
- 25. Moscona A, Porotto M, Palmer S et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo . J Infect Dis 2010; 202:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


