Abstract
In 2013, nearly 15 million units of banked blood were transfused in the United States alone. Blood shortages are expected to increase globally. Donated blood is not equal due to differences in quality and deterioration rate. There are no methods to detect time-dependent biochemical and biophysical changes of RBCs or the deterioration rate of donated RBCs. Nine randomly selected RBC units collected by the San Diego Blood Bank were examined for inter-donor variability over six weeks of storage. In vitro RBC quality was assessed weekly by conventional biochemical tests including free Hb, K+, ATP, P50, 2,3 DPG, lactate, and pH. Deformability was measured via cell filtration. Briefly, the RBC suspension (10% Hct), was forced through a 5.0μm pore membrane (106 mm2) at various flow rates. No inter-donor variability in biochemical or mechanical parameters was observed at baseline. Inter-donor variability in biochemical properties (free Hb, K+, ATP, P50, 2,3 DPG, lactate, and pH) was observed after 14 days of storage. However, significant differences from baseline in RBC mechanical properties (i.e. filterability) were observed as early as 7 days into storage at the lowest flow rates and after 28 days of storage at all flow rates. There was a net decrease in filterability over time for all donors, but the rate at which filterability decreased (i.e. deterioration rates) was different when comparing individual donors. Changes in all biochemical parameters were significant different between donors. These data suggest that filterability is more sensitive to changes in blood quality than conventional biochemical parameters.
Index Terms: Banked blood, blood storage, deformability, storage lesions, blood transfusion
I. Introduction
ACCORDING to the 2013 National Blood Collection and Utilization Report nearly 5 million individuals utilized 15 million units of banked blood [1]. Global blood shortages are expected due to reduced blood donation, a growing elderly population, and the increase in life expectancy. Reports indicate that some donors’ RBCs may be poorly storable, and inter-donor variability had been identified as one of the major determinants in variability of RBC post-transfusion recovery [2]. Individual donor variability in ATP levels had also been reported [3]. Part of the reason for such inter-donor variability may be differences in metabolic age of donor RBCs upon collection, although other donor-specific factors could be involved. Such factors may include metabolomic changes in RBCs that occur during storage. The issue is further complicated by the RBC properties’ dependence on component manufacturer [4]. A recent study tracked the performance of more than 100 transfused units, and reported significant variability in the incremental increase in Hct between RBC units of the same age [5]. Overall, inherent unit-to-unit differences are likely to be a significant confounder to transfusion efficacy with implications not only to transfusion practice, but also on the lack of regulation of RBC products [6]. Current blood transfusion standards set by the Food and Drug Administration limits blood storage to a maximum of 42 days in the proper additive solutions. Some studies show little difference between the effectiveness of blood stored for 20 days and that stored for more than 42 days, while other researchers cite cases of blood that is unsuitable for transfusion despite being stored for significantly less than the 42 day limit [7]. Clearly, there are several conflicting data points; the inconsistencies in relating RBC survival rate to the length of storage demonstrate the need to predict blood quality.
Stored RBCs undergo a series of time dependent concomitant biochemical and biophysical changes, known as the storage lesion [8, 9]. Storage lesion reduces post-transfusion RBC survival and has been linked to a variety of adverse side effects such as microcirculatory complications, transfusion-related acute lung injury (TRALI), inflammation, organ failure, and mortality [10] [11, 12]. Current studies are beginning to use time-independent metrics to predict the survival rate of transfused blood based on findings that suggest inter-donor variability as a factor of the rate of the development of storage lesion [13]. The mechanical deterioration of RBCs during storage stems from changes in shape, deformability, and aggregability [14, 15], while biochemical changes in ATP, lactate, hemoglobin (Hb), potassium, and 2,3-DPG levels are believed to account for the remaining segments of storage lesion [16]. Differences in RBC ATP levels from different donors have been previously correlated with variations in post-transfusion RBC recovery [17]. Other biochemical parameters, including hematocrit, Hb content, 2,3 DPG concentration, p50 (the concentration of oxygen required for 50% Hb saturation), extracellular potassium, pH, and lactic acid concentration, have been used as a measure of RBC quality [16]. It has been shown that additional facts that can influence post-transfusion RBC recovery include the metabolic age of the donor RBCs, as well as the age, gender, race, and overall health of the donor [2].
RBC deformability is closely correlated with the quality of transfused RBCs [18]. One relatively simple method of quantifying RBC deformability is performed by measuring the cells’ ability to perfuse through a micro-pore filter [14]. Using this method, we measured the inter-donor variability as well as deterioration rate of RBCs’ mechanical properties. There are only attempts to correlate the mechanical and biochemical changes that occur during storage with inter-donor variability [13]. This manuscript reports our findings on the biochemical and mechanical properties of human RBCs with in vitro RBC quality assays. Our findings revealed significant inter-donor variability in both the mechanical and biochemical properties of RBCs. In addition, we show that blood from different donors has significantly different deterioration rates, which may provide insight into potential dissimilarities in the function and efficacy of transfused RBCs.
II. Materials and Methods
A. RBC Collection and Processing
Venous whole blood was drawn from 9 healthy donors following AABB standards at the San Diego Blood Bank. Briefly, blood was collected into citrate phosphate double dextrose (CP2D) and then centrifuged to remove the supernatant. RBC units then received the RBC additive solution AS-1. The red cells and additive solution were mixed thoroughly and passed through a leukodepletion filter into the final storage bag. The filtered red cell product was then held in refrigerated storage (1–6°C) until 24 hours after blood draw to wait for the viral testing results. Once cleared, aliquot bags were sterilely connected to the units of blood for sampling for this study. For sampling, all but one aliquot bag was clamped off. Blood then flowed into the single open aliquot bag until full. The open tube was then irreversibility crimped closed using hermetic seal clips (Fenwal, Lake Zurich, IL). The single full aliquot bag was then separated for use.
B. RBC Oxygen Transport and Biochemical Assays
ATP and 2,3-DPG concentrations were analyzed in neutralized perchloric acid extracts of RBCs by use of kits (Boehringer, Mannheim, Germany) adapted for use with an automated spectrophotometer (Roche, Basel, Switzerland). Hb was determined by the cyan methemoglobin technique, and the supernatant Hb was expressed as percentage of total Hb. The inorganic phosphate concentration in the SM was analyzed with a phosphate determination kit (Roche, Mannheim, Germany) and an automatic analyzer (Hitachi 717 automatic analyzer, Hitachi, Tokyo, Japan). Hematocrit (Hct) was determined with centrifugation at 8,000 × g.
C. RBC Filterability Assay
Deformability was measured using the cellular filtration method [19]. Briefly, the RBC suspension was adjusted to 10% Hct with saline and forced through a Teflon membrane filter with 5.0μm pores (Millipore, Temecula, CA) at various flow rates (300, 500, 700, 900, 1100, 1300, 1500 μL/min). A reservoir on the output of the 5μm was used to prevent blood from dripping, which affected pressure measurements. Pressure transducer measurements were gathered with a BIOPAC MP150 amplifier and pre-filter (BIOPAC Systems, Goleta, California), acquired by a BNC-2110 connector box (National Instruments, Austin, TX), and then digitized. The signal was then processed and stored via a custom LabVIEW program (National Instruments).
For each flow rate, a baseline pressure was generated by passing saline solution through the filter. This signal was used to normalize pressure readings and isolate the RBC’s effect on resistance from that of the saline.
The resistance of a pore containing an RBC relative to a pore without an RBC was calculated via (1). This relative resistance (β) quantifies the resistance of a single cell passing through a pore, independent of RBC concentration, and the cell to pore volume [20]
| (1) |
Where Pi is the initial rise in filtration pressure for blood solution, P0 is the initial rise in filtration pressure for saline, V is the mean corpuscular volume to filter pore volume ratio, and h is the hematocrit [21].
D. Signal Processing
The pressure signal was amplified 5000× and then passed through a low pass filter with a −3dB frequency of 300Hz. The signal was then sampled by the ADC at a rate of 100Hz. The data was then filtered using a 2nd stage low pass Butterworth filter, with a1Hz cut off frequency.
E. Statistical Analysis
Results are presented as means ± standard deviation (SD). Two-way ANOVA was used to compare each unit to the other units of blood for every time point. MATLAB 2014b, (MATLAB 9.0 The MathWorks Inc., Natick, MA, 2014) was used to interpolate all surfaces for filterability (flow again storage time) and calculate the gradient of filterability (2)
| (2) |
Where t is the storage time and Q is the flow rate at each point on the interpolated surface in order to quantify the flow rate and storage time that results in the rate of maximum increase in the β factor. All statistical calculations were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA) with statistical significance attributed to values of P < 0.05.
III. Results
A. RBC Filterability
Filterability, as quantified by the β factor, was calculated for each of the nine different units of blood as both a function of storage time (Fig. 2) and flow rate (Fig. 3). Statistically significant differences were observed between the 9 units of blood after 5 weeks of storage at 100 μL min−1 and as early as 1 week of storage at 1500 μL min−1. Numerical differences were calculated for the 3 units of blood (Units 3, 6, and 9) with the lowest Pearson r coefficient. These numerical differences, which quantify dβ/dt, are indicative of the deterioration rate of the filterability being a function of storage time. Statistically significant differences were found in dβ/dt after as early as 1 week at only a low flow rate of 100 μL min−1; however, these differences were not observed at higher flow rates. A similar trend was observed when filterability was plotted as a function of flow rate at different storage times. Filterability was statistically significant different at all storage times (2,4, and 6 weeks) at low flow rates (100 and 300 μL min−1).
Fig. 2.
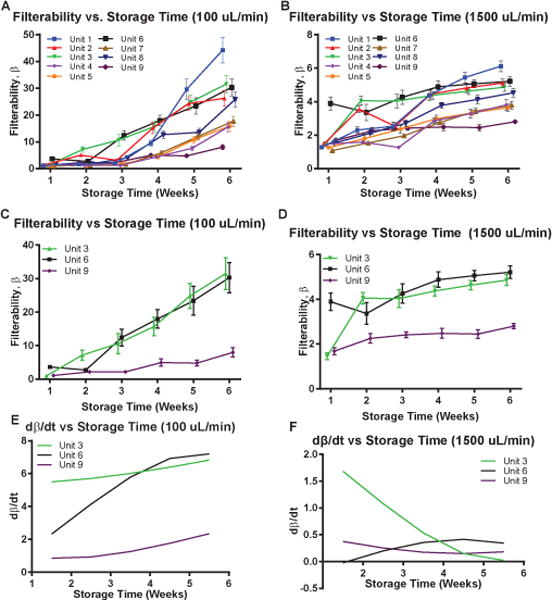
Filterability and Deterioration Rate of Filterability, as quantified by the β factor and the numerical difference of the β factor with respect to time, ∂β/∂t. Filterability vs. Storage Time for 9 different units of human RBCs at (A) 100 μL min−1 and (B) 1500 μL min−1. (C) Filterability vs. Storage Time for the 3 RBC units with the lowest Pearson r coefficient (Unit 3,6, and 9) at (C) 100 μL min−1 and (D) 1500 μL min−1. Numerical difference of the the β factor with respect to time, ∂β/∂t, calculated at (E) 100 μL min−1 and (F) 1500 μL min−1.
Fig. 3.
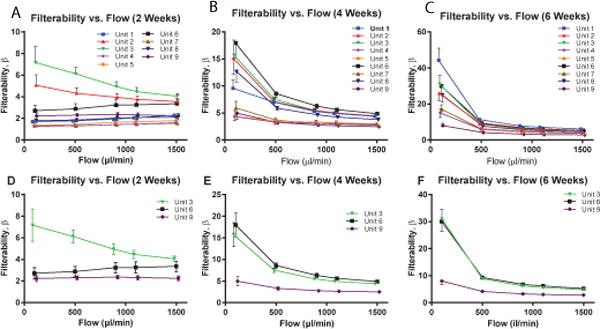
Filterability as afunction of Flow Rate at 3 Different Storage Times, as quantified by the β factor. Filterability vs. Flow Rate for 9 different units of human RBCs at (A) 2 weeks, (B) 4 weeks, and (C) 6 weeks of storage. Filterability vs. Flow Rate for the 3 different units of human RBCs with the lowest Pearson r coefficient (Units 3,6, and 9) at (D) 2 weeks, (E) 4 weeks, and (F) 6 weeks of storage.
To better quantify the behavior of filterability storage lesions, a 3-dimensional surface was interpolated for each of the 9 units of stored cells (Fig. 3). Equation (2) was used to calculate the magnitude of the filterability gradient. The magnitude was calculated at each of the points on the interpolated function, and the maximum value, which is indicative of the maximum rate of change of filterability, was found (Table 1) for each of the 9 units of stored RBCs. On average, ║∇β║ was found to be at a maximum at storage times between 4 and 6 weeks and at flow rates between 100 and 300 μL min−1.
Table 1.
Unit Specific Critical Filterability Changes.
| Storage Time Weeks |
Flow Rate μL min−1 |
|
|---|---|---|
| Unit 1 | 4.3 | 307 |
| Unit 2 | 3.8 | 123 |
| Unit 3 | 4.3 | 320 |
| Unit 4 | 6 | 100 |
| Unit 5 | 4.3 | 280 |
| Unit 6 | 2.6 | 130 |
| Unit 7 | 4.3 | 303 |
| Unit 8 | 6 | 100 |
| Unit 9 | 6 | 100 |
Critical Time and Flow Rate at which Filterability Deterioration Rates are maximized. For the surfaces interpolated in Figure 3, the gradient was calculated using the gradient function in MATLAB with increment size of 0.1 weeks and 10 μL min−1. The magnitude of the filterability gradient, (2), was calculated at every point on the surface, and the times and flow rates corresponding to the maximum magnitude of the filterability gradient were averaged and displayed here.
B. Metabolic Markers
Lactate, pH, and ATP: A summary of the metabolic markers (Lactate, pH, and ATP) of the 9 different units of blood as a function of storage time is presented in Fig. 5. Note that the statistically significant differences between at least 5 of the 9 RBC units were observed at all storage times for all metabolic markers. Numerical differences were calculated to quantify the deterioration rates of these metabolic markers, for which no statistically significant differences were observed.
Fig. 5.
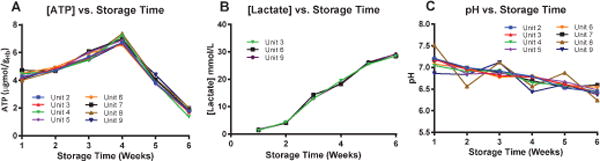
Metabolic Properties of RBCs: ATP Concentration, pH, and Lactate Concentration. (A) ATP concentration, (B) pH, and (C) Lactate Concentration for 9 different units of human RBCs. These 3 metabolic parameters were tracked over 6 weeks (42 days) of storage times, with 3 measurements taken at each time point and averaged.
C. Oxygen Affinity (p50) and 2,3 DPG
P50 and 2,3 DPG concentration were measured over storage time and are presented in Fig. 6. Statistically significant differences in p50 were observed between at least 7 of the 9 units of RBCs at all storage times; however, no statistically significant differences were observed in the numerical differences (i.e. deterioration rates). Furthermore, statistically significant differences in 2,3 DPG concentration between RBC units was observed at early storage times (between 1 and 3 weeks of storage), but disappeared after 4 weeks of storage. Likewise, statistically significant differences in the numerical difference of 2,3 DPG as a function of storage time were observed at low storage times between 1 and 2 weeks, but disappeared at later storage times.
Fig. 6.
2,3 DPG Concentration (μgmol gHb-1) and p50 (mmHg) for 9 different Units of human RBCs. (A) 2,3 DPG Concentration, and (B) p50 concentration for 9 different units of RBCS, tracked over 6 weeks (42 days) with 3 measurements taken at each time point and averaged. (C) 3 Units of human RBCs with the lowest Pearson r coefficient (Units 3,6, and 9) as well as their corresponding numerical differences, which quantify the rate of 2,3 DPG decrease in stored cells. Statistical significance in deterioration rates was observed between 1 and 2 weeks of storage time.
D. Extracellular Potassium and Hemolysis
Due to the increased RBC rigidity from storage, hemolysis and thus increased extracellular potassium are observed (Fig. 7). Statistically significant differences in both measured extracellular K+ and hemolysis in at least 5 of the 9 units of RBCs were observed after 4 weeks of storage. Furthermore, statistically significant differences were observed in the numerical differences, indicative of deterioration rates, of both extracellular K+ and hemolysis between 5 and 6 weeks of storage.
Fig. 7.
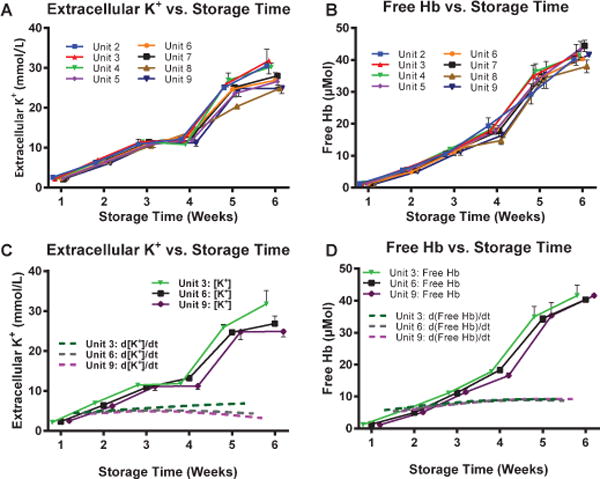
Membrane Rigidity: Extracellular [K+] and Free Hemoglobin (Hb) as a function of Storage Time. (A) Extracellular [K+] and (B) Free Hb for 9 different units of human RBCs tracked over 6 weeks (42 days) storage time. 3 stored RBC units with the lowest Pearson r coefficient and their corresponding time variant numerical differences of (C) Extracellular [K+] and (D) Free Hb. Statistical significance in deterioration rates for both parameters were observed between 5 and 6 weeks of storage time.
IV. Discussion
The principle finding in this study was that statistically significant differences were observed between units of blood for both biomechanical properties of blood (filterability), and biochemical properties of blood, including pH, lactate concentration, ATP concentration, p50, 2,3 DPG, hemolysis, and extracellular [K+]. Statistical deviations in filterability, as quantified by the variances in the observed β factor, were observed after 4 weeks of storage [22]. Furthermore, all biochemical properties of blood were found to statistically significant at all storage times after 1 week [23]. These statistical deviations between samples of blood are indicative of inter-donor variability between stored human RBCs, which are currently not taken into consideration in current blood storage protocols [16]. Furthermore, statistical variances were observed in deterioration rates of filterability, quantified as the numerical differences of the β factor in time ∂β/∂t, at low flow rates, while few statistically significant deviations were observed for deterioration rates of ATP, pH, and lactate [23]. This result suggests that the rate of formation and degradation of ATP, pH, and lactate does not vary between donors, as they are a function of the enzymatic activity present in the blood [23, 24]. However, for those biochemical properties that are a function of the mechanical rigidity of the stored RBCs, like extracellular [K+] and hemolysis, statistically significant differences were observed at late storage times [5, 7, 11]. This is likely due to these biochemical parameters being functions of RBC rigidity, and filterability, which quantifies red cell rigidity, was found to deteriorate at different rates between donors [6].
There is great clinical need in defining a quantitative metric of stored RBC quality that is more informative of the relationship between storage lesions and physiological function. As a result, the current metric of 42 days storage can be improved upon in order to minimize clinical problems that result from transfusions and ultimately decrease the number of transfusions required by patients [25]. The rate of change of filterability as both a function of time and flow (2) was found to be maximized for all RBC units between 4 and 6 weeks of storage time and between 100 and 300 μL min−1, indicating that on average, stored RBCs deterioration increases at approximately 4 weeks of storage time at low flow rates. As blood is shear-thinning fluid, viscosity decreases as shear rate increases. Therefore, at high shear rates (i.e., high flow rates), the viscous components of the stored RBCs are dominant, while at low shear rates (i.e. low flow rates), the elastic components of the stored RBCs are dominant. Therefore, increased rigidity will have greater influence on filterability at low shear rates, thus explaining why statistically significant differences were observed only at low flow rates/shear rates. As a result, this study provides a metric for testing the filterability of blood at low shear rates in order to quantify its rigidity. Because Vβ, which quantifies the rate of change of filterability, is approximately the same range for all units of blood, testing filterability of RBCs at low flow rates after 4 weeks of storage can provide a metric as to how much long the RBCs before filterability increases to levels that can influence physiological functions. Inter-donor variability in mechanical properties (filterability) is a consequence of variations in the metabolic age of RBCs between donors. RBCs with an older metabolic age have decreased lipid and water concentration as well as higher density. Because lipids are the most compliant biomolecule of the RBC membrane, decreased lipid concentration results in an increased Elastic Modulus and thus overall increased rigidity. However, this process occurs not only because of storage lesions, but also naturally throughout the life of the cell. Thus, statistically significant variations in filterability can be attributed to the fact that the red cells are collected at different times in the maturation life cycle of the cell from every donor. Statistically significant changes in the rate of filterability increase, ∂β/∂t, are a consequence of the nonlinear, viscoelastic behavior RBC membranes. Because populations of red cells in a blood unit have inherently different mechanical properties from the time of collection and because these mechanical properties deteriorate from storage lesion at different rates, their critical filterability occurs at different flow rates (shear rates). Data presented in Table 1 indicates that the storage time and flow rate when the critical filterability of each of the blood unit for different blood donors and highlights their differences.
Statistical significances in the initial (at early storage times) measured biochemical parameters, including pH, ATP concentration, lactate concentration, p50, 2,3 DPG, extracellular [K+], and hemolysis, is also a consequence of differing metabolic ages between donor cells. Red cells with an older metabolic age on average have decreased phosphate ester concentration, thus explaining statistical differences between 2,3 DPG and ATP. Statistical differences in pH, lactate, hemolysis, and extracellular [K+] can be explained by the statistical variations in the initial membrane rigidity. Membrane rigidity makes red cells more predisposed towards lysis thus increasing hemolysis and extracellular [K+]. Decreased enzymatic activity in older red cells, including PGK, PK, G6PD, and 6PGD, which are important in the RBC pentose phosphate pathway, results in decreased RBC metabolism. This results in decreased lactate production and ultimately less changes in pH, which explains statistical variations and interdonor variability in both pH and lactate concentration. However, no statistical variations were observed in deterioration rates in the metabolic properties observed in this study not only because the red cells were stored in the same chemical environment (AS-1), but also because deterioration rates are a consequence of enzymatic activity, which does not likely vary between donors.
Previous studies have shown statistically significant differences, and thus inter-donor variability, in RBC mechanical fragility (MF), % hemolysis, and Mean Corpuscular Volume (MCV) [13]. Our studies expand on this finding by not only correlating bulk mechanical properties, like filterability, with inter-donor variability in the mechanical properties of red cells, but also by providing a mathematically determined quantitative metric for testing red cell mechanical properties. Regulatory agencies and scientific societies such as the FDA and the American Association of Blood Banks (AABB) mandate that packed RBC units can be stored up to 42 days based on the premise that at least 75% of transfused RBC will be circulating 24 hours post-transfusion [26]. Ultimately, such a metric can be applied to test blood quality in a clinical setting. Clinical studies have found correlation between blood transfusions and increased adverse events post transfusion, including adverse immune responses and susceptibility to infectious complications [27]. Review of twenty clinical studies evaluating the effect of RBC transfusion on oxygen delivery showed that transfusion universally increased the hemoglobin concentration, but did not increase oxygen delivery in 75% of the studies [28]. The reason for this observation might be due to biophysical and biochemical changes occurring to RBCs during storage, therefore, supporting the need for quantitative metric for RBC quality before blood transfusion. This study provides both a method for such a measurement as well as a mathematically determined threshold for testing.
While this study provides correlations between RBC mechanical and biochemical deterioration as a function of storage times as well as evidence of inter-donor variability of donor red cells, it is unknown which of the measured biochemical and biomechanical parameters has significant effects implications on oxygen delivery to tissues. Stored blood is less able to pass through capillaries in the microcirculation resulting in decreased functional capillary density [19]. 2,3-DPG depletion results in increased RBC oxygen affinity, which prevents oxygen release to tissue. In addition, nitric oxide levels fall by up to 70% within hours of storage, resulting in vasoconstriction, reducing microcirculatory flow and oxygen delivery [29]. Future studies should aim to correlate biochemical and biomechanical properties with physiological changes in oxygen delivery and identify which parameters are most important to preserve during storage to maintain oxygen delivery in order to create both a correlation between filterability and oxygen delivery as well as a threshold value of filterability, past which the transfusion of the tested RBCs would result in transfusion related adverse effects. Future studies should aim to both confirm these results for larger populations and correlate the time and flow rate metric for filterability testing interpolated from the data in this study with changes in oxygen carrying capacity and tissue perfusion in order to determine a better threshold for testing of RBC storage lesions.
V. Conclusions
This study presented statistical variations and consequently inter-donor variability between 9 units of stored human RBCs in both mechanical properties, as quantified by filterability, and biochemical properties, including pH, lactate concentration, ATP concentration, p50, 2,3 DPG, hemolysis, and extracellular [K+]. Furthermore, this study found statistical variations in the deterioration rate of filterability after 4 weeks of storage and mathematically determined that the rate of change of filterability was found to increase fastest between 4 and 6 weeks and 100 and 300 μL min−1 for all 9 units of stored RBCs. While these studies show inter-donor variability between only 9 units of RBCs, future studies should confirm these results with a higher sample size and correlate the change in oxygen carrying capacity and tissue perfusion that results with the filterability at the mathematically determined critical storage time and flow rate.
Figure 1.
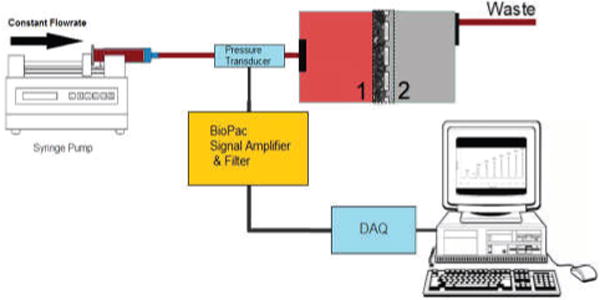
Schematic of filterability assay
Fig. 4.
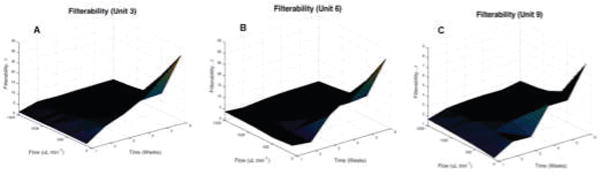
Interpolated Surface of Filterability as a function of Storage Time and Flow Rate, as quantified by the β factor. Filterability surfaces are presented for the 3 different units of human RBCs with the lowest Pearson r coefficient. Surfaces interpolated were interpolated by the MATLAB griddata function with step size of 0.1 weeks and 10 μL min−1. Surfaces are for the following units of stored human RBCs (A) Unit 3. (B) Unit 6. (C) Unit 9.
Acknowledgments
This work was supported by NIH grants from the Heart Lung and Blood Institute, T32-HL105373, P01-HL110900, R01-HL52684, R01-HL126945 and R56-HL123015.
Contributor Information
Vivek P Jani, Department of Bioengineering, University of California San Diego, La Jolla, CA 92093 USA.
Shawn Mailo, Department of Bioengineering, University of California San Diego, La Jolla, CA 92093 USA.
Ali Athar, Ali Athar is with the Department of Bioengineering, University of California San Diego, La Jolla, CA 92093 USA.
Alfredo Lucas, Department of Bioengineering, University of California San Diego, La Jolla, CA 92093 USA.
Alexander T Williams, Department of Bioengineering, University of California San Diego, La Jolla, CA 92093 USA.
Pedro Cabrales, Department of Bioengineering, University of California San Diego, La Jolla, CA 92093 USA.
References
- 1.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56(9):2173–2183. doi: 10.1111/trf.13676. [DOI] [PubMed] [Google Scholar]
- 2.Dem R, Gwinn R, Wiorkowski J. Studies on the preservation of human blood. I. Variability in erythrocyte storage characteristics among healthy donors. The Journal of laboratory and clinical medicine. 1966;67(6):955–965. [PubMed] [Google Scholar]
- 3.Dern RJ, Brewer GJ, Wiorkowski JJ. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. The Journal of laboratory and clinical medicine. 1967;69(6):968–978. [PubMed] [Google Scholar]
- 4.Zimmermann R, Heidenreich D, Weisbach V, Zingsem J, Neidhardt B, Eckstein R. In vitro quality control of red blood cell concentrates outdated in clinical practice. Transfusion clinique et biologique. 2003;10(4):275–283. doi: 10.1016/s1246-7820(03)00032-6. [DOI] [PubMed] [Google Scholar]
- 5.Pieracci FM, Moore EE, Chin T, Townsend N, Gonzalez E, Burlew CC, Barnett CC. The age of transfused blood predicts hematocrit response among critically ill surgical patients. The American Journal of Surgery. 2012;204(3):269–273. doi: 10.1016/j.amjsurg.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess JR. Scientific problems in the regulation of red blood cell products. Transfusion. 2012;52(8):1827–1835. doi: 10.1111/j.1537-2995.2011.03511.x. [DOI] [PubMed] [Google Scholar]
- 7.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sanguinis. 2009;96(2):93–103. doi: 10.1111/j.1423-0410.2008.01117.x. 2009/02. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe LC. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985;25(3):185–203. doi: 10.1046/j.1537-2995.1985.25385219897.x. 1985/05. [DOI] [PubMed] [Google Scholar]
- 9.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfusion Medicine Reviews. 2001;15(2):91–107. doi: 10.1053/tmrv.2001.22613. 2001/04. [DOI] [PubMed] [Google Scholar]
- 10.U. DEVELOPER. Transfusion of Red Blood Cells. 2016 [Google Scholar]
- 11.Almizraq R, Tchir JDR, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013 doi: 10.1111/trf.12080. n/a-n/a, 2013/01/16. [DOI] [PubMed] [Google Scholar]
- 12.Hess JR, Greenwalt TG. Storage of red blood cells: New approaches. Transfusion Medicine Reviews. 2002;16(4):283–295. doi: 10.1053/tmrv.2002.35212. 2002/10. [DOI] [PubMed] [Google Scholar]
- 13.Tarasev M, Alfano K, Chakraborty S, Light L, Doeden K, Gorlin JB. Similar donors—similar blood? Transfusion. 2014;54(3pt2):933–941. doi: 10.1111/trf.12457. [DOI] [PubMed] [Google Scholar]
- 14.Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Reviews. 1990;4(3):141–147. doi: 10.1016/0268-960x(90)90041-p. 1990/09. [DOI] [PubMed] [Google Scholar]
- 15.Card RT, Mohandas N, Mollison PL. Relationship of post-transfusion viability to deformability of stored red cells. British Journal of Haematology. 1983;53(2):237–240. doi: 10.1111/j.1365-2141.1983.tb02016.x. 1983/02. [DOI] [PubMed] [Google Scholar]
- 16.Scott KL, Lecak J, Acker JP. Biopreservation of Red Blood Cells: Past, Present, and Future. Transfusion Medicine Reviews. 2005;19(2):127–142. doi: 10.1016/j.tmrv.2004.11.004. 2005/04. [DOI] [PubMed] [Google Scholar]
- 17.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 18.Frank SM, Abazyan B, Ono M, Hogue CW, Cohen DB, Berkowitz DE, Ness PM, Barodka VM. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesthesia and analgesia. 2013;116(5):975. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293(2):H1206–H1215. doi: 10.1152/ajpheart.00109.2007. [DOI] [PubMed] [Google Scholar]
- 20.Skalak R, Impelluso T, Schmalzer EA, Chien S. Theoretical modeling of filtration of blood cell suspensions. Biorheology. 1983;20(1):41–56. doi: 10.3233/bir-1983-20104. [DOI] [PubMed] [Google Scholar]
- 21.Chien S, Schmalzer E, Lee M, Impelluso T, Skalak R. Role of white blood cells in filtration of blood cell suspensions. Biorheology. 1982;20(1):11–27. doi: 10.3233/bir-1983-20102. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick U, Adams R, Lardi A, McCollum C. Rheological properties and function of blood cells in stored bank blood and salvaged blood. British journal of haematology. 1998;101(2):364–368. doi: 10.1046/j.1365-2141.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 23.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences. 2007;104(43):17063–17068. doi: 10.1073/pnas.0708160104. 2007/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. Journal of Cellular and Molecular Medicine. 2007;11(1):148–155. doi: 10.1111/j.1582-4934.2007.00008.x. 2007/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. Journal of Trauma-Injury Infection and Critical Care. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 26.Hess JR, Hill HR, Oliver CK, Lippert LE, Rugg N, Joines AD, Gormas JF, Pratt PG, Silverstein EB, Greenwalt TJ. Twelve-week RBC storage. Transfusion. 2003;43(7):867–872. doi: 10.1046/j.1537-2995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann A, Farmer S, Shander A. Five drivers shifting the paradigm from product-focused transfusion practice to patient blood management. The oncologist. 2011;16(suppl 3):3–11. doi: 10.1634/theoncologist.2011-S3-3. [DOI] [PubMed] [Google Scholar]
- 28.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Critical care medicine. 2009;37(12):3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 29.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfusion and Apheresis Science. 2010;43(1):69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


