Abstract
Background
For a 2- to 6-year period, interventionists for the TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) randomized clinical trial delivered a family-based, behavioral weight-loss program (the TODAY Lifestyle Program) to 234 youth with type 2 diabetes. Interventionists held at least a bachelor’s degree in psychology, social work, education, or health-related field and had experience working with children and families, especially from diverse ethnic and socioeconomic backgrounds. This article describes the administrative and organizational structure of the lifestyle program and how the structure facilitated collaboration among study leadership and lifestyle interventionists on the tailoring of the program to best suit the needs of the trial’s diverse patient population.
Methods
During the pilot phase and throughout the duration of the trial, the interventionists’ experiences in delivering the intervention were collected in a variety of ways including membership on study committees, survey responses, session audio recordings, and feedback during in-person trainings.
Results
The experiences of interventionists conveyed to study leadership through these channels resulted in decisions to tailor the lifestyle intervention’s delivery location and ways to supplement the standardized educational materials to better address the needs of a diverse patient population.
Conclusion
The methods used within the TODAY study to encourage and utilize interventionists’ experiences while implementing the lifestyle program may be useful to the design of future multi-site, clinical trials seeking to tailor behavioral interventions in a standardized, and culturally and developmentally sensitive manner.
Keywords: Organizational research design, collaborative research, lifestyle intervention, lifestyle treatment
Introduction
Rates of type 2 diabetes in youth have increased dramatically along with obesity.1,2 The TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) study, a multi-site, randomized clinical trial, was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health to evaluate the efficacy of three treatments: metformin alone, metformin combined with rosiglitazone, and metformin combined with a family-based, behavioral weight-loss program.3 The TODAY study found the addition of rosiglitazone to metformin to be associated with superior glycemic control when compared to metformin monotherapy or metformin plus family-based behavioral weight control.4 Although the metformin plus family-based treatment was not associated with improved glycemic control, participants randomized to this treatment arm had favorable short-term changes in percent overweight in comparison with the other intervention groups.4
Prior to TODAY, no behavioral weight-loss program designed specifically for children and adolescents with type 2 diabetes had been developed. Traditionally, when developing an intervention, behavioral health researchers follow a “top down” approach in which decisions about the treatment and how it will be delivered are made without feedback from the individuals who will be providing the intervention.5,6 However, implementation and organizational/systems researchers have begun to recognize the benefits of including direct line staff in the development of an intervention.5–7 Despite this information, multi-center research trials rarely provide opportunities for the staff to contribute to the intervention’s development.8,9
Before the implementation of the TODAY lifestyle intervention, a variety of quality-control procedures were developed to ensure a standardized delivery of the intervention across the study’s multiple clinical sites. These procedures included training prior and throughout the delivery of the intervention, on-going supervision and an organizational structure by which interventionist suggestions for tailoring the treatment delivery were vetted and then implemented consistently across sites. This article describes the systematic process used to incorporate the interventionists’ experiences and suggestions in tailoring the intervention to meet the needs of a diverse population while quality control procedures insured that the delivery of a multi-centered behavioral intervention remained standardized.
Methods
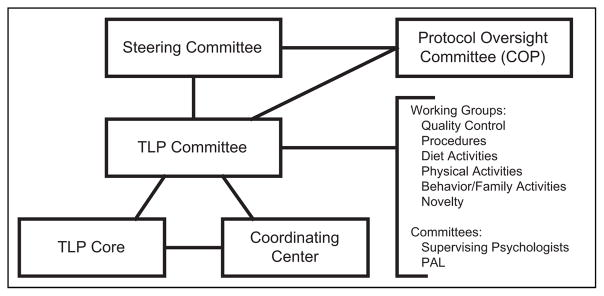
The TODAY Lifestyle Core and TODAY Lifestyle Committee comprised leading experts in pediatric endocrinology and family-based weight-loss interventions and were embedded within a larger organizational structure (Figure 1). These committees were responsible for the development and facilitation of the culturally and developmentally appropriate TODAY Lifestyle Program (TLP).10 The TLP was a standardized family-based, weight-loss intervention using behavioral modification techniques and social support methods to assist youth and their families in choosing healthier lifestyles.10 Another committee, the Physical Activity and Nutrition Leaders Committee comprised interventionists who held a bachelor- or master-level degree in psychology, social work, education, or another related field and had previous experience working with children and families, especially from low income and minority populations. This committee provided a forum in which the interventionists could discuss issues related to their experiences delivering the TLP. The committee met weekly for the first 2 years through conference calls; then bi-monthly for the next 3 years. Finally, for the remainder of the study, the committee held monthly conference calls. To allow for open discussions, these calls were chaired by interventionists who were peer-selected. These discussions provided the interventionists opportunities to share barriers experienced in implementing the TLP and to brainstorm possible solutions. The committee chairs were then encouraged to provide candid reports of issues raised by interventionists to the TODAY Lifestyle Committee. These reports aided leadership in early identification of implementation challenges so that these issues could be addressed in a consistent manner across all study sites.
Figure 1.
TODAY organizational structure elements involved in the design and implementation of the TODAY Lifestyle Program (TLP).
Another valuable aide in identifying delivery challenges was the interventionists’ training sessions. Before study enrollment began, a pilot phase of the trial was designed to provide the interventionists training on how to deliver the standardized intervention and a gauge to monitor implementation challenges.10 The interventionists had the opportunity to practice the intervention with families who were ineligible for study enrollment due to not meeting the study’s inclusion critiria.3 These session were audio taped, with the participant’s and family’s approval, and reviewed by a TODAY Lifestyle Committee member to monitor implementation challenges and to insure delivery standardization. The recordings were so useful during the pilot phase, that all intervention sessions were recorded and monitored.
Frequent, in-person training sessions were also conducted that provided an avenue to assess implementation challenges while ensuring standardization. Starting in 2006, the interventionists were asked to complete surveys prior to the in-person trainings. These surveys were designed to monitor implementation challenges, assess possible training needs, and encourage the sharing of implementation experiences. Over 4 years (October 2006 through May 2010), the interventionists were queried seven times. On average, 82% of the interventionists (range 60%–100%) returned surveys to the TODAY Lifestyle Core. Once de-identified, the responses were used to develop training topics including issues related to implementation procedures and challenges, promoting behavioral change, adjusting to changing developmental stages, and motivating study participation. During these early trainings, interventionists were encouraged to present case examples and describe their experiences implementing the TLP.
Making adjustments to a standardized trial while the trial is being conducted raises concerns of consistency in delivery and data integrity. These concerns were carefully considered whenever any modifications were made to the delivery of the intervention. It is important to note that many of the interventionists’ suggestions were not acted upon by the TODAY study leadership due to concerns that the change may affect the study’s outcome. For example, one suggestion was to collect participant’s feedback and suggestions during trial enrollment. Though fruitful information may have been collected, study leadership felt such a query would be lengthy and might compromise integrity across treatment arms. It is important to note, however, that all participants’ feedback was collected at the conclusion of the study.11
Results
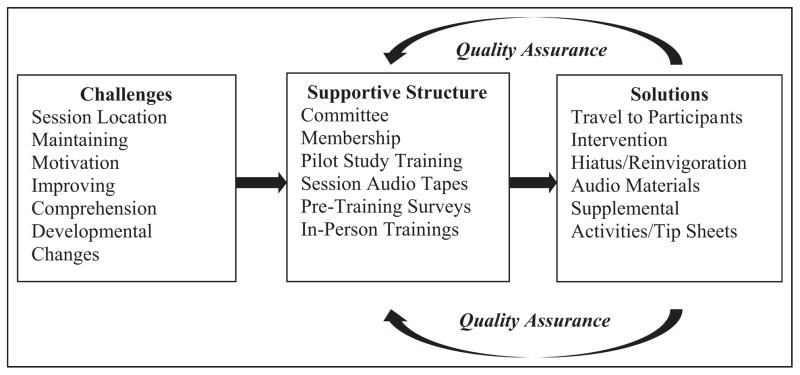
Through committee meetings and training sessions, interventionists shared their experiences implementing the intervention. Study leadership monitored the standardized delivery of the intervention, assessed challenges, and, when necessary, provided supplemental adaptations and materials (Figure 2). Since these items were designed to supplement the intervention’s key components, they were available to all lifestyle participants.
Figure 2.
Illustration of supplementary development.
Location to conduct the intervention
The lifestyle intervention was designed to be delivered in the clinic. However, one of the commonly reported challenges by the interventionists was the difficulty in scheduling TLP sessions during clinic business hours. Only 31.0% of TLP participants lived with both biological parents and 43.4% reported household annual income of less than US$25,000.12 For many participants, a parent or support person was not available during working hours and/or the family experienced barriers traveling to the clinic. To address these challenges, the study leaders agreed to allow interventionists to travel to the families’ homes or communities. Of the >12,000 TLP visits conducted during the trial, 40% were completed in the clinic, 19% were conducted in the family home, 17% at community locations, 20% by phone, and 2% by other forms of communication.
Maintaining intervention intensity and duration
TLP sessions were designed to be delivered at different intensities across the course of the study.10 As the frequency of contact decreased and the length of time in the trial increased, interventionists noted some participants’ motivation for behavioral change began to wane. To help address the dynamic nature of motivation, study procedures were developed to retain TLP participants during times of acute stressors when participation at the recommended frequency was not possible, and to capitalize on motivation for behavioral change later in the study when treatment contact was designed to decrease. These procedures included a study-approved hiatus for participants facing acute personal crises (e.g. death of a family member, temporary homelessness) or a brief re-intensification of the intervention during later phases (i.e. weekly contact for 4–8 weeks).10
Comprehension of the written materials
The educational materials developed for the TLP were written at a third- or fourth-grade reading level and provided in English and Spanish. In fact, 39.7% of study participants were Hispanic.10,12 However, the interventionists reported that some participants could not read the materials due to reading comprehension difficulties and some support persons, though fluent in Spanish, could not read Spanish or English. To alleviate these difficulties, the TODAY Lifestyle Core and interventionists worked together to produce audio-recorded versions of the materials.
Modification of the intervention to meet developmental differences across time
Participants in TODAY were between the ages of 10 and 17 years and enrolled for a minimum of 2 years and a maximum of 6 years (average: 3.9 years). As a result, interventionists could end treatment with individuals aged anywhere from 12 to 23 years of age.12 Due to the length of the study, role transitions were common within families (e.g. becoming a caregiver to an ill parent or younger sibling) and often the result of maturation (e.g. attending college, starting full-time employment, or having children). TLP interventionists experienced challenges in promoting behavioral change across these transitions.
In response to these challenges, the interventionists collaborated with TLP Lifestyle Core and Committee members to develop supplemental activities that were designed for these transitions (Table 1). A standardized procedure for proposing and using supplemental activities was developed early in the development of the TLP to ensure consistent opportunities for use of these supplemental activities across sites. Interventionists reported that these activities were well received by the participants and were energizing for the interventionists as well. Interventionists with exercise physiology training and those with nutrition backgrounds were instrumental in proposing and designing many of these supplemental activities: trips to the grocery store to learn nutrition label-reading, comparison shopping skills, the use of pedometers, and resistance bands.
Table 1.
Topics and supplemental materials for the TODAY Lifestyle Program (TLP).
| TLP intervention session topic | Supplemental activity | Tip sheet topic |
|---|---|---|
| Introduction to the TODAY Lifestyle Program (TLP) |
|
|
| TLP eating plan—what is healthy and what is not so healthy |
|
|
| Shopping and cooking—it is all in the planning/making your home a healthy place |
|
|
| Eating smart—breakfast, lunch dinner, snacks, and drinks/be a model and a coach for healthy eating |
|
|
| Problems into solutions/special events—parties and holidays/dining out |
|
|
| Learning to eat when you’re hungry—and not when you’re not/managing emotions the healthy way |
|
|
| Get physical, be active, and get healthy/get your family to be physically active/exploring interests and activities/be a healthy role model for your child |
|
|
| Sedentary behavior/get active by reducing the time you sit around |
|
|
| Praise helps to make lifestyle changes/positive reinforcement encourages lifestyle changes |
|
|
| Moving on to maintain your health/keep yourself motivated—continue healthy behaviors |
|
|
Tip Sheets were also developed for the later phase of the intervention: a single-page, educational document that illustrated a specific take-home message (Table 1). Many Tip Sheet topics were suggested by the interventionists in response to changes in their participants’ developmental and motivational needs, such as how to build physical activity into work/college routines, how to prepare healthy meals for one, and how to prioritize healthy lifestyle behaviors while facing stressors often associated with adulthood (e.g. caring for an ill parent or raising a family).
Conclusion
One limitation of this article is that no formal method of measurement was used to assess the efficiency and effectiveness of the described organizational structure. The TLP committee structure described above was designed to monitor the intervention and maintain standardization. Only in retrospect were the noted benefits observed. The inability to measure the structure’s effectiveness may limit our model’s generalizability and productivity in other clinical trials.
It is important to note that the TODAY study metformin plus lifestyle treatment was not significantly different than the metformin alone in improving glycemic control.4 While we believe the supplemental adaptations described above enhanced the implementation of the intervention, we do not believe that the adaptations impacted the overall study outcome. The intervention’s core components were safeguarded by the TODAY study leadership’s careful and thorough review of each adaptation while keeping in mind the overall impact on the standardized implementation.
The TLP was designed to be delivered in a standardized manner to families that were diverse in age, race/ethnicity, socioeconomic background, and motivation to make behavioral changes. In addition, the length of time that participants were involved in active treatment in the TLP (average: 3.9 years and maximum: 6.0 years) was an unprecedented length of time for participation in a family-based, behavioral weight-loss treatment study.12 Not surprisingly, during the course of the trial, most participants experienced significant and varied life challenges to which the interventionists needed to be sensitive and responsive while maintaining the integrity of the protocol. The TODAY organizational structure provided study leadership and the interventionists avenues for frequent communication so that the interventionists could meet their participant’s needs while insuring that the intervention’s integrity was maintained. We believe the organizational structure of the TODAY study, which allowed for on-going collaboration between interventionists and study leadership, may serve as a model for other multi-site clinical trials implementing behavioral health interventions.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NIDDK project office was involved in all aspects of the study, including design and conduct; collection, management, analysis, and interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication. The TODAY Study Group thanks the following companies for donations in support of the study’s efforts: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; and Sanofi Aventis. The authors also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members. Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was completed with funding from NIDDK and the NIH Office of the Director through grants U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254.
Appendix 1
The following individuals and institutions constitute the TODAY Study Group (*indicates principal investigator or director):
CLINICAL CENTERS
Baylor College of Medicine: S. McKay*, B. Anderson, C. Bush, S. Gunn, M. Haymond, H. Holden, K. Hwu, S. M. Jones, S. McGirk, B. Schreiner, S. Thamotharan, and M. Zarate; Case Western Reserve University: L. Cuttler*, E. Abrams, T. Casey, W. Dahms (deceased), A. Davis, A. Haider, S. Huestis, C. Ievers-Landis, B. Kaminski, M. Koontz, S. MacLeish, P. McGuigan, S. Narasimhan, and D. Rogers; Children’s Hospital Los Angeles: M. Geffner*, V. Barraza, N. Chang, B. Conrad, D. Dreimane, S. Estrada, L. Fisher, E. Fleury-Milfort, S. Hernandez, B. Hollen, F. Kaufman, E. Law, V. Mansilla, D. Miller, C. Muñoz, R. Ortiz, J. Sanchez, A. Ward, K. Wexler, Y. K. Xu, and P. Yasuda; Children’s Hospital of Philadelphia: L. Levitt Katz*, R. Berkowitz, K. Gralewski, B. Johnson, J. Kaplan, C. Keating, C. Lassiter, T. Lipman, G. McGinley, H. McKnight, B. Schwartzman, and S. Willi; Children’s Hospital of Pittsburgh: S. Arslanian*, F. Bacha, S. Foster, B. Galvin, T. Hannon, A. Kriska, I. Libman, M. Marcus, K. Porter, T. Songer, and E. Venditti; Columbia University Medical Center: R. Goland*, R. Cain, I. Fennoy, D. Gallagher, P. Kringas, N. Leibel, R. Motaghedi, D. Ng, M. Ovalles, M. Pellizzari, R. Rapaport, K. Robbins, D. Seidman, L. Siegel-Czarkowski, and P. Speiser; Joslin Diabetes Center: L. Laffel*, A. Goebel-Fabbri, M. Hall, L. Higgins, M. Malloy, K. Milaszewski, L. Orkin and A. Rodriguez-Ventura; Massachusetts General Hospital: D. Nathan*, L. Bissett, K. Blumenthal, L. Delahanty, V. Goldman, A. Goseco, M. Larkin, L. Levitsky, R. McEachern, K. Milaszewski, D. Norman, B. Nwosu, S. Park-Bennett, D. Richards, N. Sherry, and B. Steiner; Saint Louis University: S. Tollefsen*, S. Carnes, D. Dempsher, D. Flomo, V. Kociela, T. Whelan, and B. Wolff; State University of New York Upstate Medical University: R. Weinstock*, D. Bowerman, S. Bristol, J. Bulger, J. Hartsig, R. Izquierdo, J. Kearns, R. Saletsky, and P. Trief; University of Colorado Denver: P. Zeitler* (Steering Committee Chair), N. Abramson, A. Bradhurst, N. Celona-Jacobs, J. Higgins, A. Hull, M. Kelsey, G. Klingensmith, K. Nadeau, and T. Witten; University of Oklahoma Health Sciences Center: K. Copeland* (Steering Committee Vice-Chair), E. Boss, R. Brown, J. Chadwick, L. Chalmers, S. Chernausek, C. Macha, R. Newgent, A. Nordyke, D. Olson, T. Poulsen, L. Pratt, J. Preske, J. Schanuel, J. Smith, S. Sternlof, and R. Swisher; University of Texas Health Science Center at San Antonio: J. Lynch*, N. Amodei, R. Barajas, C. Cody, D. Hale, J. Hernandez, C. Ibarra, E. Morales, S. Rivera, G. Rupert, and A. Wauters; Washington University School of Medicine: N. White*, A. Arbeláez, J. Jones, T. Jones, M. Sadler, M. Tanner, A. Timpson, and R. Welch; Yale University: S. Caprio*, M. Grey, C. Guandalini, S. Lavietes, M. Mignosa, P. Rose, A. Syme, and W. Tamborlane.
COORDINATING CENTER
George Washington University Biostatistics Center: K. Hirst*, S. Edelstein, P. Feit, N. Grover, C. Long, and L. Pyle.
PROJECT OFFICE
National Institute of Diabetes and Digestive and Kidney Diseases: B. Linder*
CENTRAL UNITS
Central Blood Laboratory (Northwest Lipid Research Laboratories, University of Washington): S. Marcovina*, J. Chmielewski, M. Ramirez, and G. Strylewicz; DEXA Reading Center (University of California at San Francisco): J. Shepherd*, B. Fan, L. Marquez, M. Sherman, and J. Wang; Diet Assessment Center (University of South Carolina): M. Nichols*, E. Mayer-Davis, and Y. Liu; Lifestyle Program Core (Washington University): D. Wilfley*, D. Aldrich-Rasche, K. Franklin, D. Laughlin, G. Leibach, C. Massmann, M. Mills, D. O’Brien, J. Patterson, T. Tibbs, D. Van Buren, and A. Vannucci.
OTHER
Centers for Disease Control: P. Zhang; State University of New York at Buffalo: L. Epstein; University of Florida: J. Silverstein.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Barbara J Anderson is on the Scientific Advisory Board of Sanofi. None of the other authors have anything to disclose.
References
- 1.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 3.Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady T, Davies A. Building project capabilities: from exploratory to exploitative learning. Organ Stud. 2004;25:1601–1621. [Google Scholar]
- 6.Leplat J. Methodologies of task analysis and task design. J Ind Organ Psych. 1988;32:2–12. [Google Scholar]
- 7.Glaser EM, Van Eynde DF. Human resource development, team building, and a little bit of “kiem tau”: I. Organ Dev J. 1989;7:21–24. [Google Scholar]
- 8.Cronin MA, Weingart LR, Todorova G. Dynamics in groups: are we there yet? Acad Manag Ann. 2011;5:571–612. [Google Scholar]
- 9.Kozolowski SWJ, Chao GT. The dynamics of emergence: cognition and cohesion in work teams. Manag Decis Econ. 2012;33:335–354. [Google Scholar]
- 10.TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes. 2010;34:217–226. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walders-Abramson N, Anderson B, Larkin ME, et al. Benefits and barriers to participating in longitudinal research of youth-onset type 2 diabetes: results from the TODAY retention survey. Clin Trials. 2016;13:240–243. doi: 10.1177/1740774515613949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–167. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]